 Experimental Evidences of Stable Water Nanostructures at Standard Pressure and Temperature Obtained by Iterative Filtration
Experimental Evidences of Stable Water Nanostructures at Standard Pressure and Temperature Obtained by Iterative Filtration
Elia V1, Ausanio G4, De Ninno A3*, Germano R2, Napoli E1 and Niccoli M1
1Department of Chemical Sciences, University of Naples “Federico II”, Complesso Universitario Monte Sant’Angelo, Via Cinthia 80126, Naples, Italy
2PROMETE Srl, CNR Spin off Via Buongiovanni, 49 80046 San Giorgio a Cremano (NA), Italy
3ENEA Frascati, UTAPRAD-DIM Department C.R. 00044 Frascati, Italy
4Department of Physics and CNR-SPIN University “Federico II” of Naples, Piazzale V. Tecchio 80 80125 Naples, Italy
*Correspondence E-mail: antonella.deninno@enea.it
Key Words: Water, Nanostructures, Iterative filtration, Nafion membrane, Spectroscopy, Microscopy
Received June 13th, 2013; Revised Sept 23rd, 2013; Accepted Nov 13th, 2013; Published Jan 11th, 2014; Available online January 20th, 2014
Abstract
In a previous paper (WATER Journal Vol. 5) we have shown the modifications induced in the supra-molecular structure of water after iterative long lasting contact with a Nafion® surface. In the present paper we show that structural changes can also be induced by other kinds of perturbations such as the iterative filtration on sintered glass and disposable (Millipore) filters. Fourier Transformed Infrared spectroscopy, UV spectroscopy and Atomic Force Microscopy (AFM) have been used to study samples after the iterative filtering: the UV spectrum shows the appearance of an absorption peak at about 275 nm while the FT-IR of filtered water remains substantially unchanged with respect the untreated water. Sample of 20 ml have been then lyophilised obtaining an unexpected amount of solid residues whose nature has been investigated by FT-IR, and a few drops have been evaporated on mica sample holders at room temperature and pressure for AFM investigation. The emerging picture suggests that physical perturbations having a low energy content can promote an unexpected auto-organization in liquid water, able to survive to the evaporation with the formation of a solid phase stable at standard pressure and temperature.
Article Outline
- Introduction
- Materials and Methods
- Results and Discussion
- Conclusions
- References
- Discussion with Reviewers
Introduction
The properties of liquid water that make it necessary to support life seem to be especially based on its peculiar characteristic of auto organization. The structure of liquid water has been thought for many years to be related to the properties of the hydrogen bond network [1]. Liquid state of water has been described as a collection of independent molecules held together by weak electrostatic bonds. Such a description has been able to account for many of the properties of water, since the first formulation by Linus Pauling to explain its anomalous boiling point. However, it has been reported, by fitting data from different experimental techniques, that all the anomalies of liquid water can be also explained in terms of domains of different density [2]. Observations made by biologists about the properties of water nearby the cellular membranes reveal the existence of micro domains of structured water that extend from tens to hundreds of Angstrom from cells and macromolecules boundaries, together with independent H2O molecules responsible for the solvation processes [3-5]. Moreover, very recently, a new discipline has been proposed that provides a framework for understanding changes in the structure of water caused by various perturbations. This discipline has been named aquaphotomics [6] that is an approach which looks at water as a multi-element system that could be well described by its multi-dimensional spectra. In fact, distinct water configurations, for example dimers or larger aggregates, are known to contribute very differently to the Near Infra-Red (NIR) spectrum [7, 8]. Since these configurations are very sensitive to the amount and charges of the solutes as well as to their configuration, the NIR spectrum of water contains significant information about the solute. This findings may be extended to any perturbation affecting water even mechanical in nature. In a previous paper we have shown that a long-lasting changes in the structure of liquid water can be induced by an enduring contact with Nafion membranes [9]. The appearance of stable structures surviving to the removal of the perturbation and it implies the formation of dissipative structures able to exchange energy with the external environment.
The aim of this paper is to focus on Iteratively Filtering Water (IFW) on cellulose and sintered glass filters. In addition to pH, conductivity, density and calorimetric measurements already shown elsewhere [10-12], spectroscopic measurements in IR and UV-vis range have been done in order to collect information about both static and dynamics structure; atomic force microscopy (AFM) has been used to investigate the topography of the unexpected solid residues obtained from a few evaporated drops of water on the AFM mica sample holders.
Materials and Methods
The iterative filtration process of a given volume (1-30 ml) of Milli-Q water consists in under-vacuum filtering using the apparatus shown in Figure 1. The resultant filtrate liquid water is collected and filtered again up to 250 times. The following kinds of filters have been used:
a. Disposable filters (Millipore) made of cellulose acetate or nitrate, with porosities of 0.450, 0.200, 0.100 and 0.025 μm. Millipore filters require use of sintered glass filters as support during the under vacuum process;
b. Glass filters (Büchner funnels) with porosity between 5 and 90 μm: R1 = 90-150 μm, R2 = 40-90 μm, R3 = 15-40 μm, R4 = 5-15 μm and R5 = 1-5 μm.

Figure 1: Apparatus for the filtration under reduced pressure.
The main chemical impurities released by the glass filters are derived from alkaline oxide (Na2O) released by the glass. In contact with water, they transform into sodium hydroxide (NaOH), and then, due to atmospheric carbon dioxide (CO2), into sodium bicarbonate (NaHCO3). The concentrations of impurities deriving from the other components of the glass — SiO2, B2O3 and Al2O3 — are much lower than sodium bicarbonate. To exclude the contribution of chemical impurities of different nature from the filters, before starting the filtration procedure, they were rinsed with abundant water until they produced a filtrate with electrical conductivity of 1.2–2.0 µS cm-1 [12]. During the experiment, no extraneous chemical substances were introduced into the water, other than those ones deriving from the partial dissolution of the glass solid support. In order to quantitatively detect these pollutants in the samples, ICP (Inductively Coupled Plasma) mass spectrometry has been used: it has been found that the amount of the impurities released by the glass can account only for 10 to 30% of the changes in the physical-chemical parameters.
The physical-chemical parameters of IFW have been studied in other papers [10 – 12]. In particular: electrical conductivity, χ / μS cm-1, heat of mixing with acid (HCl), ΔQmixHCl or basic (NaOH) solutions, ΔQmixNaOH and pH have been measured. Electrical conductivity χ increases up to two orders of magnitude with the number of filtrations; ΔQmixNaOH was exothermic and linearly proportional to the electrical conductivity; the analogous ΔQmixHCl was also found to be exothermic but smaller, in absolute value. We have also observed that the greater is the number of iterations the larger is the increase of the electrical conductivity which, in turn, is also function of the dimension of the pores of the filter and of the volume of the sample [12]. Calorimetric titration with HCl have shown an enhanced interaction between H3O+ ions and the newly formed molecular aggregates thus causing an excess of free OH– and a shift of pH toward more alkaline values.
FT-IR Spectroscopy
IR Spectra on liquid samples have been recorded using a FTIR (JASCO-FT-IR-410) spectrometer in the Attenuated Total Reflectance (ATR) mode with a 45° single reflection ZnSe crystal. The spectrometer was equipped with a conductive ceramic coil mounted in a water-cooled copper jacket source, a KBr beam splitter and a TGS detector. The recording conditions for each FTIR spectrum were: 120 scans, a triangular apodization function and a resolution of 4 cm-1.
The IR spectra in the solid state have been recorded using FTIR JASCO-FT-IR-430 spectrometer in a NaCl dispersion medium: 20 ml of IFW mixed 200 mg of NaCl and lyophilized, the obtained solid is then pressed to form a pellet. As a point of reference we used pellets prepared with milli-Q water instead of IFW.
UV-Vis Spectroscopy
The UV/Vis spectra were monitored using a UV/Vis Spectrophotometer (JASCO V-560). This is a double-beam spectrophotometer with double monochromator, a wavelength range of 190-900 nm and a resolution of 0.1 nm. It compares the light intensity between two light paths, one containing the reference and the other the test sample, so that the resulting signal represents the difference between the signals detected from the two samples. The photometric accuracy is: ± 0.002 and the photometric reproducibility is: ± 0.001.
Atomic Force Microscope (AFM)
The Nanoscope III Digital Instruments by Veeco has been used to take the topography of the residues from 3 to 5 drops of liquid water evaporated on substrates of mica (sample holders) whose purity was guaranteed by careful cleavage, at room temperature and pressure. The evaporation proc-ess takes slightly more than 10 minutes. The AFM topographies were obtained in non-contact mode with an ultra-thin silicon point, with apical radius less than 5 nm, on windows of different area, setting an acquisition frequency of 2 Hz. As control we used Milli-Q water kept in contact for long time (months) with powder of Pyrex glass and let it dry on the same kind of mica sample holders.
Results and Discussion
The IR spectra of the liquid samples do not show any significant difference with normal Milli-Q water, so we can conclude that the number of absorbing dipoles per energy channel is unaffected by the applied procedure, i.e., the energy distribution in the liquid water molecules has not been changed by the iterative filtration.
UV-Vis spectra of liquid IFW show a new absorption peak appears at approximately ~ 270 nm. Being a double beam spectrometer, Fig. 2 is showing the difference spectrum of IFW and reference liquid water. A similar result has also been found in the Iteratively Nafionized Water (INW) [9] and in the Exclusion Zone [13] at a distance of about 100 µm from the Nafion surface. It must be kept into account that the leaching of organic components from glass and acetate filters have been minimized by abundant and repeated rinses before the usage; moreover, nanometric dimensions of the cellulose filters exclude the presence of moulds and bacteria in IFW.
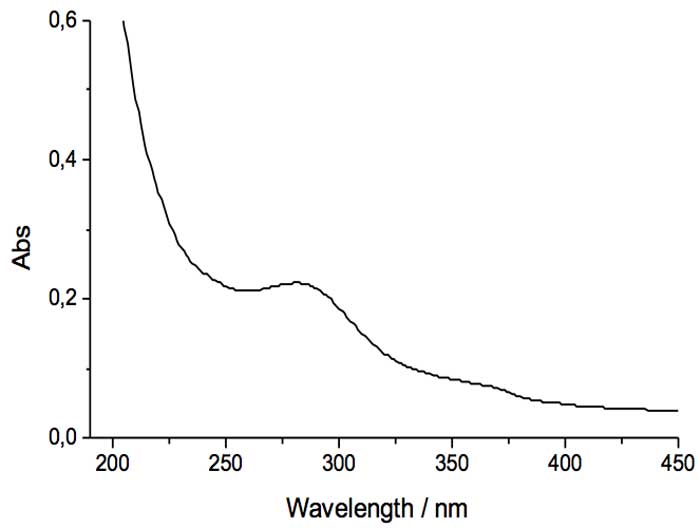
Figure 2: UV difference spectrum of IFW and reference liquid water. T=298 K.
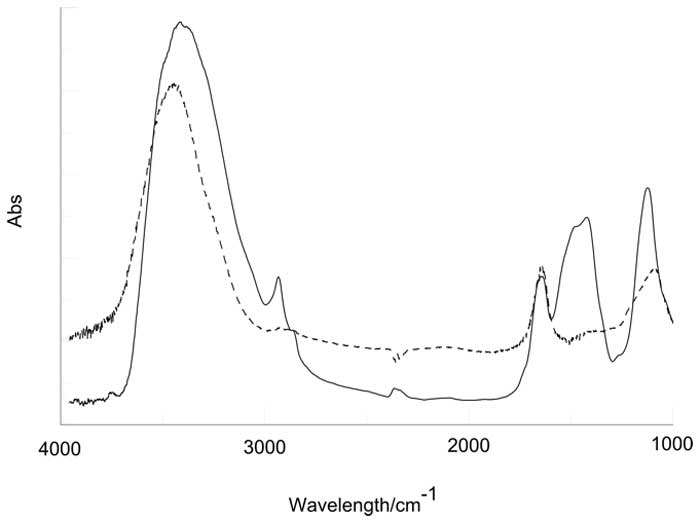
Figure 3: IR spectrum of lyophilized IFW sample (black line). The broken line is the spectrum obtained preparing the pellet with milli-Q water (see text).
Samples of 20 ml each have been lyophilized obtaining an unexpected large quantity of solid residue of the order of few milligrams. The production of such a solid residues as a consequence of the elimination of bulk water (evaporation at room temperature, evaporation at high temperatures, 90°C, lyophilization) has been already observed and described in the literature [9, 10, 14]. These findings apparently show the existence of solid aggregates of water molecules at room temperature and pressure. The nature of these structures in the solid phase has been investigated by IR spectroscopy and AFM Microscopy. Fig. 3 shows the IR spectrum of the residues of IFW sample compared to the spectrum of liquid water in the range 4000-1000 cm-1. The broad peak in the region 3600-3000 cm-1 corresponds to the O-H stretching region: while the maximum appears to be shifted to higher frequency, the spectrum shows a new peak at about 2926 cm-1 whose origin is unknown. The broad peak attributed to O-H stretching is known to be influenced by the sovra-molecular structure of liquid water and it can be de-convoluted into sub-bands which are usually assigned to different bond ordering parameters. In fact, in vapor, where molecules can be safely assumed to be independent, the sub-bands centered at 3200 and 3400 cm-1, observed in condensed water are absent; on the contrary in ice the dominant feature is the sub-band at 3200 cm-1 while the other features fade away by decreasing temperature. Since we know that component molecules in ice are mutually correlated, we can assume that the sub-band centered at 3200 cm-1 is produced by the sub ensemble of molecules which are mutually correlated. It is straightforward to see that the spectrum of lyophilized IFW sample doesn’t resemble the IR spectrum of ice even though the shift towards the low energy region indicates a higher degree of organization in the residue with respect to the liquid. A very similar behavior has been observed on INW and described in ref [9]. The peak located at 1650 cm-1, corresponding to the bending of H2O doesn’t show any difference with respect to reference water, however, other structures at lower wave numbers, whose origin is unknown, appear in the IFW spectrum.
AFM provides useful information about the topological structure of these solid residues: Fig. 4 shows the residue obtained by evaporating at room pressure and temperature five drops of a sample having an electrical conductivity χ= 141 μS cm-1. The different color codes from bright to dark indicate the height of the clusters as high as 140 nm, while the average size is of about 200 nm. The samples of IFW are compared to the deposit of a pure Milli-Q water kept in contact for some months with a powder of Pyrex glass , this assures that the same chemical impurities as in the iteratively filtering procedure have been possibly extracted from the glass. As expected, no residue is produced after the evaporation of drops of pure water.
Physical theories of liquid water considering only short-range electrostatic interactions between molecules are far from explaining the phenomena described in this and other papers recently appeared in the literature such as the emergence of a zone close to hydrophilic surfaces where solutes are not admitted. Since the perturbations applied to the system have a an energy content very much smaller than the energy required to create an organization on a mesoscopic scale, it is necessary to abandon the picture of a direct energy transfer between the perturbation and the single water molecule. The inspection of the IR spectrum of the liquid in the range 2800-3800 cm-1, related to the OH stretching vibrations, confirms that the number of dipoles oscillating at a certain frequency remains unchanged when subjected to iterative filtration. This allows to exclude a direct energy absorption by the dipoles. Furthermore, the alterations of the transport parameters [11, 12] as well as the size of the aggregates found in INW [9] suggest an increase of the aggregation of H2O molecules.
1) In case water is left in contact with finely divided glass powder for very long time, a solubilization of the glass components happens. Na2O is the most soluble component of glass and interacts with CO2 to form NaHCO3,which is responsible for the increase of conductivity (χ=84 μS cm-1).
The astonishing solid residues observed with AFM show a cauliflower structure whose mean dimension is of few hundreds of nanometers. This observation, together with the modification of the IR spectrum of the solid phase with respect to the liquid phase lead us to the conclusion that the modification of the supra-molecular arrangement induced by the iterative filtration are able to survive to the phase transition. It has been suggested elsewhere [15,16] that the presence of nanobubbles of atmospheric dissolved gases can be responsible for the formation of cluster and aggregates which behaves like nanoparticles. It has been proposed that the hydrophobic attraction, responsible for the formation of aggregates in liquid solutions, is due to the bridging of nanobubbles. The main objection is that the theoretically short lifespan of bubbles due to their high internal pressure. Moreover the presence of domains on a hydrophobic glass surface immersed in solution has been proved only in liquid phase [16]. Nanobubbles can be produced in water either by inhomogeneous nucleation around impurity sites or at liquid-glass interface, or by homogeneous nucleation after a compression-expansion cycle. However, the conductivity of water before the iterative filtering (1-2 µS/cm) is not compatible with the amount of impurities necessary to produce the amount of solid residue resulting from evaporation process and it is not explainable that such an amount of chemical impurities could be extracted from the glass during the filtering. Filtering under vacuum itself may allow the formation and the removal of bubbles at each cycle but it is quite hard to envisage a mechanism of stabilization of such nanobubbles capable to form aggregates of several hundreds of nanometers.
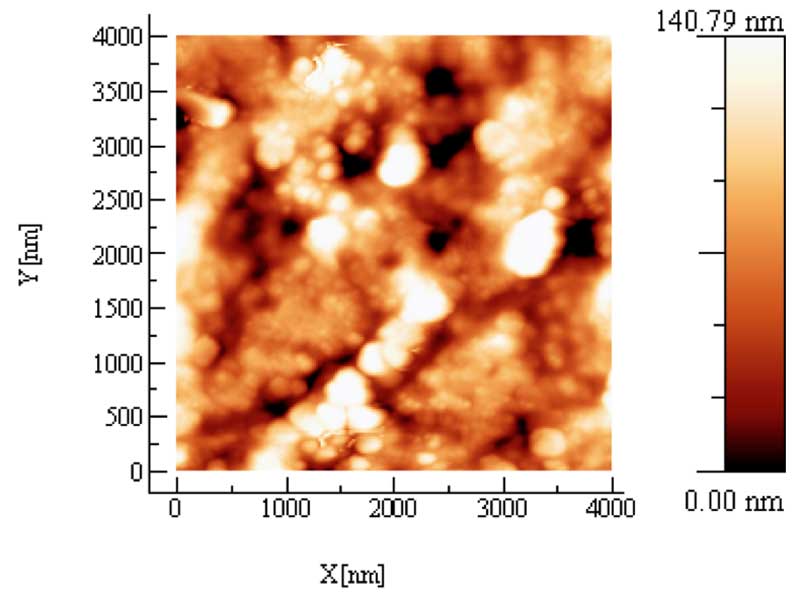
Figure 4a: AFM topography of a few drops, 3-5,of evaporated Iteratively Filtered Water. The XYscale of the picture is 4×4 μm. The different colorcode from bright to dark indicates the Z height of the clusters.
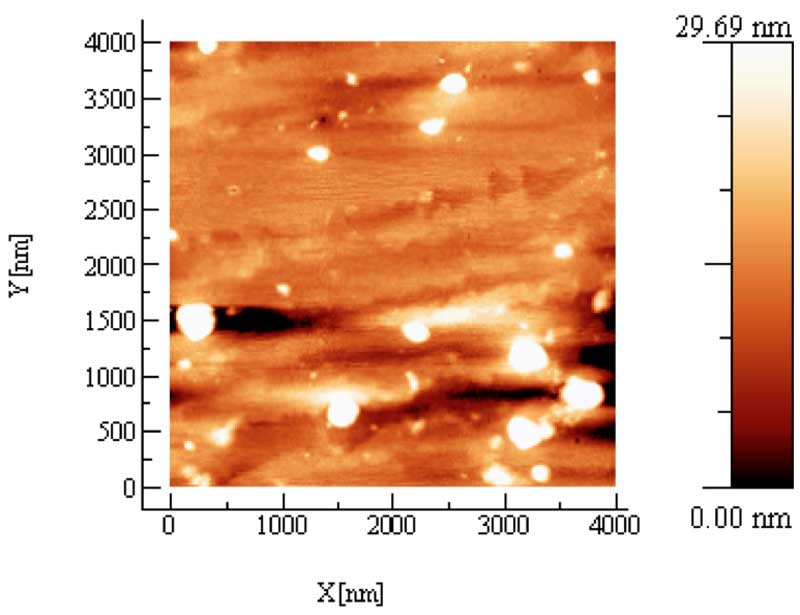
Figure 4b: AFM topography of a few drops, 3-5, of liquid Milli-Q water kept in contact with glass powder for long time (control). The XY scale of the picture is 4×4 μm. The different color code from bright to dark indicates the Z height of the clusters.
Quantum Electrodynamics (QED) has shown [17,18] that an ensemble of independent water molecules, above a density threshold and below a critical temperature get into a coherent state where their electronic clouds oscillate between a ground state and an excited state separated by an energy gap: Egap=0.17eV≈6.8KT at room temperature, then well protected against the thermal noise. These molecules oscillate coherently (i.e. with the same phase) between the two levels with a proper frequency ν inside a region having a size determined by the wavelength of the oscillation. In case of water, this electronic coherence gives rise to Coherence Domains (CD) having a span of about 0.1 micron. However, other kind of coherence based, for instance, on vibrational or rotational levels may be envisaged [19].
In case of rotational levels, the energy spacing is in the order of 20 cm-1 (~ 0.0025 eV) which corresponds to a CD length of about 500 microns; however, thermal energy destroys the coherence in case Egap<KT≈0.025eV so that non-electronic coherence cannot survive. Nevertheless, the experimental findings show the existence of dissipative structures able to exchange energy and matter with the environment and attain to higher level of organization collecting low grade energy to reduce the entropy of the system, so that further experimental investigations are necessary for a full understanding of this phenomenon.
Conclusions
We suggest that modifications in the aggregation of CDs may be induced by weak mechanical perturbations such as those described here and in the previous paper [9] giving rise to collective metastable states which release energy in times much longer than the relaxation time of the excited molecular levels and are, thus, observable even at room temperature and pressure.
We have shown that an iterative process of filtering can induce a permanent change of the structure of liquid water. We have observed that such a process can induce the modification of the UV spectrum with the emergence of an absorption peak at about 270 nm, and a change of the IR spectrum of the unexpected solid residues obtained after lyophilization. We have also shown the existence of a solid residue after the evaporation of a few drops of treated liquid water on mica surface of the AFM sample holders, that is a solid phase of water stable at standard pressure and temperature.
References
1. Stanley HE, Teixeira J. (1980) Interpretation of the unusual behavior of H2O and D2O at low temperatures: Tests of a percolation model. J. Chem. Phys. 73, 3404.
2. Vedamuthu M, Singh S, Robinson GW. (1994) Accurate Mixture-Model Densities for D2O. J. Phys. Chem. 98, 8591.
3. Chaplin M. (2001) Water: its importance to life. Biochem. and Molec. Biol. Education 29, 54.
4. Wiggins P. (2008) Life Depends upon Two Kinds of Water. PLos ONE 3(1) e1406.
5. Cartlidge E. (2010) Water’s mysteries explained. New Scientist 205, 2746, 32.
6. Tsenkova R. (2009) Introduction: Aquaphotomics: dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J. Near Infrared Spectrosc. 17, 303.
7. De Ninno A, Del Giudice E, Congiu Castellano A. (2013) The supramolecular structure of liquid water and quantum coherent processes in biology. J of Phys: Conf. Series 442, 012031.
8. Gowen AA, Amigo JM, Tsenkova R. (2012) Paper presented at the XIII Conference on Chemometrics in Analytical Chemistry (CAC 2012), Budapest, Hungary, June 25–29 2012. Analytica Chimica Acta 759, 8.
9. Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M. (2013) Experimental Evidence of Stable Aggregates of Water at Room Temperature and Normal Pressure After Iterative Contact with a Nafion® Polymer Membrane. WATER Journal 5, 16.
10. Elia V, Napoli E. (2010) Dissipative Structures In Extremely Diluted Solutions Of Homeopathic Medicines: A Molecular Model Based On Physico-chemical And Gravimetric Evidences. Int J Design Nature 5, 39.
11. Elia V, Napoli E, Niccoli M. (2010) Thermodynamic parameters for the binding process of the OH− ion with the dissipative structures. Calorimetric and conductometric titrations. J Therm Anal Calorim. 102, 1111.
12. Elia V, Napoli E, Niccoli M. (2013) Calorimetric and conductometric titrations of nanostructures of water molecules in iteratively filtered water. J Therm Anal Calorim. 111, 815.
13. Chai B, Zheng J, Zhao Q, Pollack G. (2008) Spectroscopic Studies of Solutes in Aqueous Solution. J. Phys. Chem. A 112, 2242.
14. Lo SY, Geng X, Gann D. (2009) Evidence for the existence of stable-water-clusters at room temperature and normal pressure. Phys. Lett. A 373, 3872.
15. Vallèe P, Lafait J, Legrand L, Mentré P, Monod M-O, Thomas Y. (2005) Effects of Pulsed Low-Frequency Electromagnetic Fields on Water Characterized by Light Scattering Techniques: Role of Bubbles. Langmuir 21, 2293.
16. Tyrrell JWG, Attard P. (2002) Atomic Force Microscope Images of Nanobubbles on a Hydrophobic Surface and Corresponding Force−Separation Data. Langmuir 18, 160.
17. Arani R, Bono I, Del Giudice E, Preparata G. (1995) QED Coherence and the Thermodynamics of Water. Int. J. of Modern Phys. B 9, 1813.
18. Bono I, Del Giudice E, Gamberale L, Henry M. (2012) Emergence of the Coherent Structure of Liquid Water. Water. 4(3):510-532.
19. Del Giudice E, Tedeschi A, Vitiello G, Voeikov V. (2013) Coherent structures in liquid water close to hydrophilic surfaces. J.of Phys: Conf. Series 442, 012028.
Discussion with Reviewers
Anonymous Reviewer: I see an issue with the IR difference peaks; there are many uncommented differences not only the 2926 cm-1 “unknown” peak; but even this peak is extremely significant because, as each student of chemical analysis may tell you, the 2926 cm-1 peak is indicative of the existence of hydrocarbon impurities in your water, being the value corresponding to the stretching of C-H bond. Please check in each IR atlas; this corresponds to the fact that your solution is not sufficiently pure.
V. Elia, G. Ausanio, A. De Ninno, R. Germano, E. Napoli and M. Niccoli: As the referee points out, the IR spectrum of the lyophilized IFW sample in Figure 3 shows a very complex structure, much more so than expected. The peak at about 2950 cm-1 may be attributed to a C-H alkyl bond of methyl/methylene bridge, however when analyzing IR spectra, it is very important to take advantage of all of the information you have. Beside the two peaks clearly attributed to water molecules (i.e. O-H stretching broad band 3000-3700 cm-1 and O-H bonding centred at 1650 cm-1) you can see five more peaks:
- 2950 cm-1
- 1506 cm-1
- 1409 cm-1
- 1129 cm-1
- 1012 cm-1
The first one can be attributed to methyl group but, in this case there should be other peaks at 1260, 2870 and 1380 cm-1 which are actually missing, so that methyl can be excluded. Similar considerations can be also applied to methylene. The absorptions in the range ~1400-1500 cm-1 can be attributed to any aromatic C=C bond and strong absorption in the range ~1000-1100 cm-1 to C-O bonds in alcohols. The spectrum looks quite complex because the intensity of an absorption bands depends on the dipole strength and on the number of bonds present, thus the task is hard even for a student of chemical analysis. However, the intensity of the bands is so strong that the sample looks as a molar solution of hydrocarbons. Since the IR spectra of the liquid samples do not show any significant difference with normal Milli-Q water, we should assume that the contaminants have been introduced in the procedure of solid pellet preparation which is described here once more for the reader’s convenience: 1) 200 mg of NaCl are added to a sample of 20 ml of IFW; 2) the obtained liquids is then lyophilized: 3) the solid residue is pressed in a tablet and measured in the IR spectrometer. A similar procedure has also been used for a sample of 20 ml of Milli-Q water but in such a case any anomaly in the spectrum has been observed.
The appearance of a similar peak at (about) 2950 cm-1 has also been observed in IR spectrum of solid samples of ultra-diluted solutions in different laboratories but even in such a case an unambiguous attribution is missing [V. Elia, G. Ausanio, F. Gentile, R. Germano, E. Napoli, M. Niccoli doi:1016/J.homp.2013.08.004 and Lo S.Y., Geng X., Gann D. Phys Lett. A 373 (2009) 3872-3876]. The result being so puzzling, any conclusion can be reached by this experimental finding alone, however, we have tried to consider as many experimental techniques as possible in order to get a wider description of the effects of the iterative filtration.
Reviewer: The AFM technique is essentially a physical technique not a chemical analysis technique; the image you have submitted corresponds only to the presence of some topographic variations not to their composition (water? Why? What forbids a different composition given the IR “unknown” spectrum?); but there is something more: obviously the physical state of the topographic variation could be examined by AFM but this may be done using different AFM methods than the common one you have applied; from those images no conclusion on the physical state of eventual residue could be obtained.
Elia, Ausanio, De Ninno, Germano, Napoli and Niccoli: We totally agree with the referee: of course AFM is not a technique of analytical chemistry, in fact from the topography one cannot deduce the composition of the solid residue. And we didn’t. Once again, this experimental finding alone does not give us any information about the chemical composition of the solid residue, however the extraordinary large amount, 1-2 mg of residue out of 20 ml of liquid should be compatible with a massive pollution which has not been detected by ICP mass spectrometry as explained in the text. Moreover the liquid sample before the lyophilization had shown large changes of electrical conductivity, pH and heat of mixing with NaOH alkaline solutions unable to coexist with the quantitative chemical analysis results. Hence the pollutants were not present in the liquid phase after the iterative filtration process. It must also pointed out that samples of Milli-Q water kept for months in contact with Pyrex glass powder in order to extract the same chemical components extracted during the iterative filtration process, don’t show any anomaly. A similar behavior has been also observed for ultra-diluted solutions [Elia V., Napoli E. Int. J. Des. Nat. 2010;5:39-48]. The calculated weight of the solid residue obtained evaporating water at 90° C turns out to be very much lower than the weight of the observed residue. Furthermore, the difference between the expected and experimental weight linearly depends on the value of the electrical conductivity of the system and that the difference vanishes in case of Milli-Q water sample prepared exactly with the same procedure.
AFM provides a topography of the solid leaved behind by the lyophilization process however any conclusion can be reached by this experimental finding alone. The ultimate goal of this research is to contribute to depict a picture of the weird properties of liquid water submitted to an iterative filtering process using several experimental techniques both of analytical chemistry and physics. The evaluation of the results of an experiment should be taking advantage of all of the information you have without assuming that each individual piece of information proves a theory. It is well known that nano-structured materials show different properties with respect to bulk materials but this fact is not used to claim the reconsideration of the ordinary properties of the matter. In this paper we are dealing with nano-aggregates whose formation laws is still missing, the observation of their properties does not lead us to amend in any way neither the average properties of the liquid bulk water nor its common state diagram.
