 Experimental Evidence of Stable Aggregates of Water at Room Temperature and Normal Pressure After Iterative Contact with a Nafion® Polymer Membrane
Experimental Evidence of Stable Aggregates of Water at Room Temperature and Normal Pressure After Iterative Contact with a Nafion® Polymer Membrane
Elia V1, Ausanio G4, De Ninno A3*, Gentile F1, Germano R2, Napoli E1 and Niccoli M1
1Department of Chemical Sciences, University of Naples “Federico II”, Complesso Universitario Monte Sant’Angelo, Via Cinthia 80126, Naples, Italy
2PROMETE Srl, CNR Spin off Via Buongiovanni, 49 80046 San Giorgio a Cremano (NA), Italy
3UTAPRAD-DIM Department C.R. ENEA Frascati, 00044 Frascati, Italy
4Department of Physics and CNR-SPIN University of Naples “Federico II”, Piazzale V. Tecchio 80 80125 Naples, Italy
*Correspondence E-mail: antonella.deninno@enea.it
Key Words: Water, Nafion membrane, Nanostructure, Conductivity, IR spectroscopy, UV-vis spectroscopy, Fluorescence microscopy, Light scattering, AFM
Received February 22nd, 2013; Accepted April 16th, 2013; Published May 15th, 2013; Available online May 19th, 2013
Abstract
Long-range effects in interactions between aqueous solutions and charged surfaces have been frequently reported. Six different experimental techniques have been used to study the properties of water after prolonged contact with a hydrophilic polymer. The presence of supra-molecular aggregates of water, hundreds of nanometers in size, have been observed at ambient pressure and temperature after iterative long-lasting contact of Milli-Q water with a Nafion surface. These isolated aggregates of water also survive lyophilization. Analytical determination by Ion Chromatography allows us to exclude the role of contaminants. This suggests that water may possess an exceptional self-organization capability triggered by the contact with a hydrophilic surface.
Article Outline
- Introduction
- Materials and Methods
- Results and Discussion
- Conclusions
- Acknowledgements
- References
- Discussion with Reviewers
Introduction
Water close to hydrophilic surfaces shows very interesting properties, which differ significantly from those of ordinary bulk water. That water is detectable close to hydrophilic surfaces, such as Nafion®, and exhibits properties quite different from those found in normal bulk water:
a) is unable to host solutes, and this is the root of the name Exclusion Zone (EZ);
b) its viscosity is much higher than that of normal water, suggesting the presence of a strong interaction among molecules;
c) it is an electron-donor, a chemical reducer, whereas normal water is a mild oxidant. Consequently the EZ water/normal water interface is a red-ox pile, where the red-ox potential could have a jump of a fraction of a volt [1];
d) it exhibits a fluorescent response in the UV region at 270 nm [2, 3].
EZ water should therefore imply a major reorganization of the molecular structure of water, in particular (see property d) with respect to the electronic structure. The observed depths of the layers of EZ water on the surface could reach values as high as 500 μm.
The physical-chemical parameters of Iteratively Nafionated Water (INW), that is Milli-Q water kept in contact with Nafion, following the procedure described in the next section, have been examined in previous papers. In particular the following properties have been measured: electrical conductivity χ, the heat of mixing Qmix with acid (HCl), or basic (NaOH) solutions, and pH. An increase of electrical conductivity, χ, up to two orders of magnitude has been observed together with a linear correlation between Qmix versus χ and between pH versus logχ. It has been suggested there, that water is a complex liquid capable of self-organization induced by mechanical and/or electromagnetic perturbations, even of a small amplitude. The stability of such structures would be achieved through the dissipation of energy, making the formation of the structures a spontaneous phenomenon [4].
The aim of this paper is to describe the changes in the properties of liquid water after a prolonged contact with Nafion, investigated by performing a series of structural measurements: FT-IR, UV-vis spectroscopy and light scattering and two types of microscopies: fluorescence microscopy and atomic force microscopy, in addition to the pH and conductivity measurements.
Materials and Methods
Nafion membranes having a surface of 60-120 cm2 and a thickness of 50-180 μm, were initially washed 5 times using 20 ml of Milli-Q water. Then, the membrane was placed in an open Petri dish (made either of Pyrex glass or Polystyrene) in contact with 10-20 ml of Milli-Q water (χ = 1-2 μS cm-1), manual stirring was performed repeatedly to allow 2-3 mm of liquid to lap against the membrane. Then the conductivity was measured and the procedure was repeated turning over the membrane. The procedure was repeated manually some tens of times, producing an increase of conductivity after each step. After some hours the membrane was removed from the Petri dish and allowed to dry in air for several hours, then it was again placed in the previously used water and the whole procedure was repeated again. In order to obtain a significant increase in conductivity (up to 100-300 μS cm-1) the procedure was repeated from 10 to 20 times, the kinetic being quite variable from time to time. It has also been observed that the volume of the treated water had an influence on the increase of the conductivity in the sense of a higher increase for smaller volumes [4].
After the above procedure, some samples were subjected to a lyophilization procedure to examine the (possible) solid phase residues.
Conductivity
Conductivity χ has been measured at a controlled temperature of 25 ± 1°C, and further temperature-corrected using a pre-stored temperature compensation for pure water. Systematic measurements have been performed on the samples using a YSI 3200 conductometer with a conductivity cell constant of 1.0 cm-1. For a given conductivity measuring cell, the cell constant was periodically determined by measuring the conductivity of a KCl solution with a specific conductivity known to a high accuracy, at several concentrations and temperatures. The specific conductivity χ (μS cm-1) was then obtained as the product of the cell constant and the conductivity of the solution.
FT-IR Spectroscopy
IR spectra of liquid samples have been recorded using the Attenuated Total Reflectance mode with a 45° single reflection ZnSe crystal on a FTIR Jasco/410 spectrometer, equipped with a conductive ceramic coil mounted in a water-cooled copper jacket source, a KBr beam splitter and a TGS detector. The recording conditions for each FTIR spectrum were: 120 scans, a triangular apodization function and a resolution of 4 cm-1. IR spectra in the solid state have been recorded in a NaCl dispersion medium for lyophilized samples, using an FT-IR Jasco-FT-IR-430 spectrophotometer.
UV-Vis Spectroscopy
The UV/Vis spectra have been monitored using a Jasco Spectrophotometer model V-560 UV/Vis. This is a double-beam spectrophotometer with double monochromator, a wavelength range of 190-900 nm and a resolution of 0.1 nm. The photometric accuracy and reproducibility of the instrument are respectively ± 0.002 and ± 0.001; the recording conditions for each UV/Vis spectrum were the following: response: slow; scanning speed: 100 nm/min; sampling interval: 1 nm/data. The optical path of the cuvette was 1cm.
Fluorescence Microscopy
The illumination source of the microscope is a 100 W Hg lamp. A specific wavelength of excitation and emission of light has been selected through a set of dichroic filters. Images generated from the emission of fluorescence have been observed both by an ocular and through a Hamamatsu ccd/cmos 20x photo-camera via an inverted Olympus x71. The max resolution of the photo-camera was 1920 x 1440 pixel with a ratio of 8.26 pixel/micron. 1% in weight of polystyrene latex beads solution dispersed in a 1 ml of sample (INW) and in 1 ml of Milli-Q water (control) have been used in order to observe a Brownian motion of the particles dispersed in water. The beads of carboxylate-modified polystyrene had a size of 200 nm and each particle carries fluorescent green probes purchased from The Thermo Scientific. These ones have internally dyed microsphere suspensions that feature bright, high contrast colors emitting bright and distinct colors when illuminated by 465 nm light. Trace amounts of surfactant to inhibit agglomeration and promote stability are present on the surface of each bead.
Light Scattering
Light scattering measurements were per-formed with a MALVERN Zetasizer Nano-ZS. The size information has been obtained from the correlation function calculated by the instrument software. Measurements were performed at T=25°and/or 65°C.
Atomic Force Microscopy (AFM)
A Veeco Digital Instruments Nanoscope IIIa has been used to perform the AFM topography of the solid, obtained as residues from evaporated drops of samples on substrates of mica. The AFM images were obtained by operating in non-contact mode. The AFM was equipped with an ultra-thin silicon point, with an apical radius of less than 5 nm, and analyses was evaluated on windows of different areas with an acquisition frequency of 2 Hz. As a control, we used water kept in long-standing contact (months) with Pyrex glass powder and allowed to dry on a similar glass slide.
Results and Discussion
The purpose of this study is to validate the hypothesis of a permanent alteration in the supramolecular structure of liquid water induced by the contact with a strongly hydrophilic surface, as already discussed in Elia et al. paper [4]. The physicochemical investigations carried out revealed un-expected properties of the samples both in the liquid and in solid phase. The most astonishing effect is the increase of the electrical conductivity χ of more than two orders of magnitude. The linear relation between log χ and pH already previously described [4] has been confirmed. This behavior may be justified by the proton hopping mechanism [5,6] favored by the presence of molecular aggregates of water molecules [7-9]. Likewise, the variation in pH has been explained by strong affinity of OH– to the nanostructures, resulting in an ex-cess of free H+ close to the water aggregates. The variation in pH is incredibly large, varying from about pH = 6 for pure Milli-Q to about pH = 3, which implies an increase in the H+ concentration of three orders of magnitude. This finding, in particular, poses a significant problem to the hypothesis that the impurities are responsible for these effects. In fact, a pH = 3 means a proton concentration [H+]=10-3 M (mol/L) and a non relevant OH- concentration in the order of 10-11 M (mol/L) according to the water dissociation constant. In order to neutralize the cations, a hypothetical negative counter ion concentration should be then about 10-3 M, that is almost 3 orders of magnitude higher than the level of the analytically determined impurities. Actually, the Nafion membrane can only release F- and HSO4-, while soluble impurities will be reduced to zero concentration after several cycles of washing. Analytical determination of the two ions, using Ion Chromatography, gives a concentration of 2.8·10-6 mol /L for F– and 2.5·10-6 for HSO4- ions, 3 orders of magnitude lower than the values able to justify both pH and conductivity measured values.
Structural Investigations in the Liquid Phase
IR investigation of the structure of INW samples in the liquid phase reveals a spectrum matching the normal one of pure water, while UV-Vis spectroscopy shows a new absorption peak in the 225-325 nm wavelength region, at approximately ~ 270 nm, see Figure 1a. A similar result was found for the Exclusion Zone at a distance of about 100 μm from the Nafion surface [10]. The absorbance at the wavelength of the relative maximum shows a roughly linear correlation with the electrical conductivity, see Figure 1b.
The examination of the INW water samples with fluorescence microscopy reveals an astonishing fact: the appearance of large-sized structures on which the marked polystyrene spheres appeared to be clust-ered, immersed in the surrounding water. Figure 2 (left) shows the image of the control obtained with Milli-Q water, while Figure 2 (right) shows the irregular shape of the structures in which the polystyrene beads were confined in INW samples. Images indicate that the latex beads are dispersed in an anisotropic medium, which means it is highly probable that the viscosity inside the aggregates is different from that in the bulk liquid.
Light Scattering provides information about the existence of long-range correlations between molecules of a possible solute in water. The speed at which particles are moving, due to Brownian motion, is measured by dynamic light scattering. This is done by measuring the rate at which the intensity of the scattered light fluctuates when detected by a suitable optical arrangement. The light scattering theories used to calculate the light scattering take into account the dimension of particles compared to the wavelengths of the used laser. However, in order to use a theory valid for most spherical particle scattering systems, several ad hoc hypotheses are required, such as: a) the definition of the hydrodynamic diameter of the centre of scattering (the diameter of a sphere that has the same translational diffusion coefficient as the particle); b) the assumption that viscosity be constant throughout the sample; c) the size distribution is obtained measuring the relative intensity of light scattered by particles of various size. The assumption of such “ad hoc” hypothesis just allows to detect the presence of large-sized aggregates in liquid water but a reliable quantitative analysis of their dimensions. Notwithstanding that, we were able to observe a linear correlation, beyond experimental error, between the aggregate size and the conductivity of the samples, see Figure 3.
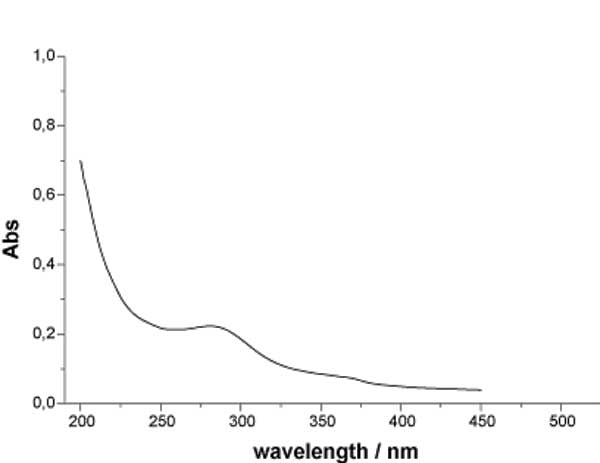
Figure 1a: UV spectra of INW at 298 K.
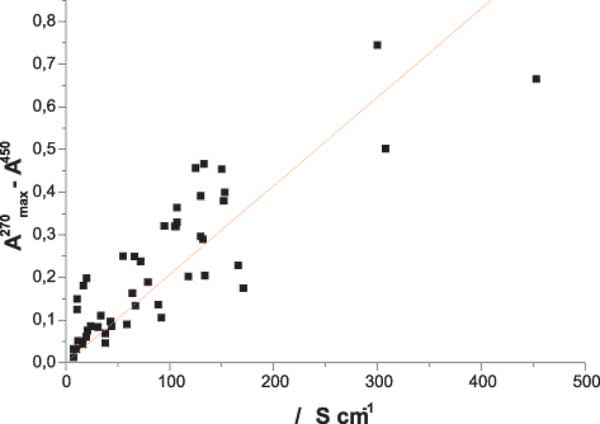
Figure 1b: The linear correlation between absorbance at the wavelength of the relative maximum, A270max, minus A450 versus the electrical conductivity.

Figure 2a: Fluorescent microscope pictures of polystyrene spheres in Milli-Q water. 1 μm = 8.26 pixels.

Figure 2b: Irregular shape of the structures observed with fluorescence microscopy in INW. 1 μm = 8.26 pixels.
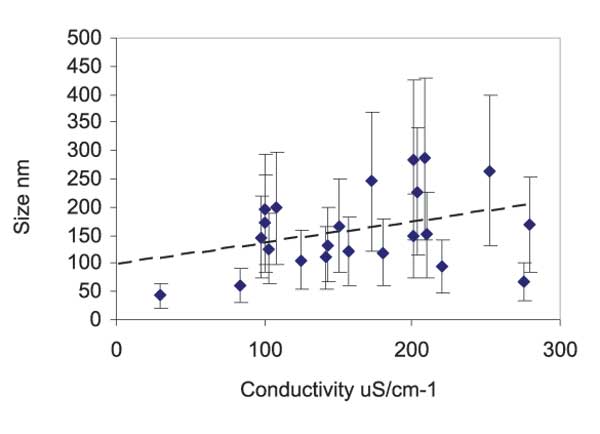
Figure 3: Aggregate size as determined by Light Scattering measurements, versus the conductivity of the sample. Error bars on the Y axis were evaluated through the fit of a multiple exponential to the correlation function (CONTIN) from the Dispersion Technology Software 5.10 by Malvern.
The size of the aggregates appears to be reduced by a factor of 10 after filtering with a 0.22 μm single-use Millex-GS and 0.20 cellulose acetate filters. We also examined the possibility that the aggregates detected by light scattering were actually nano-bubbles present in the liquid phase, as conjectured in the literature [11, 12]. However, an analysis conducted on samples heated up to 65°C did not show any changes in the number or size of the observed aggregates, which therefore have negligible dependence on temperature in the range of existence of liquid water.
Structural Investigations in the Solid Phase
Samples of iteratively nafionated water (INW 20 ml) were lyophilized, yielding an unexpectedly large amount of solid residue of about 1-2 milligrams. The production of unexpected solid residues after elimination of bulk water (evaporation at room temperature, evaporation at high temperatures, 90°C) has been described in two papers (though using fundamentally different methodologies) where the hypo-thesis that water molecule aggregates are present at room temperature and pressure is considered [13, 14]. In order to examine them by AFM, some deposits were formed on mica substrates, whose purity was guaranteed by careful cleavage, by evaporating, at room temperature and pressure, only 3-5 drops of the liquid. Atomic Force Microscopy images of these deposits were taken in non-contact mode. Figure 4 shows the residues from five drops of a sample having χ= 321 μS cm-1: the Z-height is much higher compared with the reference sample of pure water kept in contact for days with polystyrene Petri dish.
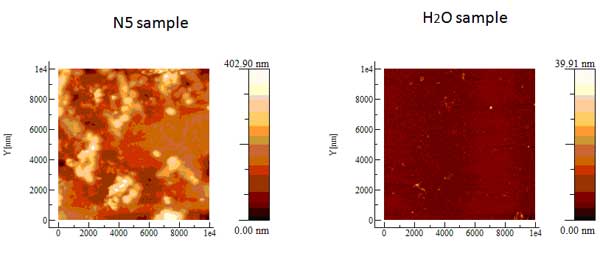
Figure 4: The size of the picture is 10×10 μm. Residues from five evaporated drops of a sample of INW (Electrical conductivity χ= 321 μS cm-1) (left) and of the blank (right). The bright-to-dark colour coding corresponds to the height of the clusters, ranging from 0.040 μm (the control – right) to 0.403 μm (the sample – left).
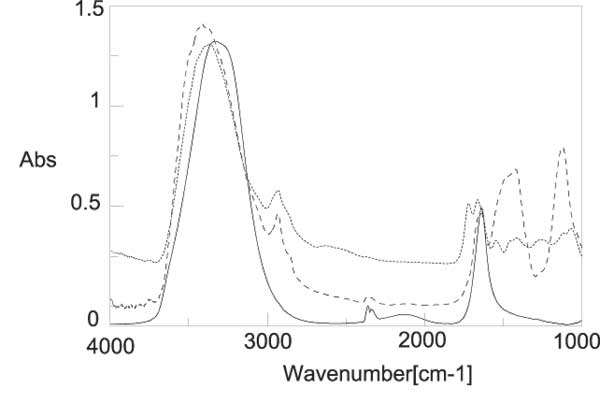
Figure 5: IR spectra residue of INW (dotted line) and liquid water (black line) at room temperature. The main differences are the overall red-shift of the broad OH stretching peak and the appearance of an absorption line at about 2926 cm-1.
The solids obtained by the lyophilization, of 20 ml of nafionated water, were used to make NaCl tablets suitable for IR spectroscopy. Figure 5 shows the IR spectrum of the residues of INW samples, compared to the spectrum of liquid water in the range 4000-1000 cm-1. The main stretching band of liquid water is shifted to a lower frequency and a wide bump appears at around 2926 cm-1. Figure 6 shows the comparison between the OH-stretching region of the spectrum for the solid residue and liquid water at NTP. It is worthwhile noting that the de-convolution of the OH stretching peak between 2880 and 3800 cm-1 shows the total disappearance of the higher energy component at around 3600 cm-1 with respect to bulk water [15-16]. This feature is peculiar of the OH stretching in ice. Other features appearing at 1418 cm-1 and 1105 cm-1, that are not present in liquid water, are difficult to attribute univocally at the present stage of knowledge.
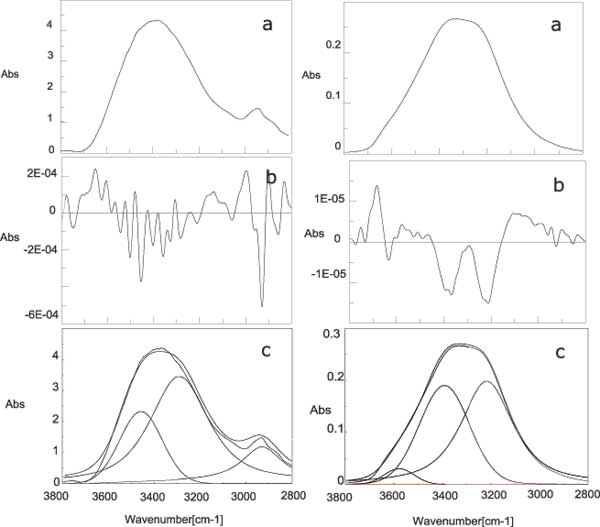
Figure 6: Comparison between the spectra of lyophilised INW (left panel) and liquid water (right panel) at normal temperature and pressure. a) spectrum of the OH stretching region; b) second derivative spectrum; c) deconvolution of the spectrum.
Conclusions
Physical theories of liquids considering only the electrostatic interactions between molecules have been used to explain the solvation of polar molecules in water. However, the same short range interactions are not able to explain any form of spatial organization above this scale. The peculiar properties of the characteristic self-organization of water make it fundamental not only for the colloid science but also for life science.
It has been widely accepted for many years that the structure of liquid water is due to the properties of the hydrogen bond network [17]. This network has been described as a collection of independent molecules held together by weak electrostatic bonds. Such a description has successfully accounted for many of the properties of water, starting from its first formulation by Linus Pauling to explain water’s anomalous boiling point. However, some authors maintain, on the basis of data from different experimental techniques, that all the anomalies of liquid water can be explained in terms of domains of different density [18]. The existence of water in two different populations, made up by molecules having different degree of mutual correlation, has been also demonstrated in the framework of Quantum Electrodynamics (QED). An electromagnetic field emerges from the collective behavior of the water and is responsible for the long range forces which account for the physical properties of the liquid state. Liquid water can be described as a superposition of two quantum states: a lower energy state, characterized by both coherent quantum oscillations and spatial correlation on mesoscopic scale and a higher energy state populated by non correlated, single molecules, like in the vapor phase [19-20].
In order to explain long range cohesion of liquid water we are compelled to consider aggregates of structured H- bonded molecules, nevertheless, life scientists describes hydration processes as interactions of independent water molecules with ions or non polar molecules. In addition, the observations concerning the properties of water close to the cell membrane suggest that two kinds of water coexist as micro-domains throughout the liquid temperature range [21-23].
The hypothesis of the formation of molecular aggregates of water molecules, adopted by a large body of work published over the past fifteen years, appears to be supported here both by the thermodynamic parameters and by structural observations in the liquid phase. IR Spectroscopy and Atomic Force Microscopy confirm also the possibility that such molecular aggregates survive in the solid phase.
We have shown that a permanent change in the structure of liquid water can be induced by a purely physical method: an iterative procedure including a long-lasting contact with Nafion membranes. The size of the observed nanostructures, and the substantial alterations of transport properties, cannot be explained in the framework of short range interactions, so that an electrodynamics approach is mandatory. The appearance of stable structures that survive the phase transition, from liquid to solid state, also implies the existence of coherent space-time dissipative structures, capable of exchanging energy and matter with the environment and attaining a higher level of organization.
Acknowledgements
We want to thank A. Congiu Castellano for the interesting discussions and the valuable help for FTIR measurements, and we are grateful to the Biological Macromolecules Laboratory of the Physics Department, of the University “La Sapienza” of Rome, for their hospitality.
References
1. R. Germano R., Del Giudice E., De Ninno A., Elia V., Hison C., Napoli E., Tontodonato V., Tuccinardi F. P. and Vitiello G. Key Engineering Materials, 543, 455-459 (2013).
2. Zheng J. M., Chin W. C., Khijniak E. and Pollack G. H. Adv. in Colloid and Interf. Sci. 23 (2006) 19.
3. Zheng J., Wexler A. and Pollack G. H. J. Colloid. Interface Sci. 332 (2009) 511.
4. Elia V., Napoli E. and Niccoli M. J. Therm. Anal. Calorim., doi 10.1007/s10973-012-2576-z.
5. De Grotthuss C. J. T. Ann. Chim. LVIII 58 (1806) 54.
6. Gileadi E. and Kirowa-Eisner E. Electrochim. Acta. 51 (2006) 6003.
7. Cacace C.M., Elia L., Elia V., Napoli E. and Niccoli M. J. Mol. Liq. 146 (2009) 122.
8. Elia V., Napoli E. and Niccoli M. J. Mol. Liq. 148 (2009) 45.
9. Elia V., Napoli E. and Niccoli M. J. Therm. Anal. Calorim. 102 (2010) 1111.
10. Chai B., Zheng J., Zhao Q. and Pollack G. H. J. Phys. Chem. A 112 (2008) 2242.
11. Colic M. and Morse D. Coll. and Surf. A 154 (1999) 167.
12. Katsir Y., Miller L., Aharonov Y. and Ben Jacob E. J. Electrochem. Soc. 154 (2007) D249.
13. Lo S. Y., Geng X. and Gann D. Phys. Lett. A 373 (2009) 3872.
14. Elia V. and Napoli E. Int. J. Design Nature 5 (2010) 39.
15. Maréchal Y. J. Chem. Phys. 95 8 (1991) 5565.
16. De Ninno A. and Congiu Castellano A. J. Mol. Struct. 1006 (2011) 434.
17. Stanley H. E. and Teixeira J. J. Chem. Phys. 73 (1980) 3404.
18. Vedamuthu M., Singh S. and Robinson G. W., J. Phys. Chem. 98 (1994) 8591.
19. Arani R., Bono I., Del Giudice E. and Preparata G. Int. J. of Modern Phys. B 9 (1995) 1813.
20. Bono I., Del Giudice E., Gamberale L. and Henry M. Water (2011) 4(3), 510-532.
21. Chaplin M. Biochem. and Molec. Biol. Education 29 (2001) 54.
22. Wiggins P. PLos ONE 3 (1) (2008) e1406
23. Cartlidge E. New Scientist 2746, 2010, mg20527466.200
Discussion with Reviewers
Anonymous Reviewer: In the present paper, the authors describe the findings coming mainly from spectroscopic and microscopic techniques. How do they look at their preceding, mainly thermodynamic work, which was aimed at the proposition of hypotheses to explain the peculiar properties of differently treated water? Have they had to change anything in what was inferred before?
V. Elia, G. Ausanio, A. De Ninno, F. Gentile, R. Germano, E. Napoli and M. Niccoli: In the previous paper about physical-chemical properties of water in contact with hydrophilic polymer, some of us proposed an hypothesis coherent both with the thermodynamic and transport data: water is capable of self organization induced by small energy perturbations such as mechanical and/or electromagnetic disturbance. Examples of such disturbance can be considered: dilution and agitation as in EDS (Extremely Diluted Solutions); Iterative Filtration as in IFW (Iteratively Filtered Water); long time contact with hydrophilic polymers as in INW (Iteratively Nafionized Water). In the above mentioned circumstances, we have observed the formation of stable structures ongoing after the removal of the perturbation and suggested the formation of a stable far-from-equilibrium state achieved trough the dissipation of energy subtracted to the environment. The results of the present work mainly confirm those findings adding several information about the arrangement of the liquid water. In particular, the existence of a proton-hopping mechanism enhanced by the existence of aggregates having an homogeneous space charge seem to be supported by the results of light scattering.
Reviewer: The authors find it highly prob-able that the viscosity inside the aggregates is different from that in the bulk liquid. I think it would be interesting to discuss that point more deeply. If the aggregates were more structured than pure water, then how could the process occur with a loss of entropy?
Elia, Ausanio, De Ninno, Gentile, Germano, Napoli and Niccoli: Images obtained by fluorescence microscopy show that nano-particles of polystyrene, marked with flourescein, stick on the surfaces of the structures existing in liquid water and that their thermal motion appears greatly slowed. This fact is evidence for the existence of a segregation between two zones in liquid INW: the one where particles can freely move subjected to the Brownian motion and the other one where their motion is hindered by a higher viscosity. The detail that the organization inside the aggregates might be greater than in the bulk is not in contradiction with the hypothesis of a loss of entropy as in the case of Excluded Zones by J.Pollack (EZ), in fact the decrease of entropy due to the local ordering may be compensated by the dissipation of energy in form of heat, thus contributing to the overall increase of the entropy of the Universe. Moreover, the characteristics of these systems lead to the hypothesis that they are far from equilibrium systems: the experimental observations are not compatible with the reaching of the minimum energy (equilibrium), in fact, if it were the case, these aggregates should be always present in ordinary liquid water as a consequence of its past events.
Reviewer: Figure 2b illustrates the appearance of clusters of polystyrene spheres marked with fluorescent probes. Do these aggregates appear due to the exclusion of spheres from the aggregates of structured water (presumably Exclusion Zone-like water) or on the contrary are the spheres attracted by structured water ensembles?
Elia, Ausanio, De Ninno, Gentile, Germano, Napoli and Niccoli: Both hypothesis are compatible with the experimental observations. However, in either case the spheres are attracted on the surface of the clusters or they are dispersed in the bulk, it can be said that either the clusters exclude the solutes or segregate them on their surface.
Reviewer: After lyophilization of INW a solid residue was left. Was it soluble in pure untreated water and if so, did this solute make the solution similar to INW?
Elia, Ausanio, De Ninno, Gentile, Germano, Napoli and Niccoli: The solid residue obtained by lyophilization is soluble in pure water. The solution obtained exhibits the same chemical-physical characteristics measured before the lyophilization process.
Reviewer: I am somewhat surprised that in the section “Conclusions” that is finished by the statement “The appearance of stable structures that survive the phase transition from liquid to solid state also implies the existence of coherent space-time dissipative structures, capable of exchanging energy and matter with the environment and attaining a higher level of organization.” no reference to the works of G.Preparata and E. Del Giudice is given. These physicists basing on the first principles of quantum electrodynamics suggested that coherent structures (coherent domains) should exist in liquid water. Do the authors see any relationship between theoretical predictions of Preparata and Del Giudice and their experimental results?
Elia, Ausanio, De Ninno, Gentile, Germano, Napoli and Niccoli: The phenomenology observed with INW is the same observed by using similar weak mechanical energy perturbations (EDS, IFW, see above). This phenomenology is completely unexpected in the current theoretical framework: short range interactions based on electrostatic forces are unable to justify any form of large scale ordering. QED provides the appropriate basis to understand the co-operative effects and the appearance of two different energetic states. Moreover, the two-state picture of liquid water emerging from G.Preparata and E. Del Giudice’s seminal works has been very successful in explaining the intricate experimental phenomenology that we have observed (Volume Effect, Time Effect, oscillating phenomena …). However, the straight identification of the observed nanostructures with the Coherence Domains described by Preparata- Del Giudice is not possible. Actually, in their calculations the amount of water molecules belonging to the coherent fraction is only function of the temperature T, while our observations show that the size and number of aggregates can be changed with an external energy supply much lower than KT.
