 Large Supramolecular Water Clusters Caught on Camera – A Review
Large Supramolecular Water Clusters Caught on Camera – A Review
Ho M-W1*
1Institute of Science in Society, 29 Tytherton Road, London N19 4PZ, UK
*Correspondence E-mail: m.w.ho2@i-sis.org.uk
Key Words: Stable water clusters, Supramolecular clusters, Quantum coherent domains
Received September 9th, 2013; Revised Dec 16th, 2013; Accepted Dec 20th, 2013; Published January 20th, 2014; Available online January 26th, 2014
Abstract
This article reviews recent reports on large water clusters containing millions to billions of water molecules directly imaged under the transmission electron microscope and atomic force microscope, which are created by repeated dilution of aqueous solutions of polar solutes and also apparently by other means, and offers an explanation of the structures based on special spherical dipoles originating from quantum coherent domains predicted from quantum electrodynamics theory.
Article Outline
- Introduction
- Stumbling from High Energy Physics into Water
- Characterizing the Water Clusters
- Relationship to Other Supramolecular Structures
- Criticism of Lo et al.’s Work
- Symmetrical Spherical Dipoles From Coherent Domains
- How This Finding Could Revolution-ize Biology and Medicine
- References
- Discussion with Reviewers
Introduction
Stable water clusters tens of nanometres to millimetres in dimensions can be seen under the transmission electron microscope (TEM) and the atomic force microscope (AFM). These appear by drying specially prepared distilled water at room temperature and pressure (Lo A. et al., 2012). The clusters consist of millions to billions of water molecules and come in a wide variety of shapes and sizes (Figure 1). They make up structures that are flexible, and can be deformed by the tips of the atomic force microscope probe if scanned in the contact mode. Otherwise, they remain stable for weeks, even months at room temperature and pressure. They have all the characteristics of ‘soft matter’ – liquids, liquid crystals, colloids, polymers, gels, and foams – that form mesoscopic structures much larger than the molecules themselves, but small compared with the bulk material (Web ref. 1 ).
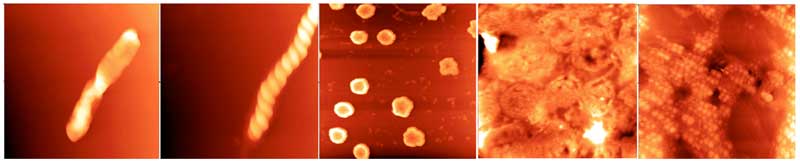
Figure 1: Different forms of supramolecular water clusters imaged with AFM; all fields are 5 microns square (rearranged from Lo A et al., 2012).
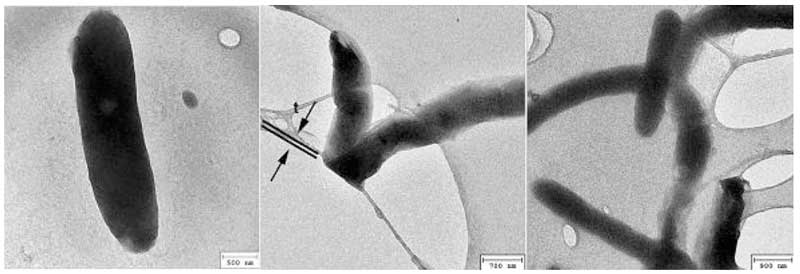
Figure 2: Dark rods under TEM (rearranged from Lo A et al., 2012).
Close-up, there appears to be a common fine structure to the clusters; they are all made up of small spheres tens of nanometres in diameter (see Fig. 1, right panel) lined up in strings that are further aggregated into rods (left panel) two of which wind around each other into a double-helix (second panel from left), or loops (middle panel) and wreaths (second panel from left). These and other observations suggest to the researchers that the spheres are dipoles, enabling them to line up end to end to form an infinite variety of shapes and sizes. Significantly, no diffraction pattern characteristic of crystals was recorded, neither the spheres making up the clusters nor the clusters are crystalline.
One interesting observation is that under the TEM, the rods appear dark (Figure 2), indicating that they diffract electrons strongly. The water was dried on a substrate of holey carbon film, as can be seen, some of the rods are curved (right panel) and in the middle panel, a rod was folded over the edge of a hole with half of it under the film, being flattened by the sharp edge, as consistent with the flexibility and deformability of soft matter.
These supramolecular clusters are the subject of the present review. Not only are they the first direct microscopic images of supramolecular clusters; the images are also of the highest quality obtained so far. Furthermore, Lo and his team have carried out an impressive series of physical analyses described in various published and unpublished reports over a period of more than 15 years, which need to be brought together. The purpose of the present review is indeed to bring all the information together. As a result, I am able to suggest a model of the structures that fit in with all the observations and connect their findings to an emerging paradigm on cell biology and medicine based on water, and most important of all, serve as a working hypothesis for further investigations.
Other research groups have produced microscopic images of supramolecular clusters of water, notably Upadhyay and Nayak (2011) and Elia et al. (2013a,b,c); however, the procedures used are different and the quality of the images are such as to make direct comparisons difficult. For example, the ‘extremely dilute solutions’ investigated by Elia et al. (2013b) all contain concentrations of Na+ > 10-4M, which are 100 to 1000 times those used by Lo and his team, and just above the concentration at which Lo identified a transition from ionic to dipole interactions in water (see below). Nevertheless, these and other results will be mentioned in the relevant contexts.
Stumbling from High Energy Physics into Water
The amazing supramolecular water clusters were actually discovered by Shui-Yin Lo, former high energy physicist and now head of the Quantum Health Research Institute Pasadena, California. He “stumbled” into water research by accident after filing a patent with colleagues at the University of Melbourne on a new type of particle beam (Gann and Lo S-Y, 2009). He was given leave of absence, and ended up a visiting professor at Caltech Pasadena to continue his work with the help of chemist David Gann. Gann was trying to develop a catalyst to reduce smoke and pollutants in the oil-drilling industry, and came across one based on water, a ‘homeopathic’ dilution of a chemical catalyst much more effective than the catalyst itself. Intrigued, he enlisted Lo’s help to make sense of the phenomenon, and there was no turning back.
Yin started to study all the available literature on water, and to carry out experiments and physical measurements. An earlier paper (Lo S-Y, 1996) described the discovery of an “anomalous state of ice” and presented a theory of how the water structures could form from dipole interactions, together with TEM images of clusters similar to those presented in Lo et al. (2012). A more recent publication (Lo S-Y et al., 2009) referred to “stable-water-clusters” prepared by serially diluting a solution of 99.99% pure sodium chloride with vigorous shaking (succussion) in ultrapure distilled deionised water (resistivity 18.2 MΩ cm) in a low-dust room, then placing drops to dry on a clean glass slide or some other substrate. The residue left behind is actually visible under an ordinary light microscope. As a control, drops of pure water were place on similar glass slides to dry under the same conditions, but no structures were seen.
Characterizing the Water Clusters
The Clusters are Giant Dipoles
To find out if the clusters are electrically charged, they were subjected to a backward scan simultaneously on the AFM that, instead of giving surface contours as in the forward scan, records voltages instead, acting as an electric force microscope (EFM) (Lo S-Y et al., 2009). The results are shown in Figure 3 for a large cluster that looks a cloud. The voltage contours are approximately the converse of the surface contours, indicating the presence of electric fields up to ~4 mV (as judged by eye).
As explained in Lo S-Y (1996) and Lo S-Y et al. (2009), when the density of ions is high in the NaCl solution, the dominant interactions are those among ions. As the concentration of NaCl decreases, the interactions among ions are diminished at the expense of dipole interactions. The point at which dipole-dipole interactions dominate is found experimentally to be ~10-4M. At concentrations below this transition point, water molecules will attract one another to form clusters that have permanent dipole moment. A great variety of “snowflake-like shapes” were found. When the density of clusters was reduced, the clusters spread out in straight lines, the horizontal interacting with the vertical at 102º (Figure 4). However, it is not possible to predict which structures will result at any one time, which is consistent with the well-known uniqueness of individual snowflakes.
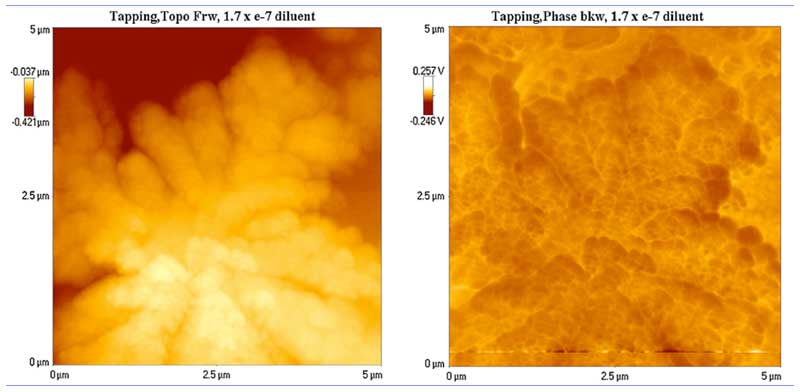
Figure 3: Surface contours (left) and electrical potential (right) of a large water cluster(rearranged from Lo S-Y et al., 2009).
Remarkably, a distillate of the 10-7M NaCl also gave a cluster similar to that from drying a drop of the solution; indicating that the clusters remain intact (perhaps due to strong dipole interactions) upon heating, and furthermore, do not contain contaminating silicon or other ions. This result is important, and needs to be replicated in a systematic way. The lack of silicon is important as silicon is a main contaminant in homeopathic dilute preparations, forming nanoparticles that have been hypothesized to be responsible for the ‘memory’ of water (Upadhyay and Nayak, 2011), and certainly give images that are very different from those depicted here.
Spectroscopic Measurements
The infrared spectrum of the clusters differs from that of pure water (Lo S-Y et al., 2009). Pure water shows absorption peaks at 3283.5 and 1634.5 cm-1. The absorption peaks for stable water clusters were 3371.4, 1639.5, 1342 and 822.5 cm-1.
On Raman spectroscopy, the dominant peak of liquid water at 3426.42 cm-1 is replaced by three peaks at 3145.33, 3049.51 and 2860.34 cm-1; in addition, smaller peaks were found at 2011.24, 1754.81, 1709.31 and 1404.86 cm-1, which may be related to the Raman peak of liquid water at 1651.52 cm-1.
No attempt was made to assign the peaks to definite structures (Lo S-Y et al., 2009).
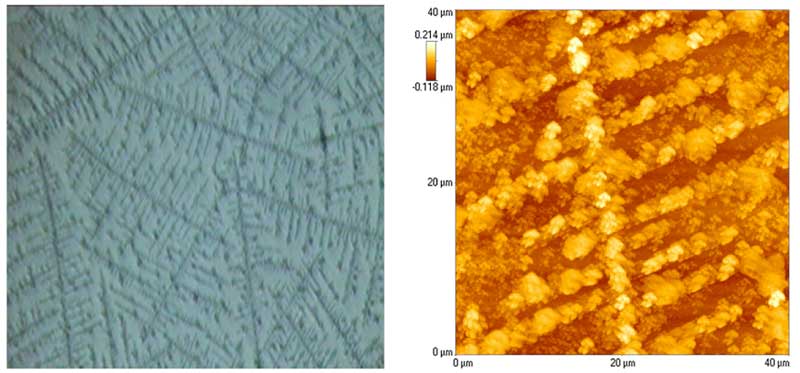
Figure 4: Large array seen under the ordinary light microscope (left) and atomic force microscope (right) (rearranged from Lo S-Y et al., 2009).
Yet further characterizations are described in a conference paper (Lo S-Y, 1998). The water clusters could be detected in solution by photon autocorrelation using a helium-neon laser on a glass bottle containing the solution. The interference between the light scattered by the clusters and the transmitted light enables the size of the clusters to be estimated. Three major sizes were centred at 15 nm, 300 nm and several microns. Interestingly, the clusters also formed with different initiating polar solutes such as isomaltose, cellulose, and sophorose, as imaged on transmission ele-ctron microscopy, indicating that cluster formation is a fundamental property of water itself, rather than that of the initiating solute. (Whether the different clusters could be carriers of homeopathic memory was not addressed by the author.)
To rule out the presence of contaminants being responsible for the clusters, some particles found in pure water were analysed with x-ray spectra compared with the water clusters. The particles in pure water registered a strong peak of Si, as well as Na, Zn Al, Cl, K, and Ca. In contrast, the water clusters were free of Si as well as the other ions.
The clusters could be observed under tapping mode on the AFM either dried or immersed in liquid water (indicating that the glass surface stabilizes the clusters even in contact with liquid water). On AFM, the main sizes were 10 nm, 100 nm and 1,000 nm.
Electric Field Directs Crytallization
Direct evidence of the electret (dipolar) nature of the clusters was provided by crystallization of monosodium phosphate NaH2PO4 (Lo S-Y, 1998). The solid was dissolved in water containing the clusters and a small amount placed on a glass microscope slide and allowed to evaporate and crystallize. The slide was then examined with an optical microscope, and compared with a control sample prepared in deionized water (Figure 5). The monosodium phosphate crystals from the solution containing clusters formed a pattern of straight radiating lines (Fig. 5a) while the crystals from deionized water failed to line up at all (Fig. 5b). Applying a small electric field of 20V/in or 100V/in during the crystallization process did not have any effect on the deionized water sample (Fig. 5d), while the crystals from the water with clusters aligned along the external field lines (Fig. 5c).
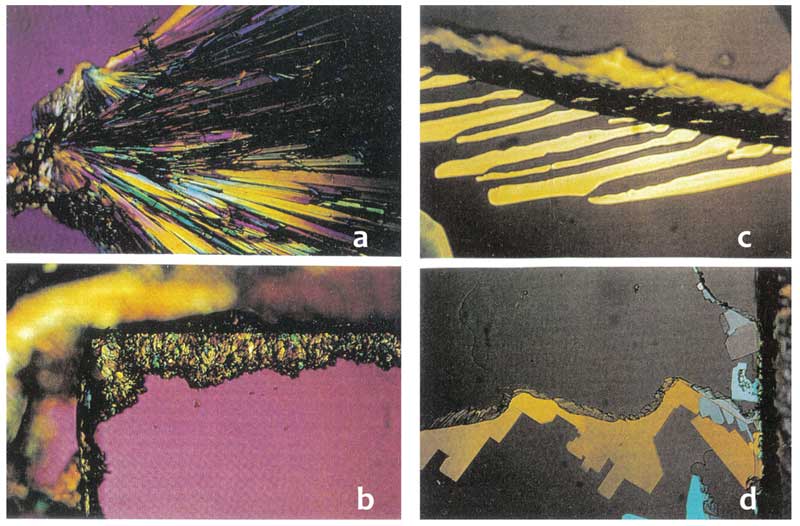
Figure 5: Crystallization of sodium monophosphate from solution with water clusters (a, c) compared with deionized water (b, d); a & c without external field, c & d in the presence of external electric field (rearranged from Lo S-Y 1998).
X-ray diffraction on powdered monosodi-um phosphate crystals formed from control deionized water and water containing clu-sters showed that while monohydrate was formed in deionized water from the original anhydrous sample, dihydrate crystals were formed in the water with clusters.
Deviation from Linear Relationship Between pH and Electric Potential
An electric field tens of mV can be measured between two stainless steel electrodes immersed in the solution containing the clusters. In ordinary ionic solutions, no electric field is established between two identical electrodes. But in the case of the cluster solutions, the electric dipoles of the clusters can line up to establish an electric field (in the form of an electret) without any electrochemical reaction occurring.
Using a pH meter, it is possible to measure the electric potential as well as the pH of a solution, and the two can be plotted against each other (see Lo S-Y, 1998 for a detailed description).
A wide range of pH from 1 to 12 was tested. The test solutions were prepared either with concentrated cluster solution (UV absorbance of 2 at 195 nm, where the clusters show a characteristic maximum absorption peak) or deionized water. The pH was adjusted with NaOH or HCl. The maximum deviation of the cluster water from a linear relationship was found at neutral pH, where dipoles strongly dominate the system’s electric potential, and diminished at both ends when ionic contribution dominates the potential. The addition of KCl up to a concentration of 0.01M to the cluster water solutions did not significantly change the shape of pH vs electrode potential mV curve. Thus, the electric potential of the stable dipole clusters was not affected by the presence of additional ionic species. In contrast, a linear relationship between electric potential and pH was found in deionised water over the entire range of pH tested. The average difference in measured electric potential between ordinary water and cluster solutions was 92.4 mV for NaCl and 103.4 mV for KCl.
This finding is in agreement with the ob-servation of electric potential associated with the structures on EFM (Fig.3b) and also with the crystallization patterns induced in NaH2PO4 (Fig. 5). Interestingly, Germano et al. (2013) has succeeded in electrolysing bi-distilled water to which a small amount of H2O2 was added. Two platinum electrodes were used, between which some tens of millivolts were measured, similar to that measured by Lo S-Y (1998) using stainless steel electrodes.
Relationship to Other Supramolecular Structures
Supramolecular structures of water per se is not new; many different forms have been inferred in bulk water under ambient conditions beginning with the convention-ally accepted H-bonded networks, the theory that water exists simultaneously in two states of different densities (for which there is now good evidence) (Huang et al., 2009), the idea due to Martin Chaplin (1999) that the two states correspond to an expanded and collapsed form of a 280-molecule quasicrystalline icosahedral structure (re-viewed in Ho, 2012a, pp.15-47), and the remarkable interfacial water that forms a macroscopic exclusion zone (EZ) next to a hydrophilic surface, rediscovered by Pollack and his team (Zhang and Pollack, 2003) (detailed review in Pollack, 2013, pp.45-69).
A substantial body of work carried out in the laboratory of A.I. Konovalov, kindly drawn to my attention by a referee (Ryszhkin et al., 2009, 2011, 2012, and references therein) shows that highly serially diluted solutions, regardless of whether they involve organic salts, amphiphilic or lipophilic substances, spontaneously form clusters 100 to 300 nm in size with surface potential -2 to -20 mV. This is in line with findings described here, although their data are complicated by the tendency of the organic molecules to form micelles, so a detailed comparison is not feasible at present. A most significant finding is that the nanostructures fail to form when the diluted solutions are placed in a container shielded by permalloy to exclude electromagnetic fields (Ryszhkin et al., 2011). This suggests that intera-ction with the ambient electromagnetic field is essential for the formation of supramolecular water clusters; as predicted from quantum electrodynamic field theory (see later). The team has characterized the nanostructures in terms of conductivity, size, surface tension and surface potential at a wide range of concentrations, but has not produced direct images of the nanostructures.
Elia et al. (2013a) successfully imaged large water clusters in water repeatedly brought into contact with Nafion. The “Nafionated water”, which they equate with EZ water (Pollack 2013) showed an increase in electrical conductivity χ up to two orders of magnitude, together with a linear cor-relation between the heat of mixing Qmix and χ, and linear anticorrelation between pH and logχ. Like EZ water, it has an absorption peak at 270 nm, which shows a roughly linear correlation with the χ. At the same time, there was a drop in pH from ~6 to 3, representing three orders of magnitude increase in proton concentration. The in-crease in conductivity was attributed to proton conduction in another publication (Elia et al., 2013c), suggesting that protons are present in the clusters. In other words, their clusters include not only EZ water but also water immediately next to it, which has been shown to be enriched in protons (Zhang and Pollack, 2003, Pollack, 2013).
Fluorescence microscopy revealed large structures on which the polystyrene spheres added to the solution appeared to be clustered (Elia et al., 2013a). Lyophilization of 20 ml of the Nafionated water gave 1 to 2 mg residue, and AFM confirmed the presence of micron size structures that look superficially similar to the large water clusters identified by Lo A et al. (2012) and Lo S-Y et al. (2009). In yet another publication, Elia et al. (2013b) claimed to have created similar supramolecular clusters in solutions diluted with vigorous shaking, though the resemblance is poor, and the solutions contained 100 to 1,000 times as much Na+ as those used by Lo’s team.
Lo’s clusters were created by another procedure; with absorption maximum reported at 195 nm (see above) and without a decrease in pH observed in Elia et al. (2013a). (Actually, an absorption maximum below 200 nm does exist in the Nafionated water of Elia et al. (2013a), though the scale of the absorption curve ends at 200 nm.) In another publication (Lo S-Y et al. 1996) however, the stable water clusters were measured with UV absorption peaks at 230.6 nm and 276.7 nm, with peaks of excitation (to fluorescence) at 220 nm and 270.2 nm, so at least some of the preparations may be more similar to Pollack’s EZ water, which shows a characteristic peak absorption at around 280 nm (Pollack, 2013). A variety of initiators – both ionic and non-ionic (but polar) – appeared to create similar clusters, suggesting that water itself is the main determinant in the formation of both the clusters and of the EZ.
Criticism of Lo et al.’s Work
Not surprisingly, Lo et al. (2009) were criticised for their claim to have produced stable water clusters (Kožišek et al., 2013), for that is the usual sceptical reaction to anything new and significant. The same criticism applies to most if not all other individual claims to have produced supramolecular water clusters mentioned in the section above. But the fact that, taken together, the very different approaches converge on the same result is sufficiently persuasive that the supramolecular clustering of water is at least a good working hypothesis for further research, and certainly should not be dismissed. I shall not go into the details of the criticisms, as Lo (2013) has answered them quite adequately. Rather, I shall go on to propose a structure for the supramolecular clusters based on predictions from quantum electrodynamics field theory.
Symmetrical Spherical Dipoles From Coherent Domains
Lo’s water clusters are closely linked to the coherent domains (CDs) of water predicted by Emilio Del Giudice and colleagues (Arani et al. 1995) to form spontaneously as the result of interaction between the ambient electromagnetic field and water in accordance with quantum field theory as applied to condensed matter (reviewed in Ho, 2012a, pp,51-81).
To summarize in brief, standard quantum theory does not predict quantum coherence for liquid water, largely because it ignores both quantum fluctuations and the interaction between matter and electromagnetic field; these are only taken into account in quantum electrodynamics field theory. But conventional quantum electrodynamics field theory applies only to gases.
Theoretical physicists Giuliano Preparata (1942 – 2000), Emilio Del Giudice, and colleagues extended conventional quantum electrodynamics theory to the condensed phase of liquids; they showed that interaction between the vacuum electromagnetic field and liquid water induces the formation of large, stable coherent domains (CDs) of about 100 nm in diameter at ordinary temperature and pressure, and these CDs may be responsible for all the special properties of water including life itself (Arani et al., 1995; Del Giudice, 2007; Del Giudice et al., 2010; see also Ho, 2011).
Each CD of water is a resonating cavity produced by the electromagnetic field that ends up trapping the field because the photon acquires an imaginary mass, so the frequency of the CD electromagnetic field becomes much smaller than the frequency of the free field with the same wavelength.
Consequently, under ambient conditions, water is an approximately equal mixture of CDs surrounded by incoherent regions. (It is more accurate to say that the water molecules are dancing between the CD and non-CD configurations, so both the CD and non-CD molecules are interchangeable.) This picture, according to Del Giudice and colleagues, is reflected in the many observations supporting a two-state model of liquid water (a dense state and less dense state co-existing simultaneously).
The small spheres of water (“balls”) that make up all the diverse structures of supramolecular water clusters created by Lo’s team (Figure 1) have the dimensions of the coherent domains predicted, i.e., ~100 nm in diameter. This is in line with the finding that the precise nature of the initiator is unimportant, because it is largely a property of water itself, with the initiator molecules playing a catalytic role.
However, these spherical coherent domains are not dipoles in the ordinary sense of the word as stated by A. Lo et al. (2012). Instead, quantum field theory predicts that the CD is oscillating between the ground state and an excited state of 12.06 eV, just below the first ionization potential of 12.56 eV, and therefore contains close to a million almost free electrons (the proportion of excited state molecules within the CD is estimated to be 0.13 (Del Giudice et al., 2013)). That means CDs are negatively charged at the periphery (close to or at the surface of the sphere). At the same time, positively charged protons are most likely extruded outside the domain, in common with what happens in EZ water (Pollack, 2013, pp.52-54). Del Giudice et al. (2010) hypothesize that EZ water is a macroscopic coherent domain stabilized on the hydrophilic interface (where they may transform into the specific layered structure proposed by Pollack 2013, pp. 59-61).
Consequently, these spherical CDs can mimic dipole interactions through negative charges on their periphery attracting positive charges just outside (see Figure 6) to form a three-dimensional potentially perfectly symmetrical giant electret (di-pole), i.e., there will be a dipole electric field measured in any direction as found by Lo S-Y (1998), and also German et al. (2013). Note also the 6-fold symmetry arising from close-packing of spheres, which will give rise to ‘snowflake’ like clusters.
Another prediction from quantum electrodynamic field theory (Del Giudice et al., 1988, 2010; Del Giudice and Vitiello, 2006) is consistent with the structure of the clusters proposed here, and may account for their apparent stability. The coherent oscillations maintained by the electromagnetic field trapped within the CDs can occur not just between the coherent ground state and excited state of the water molecule, but also between two rotational levels, which produce correlations as large as several hundred microns, giving rise to a common dipole orientation, but a net zero polarization field (on account of its symmetry), unless and until the rotation symmetry is broken. The combination of the two coherent oscillations, therefore, produces phase-locked coherent inter-actions among the CDs, resulting in stable supramolecular clusters with the electret structure depicted in Figure 6.
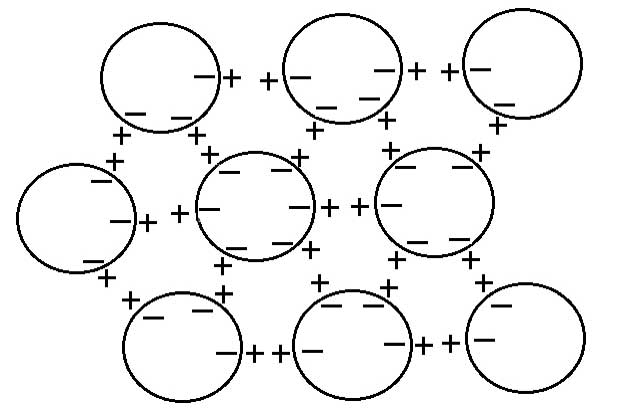
Figure 6: Spherical coherent domains forming a 3-dimensional dipole structure; note the 6-fold symmetry resulting from close-packing of sphere (see text for explanation).
The initiating solute has the role of aligning the CDs, while the sequential dilution with succession stimulates the coherent phase-locking of rotational oscillations among CDs. As the solution becomes diluted with vigorous shaking, the cluster breaks up into small pieces, seeding more clusters that align with one another or coalesce into larger ones. In the bulk solution, overall symmetry will be maintained, but two electrodes placed into it will still measure a net electric field, because the electrode surfaces effectively breaks symmetry.
This metastable state will also spontaneously break symmetry to favour one direction over all others when drops are placed in contact with a solid substrate, thereby giving rise to a wide variety of aggregates or clusters. A. Lo et al. (2012) have done detailed calculations to model the formation of double helix water clusters assuming two ‘rods’ each made up of 4 parallel strings of aligned dipole ‘balls’ winding round each other. That model should be equally valid for the spherical dipoles proposed here. Incidentally, the strong negative charge at the periphery of the coherent domains also explains why they should diffract or scatter electrons on TEM, and appear dark (Fig. 2).
A somewhat different explanation of supramolecular structures based on a complex array of starting aggregates of solvents with or without solutes in combination with rotating coherent domains was presented by Yinnon and Yinnon (2011), but they have not offered any specific hypothesis regarding the structure of the clusters.
How This Finding Could Revolutionize Biology and Medicine
Shui-Yin Lo attaches special significance to the double-helix clusters (“double helix water”) found in some preparations of clustered water, which he believes – in the absence of real evidence – to be the precursor the double-helix in DNA and to be the basis of the acupuncture meridians of traditional Chinese medicine. He also believes double helix water is especially beneficial for health, because it “works like a needle in acupuncture” (Lo S-Y, 2012). (See an alternative, though not mutually exclusive hypothesis on acupuncture mer-idians based on superconducting water aligned with collagen fibres in Ho, 2012b). Impressive changes to the thermographic images of the meridians can be observed before and after drinking double helix water, and preliminary results suggest that double helix water may be beneficial for autism, diabetes, thyroid, brain, and digestive systems (Lo SY, 2012, Bonavida and Stavroula, 2012, Velasquez, Chu and Lo SY, 2012). Obviously, a lot more work needs to be done to substantiate those preliminary observations; as well as the proposed structure of the supramolecular clusters presented here. Shui-Yin Lo and his team have opened up an exciting avenue for future research that could indeed revolutionize biology and medicine (see also Ho 2012a, Pollack 2013).
References
1. Arani R Bono I, Del Guidice E and Preparata G (1995). QED coherence and the thermodynamics of the water. Int J Mod Phys B 9: 1813-1841.
2. Bonavida B and Stavroula B (2012). Stable water clusters-mediated molecular alterations in human melanoma cell lines. Forum on Immunopathological Diseases and Therapeutics 3: 253-239.
3. Chaplin MF (1999). A proposal for the structuring of water. Biophy Chem 83: 211-221.
4. Del Giudice E (2007). Old and new views on the structure of matter and the special case of living matter. Journal of Physics: conference Series 67: 012006.
5. Del Giudice E, Fuchs ED and Vitiello G (2010). Collective molecular dynamics of a floating water bridge. WATER Journal 2: 69-82.
6. Del Giudice E, Preparata G and Vitiello G (1988). Water as a free electric dipole laser. Phys Rev Letts 61: 1085-1088.
7. Del Giudice E, Spinetti PR and Tedeschi A (2010). Water dynamics at the root of metamorphosis in living organisms. Water 2: 566-586.
8. Del Giudice E and Vitiello G (2006). Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transition. Physical Review A 74: 022105.
9. Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E and Niccoli M (2013a). Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with a Nafion polymer membrane. WATER Journal 5: 16-26.
10. Elia V, Ausanio G, Gentile F, Germano R, Napoli E and Niccoli M. (2013b). Experimental evidence of stable water nanostructures in extremely diluted solution, at standard pressure and temperature. J Hom (in press).
11. Elia V, Napoli E and Niccoli M (2013c). Physical-chemical study of water in contact with a hydrophilic polymer: Nafion. J Therm Anal Calorim 112: 937-944.
12. Gann DL and Lo S-Y (2009). Double-Helix Water, D & Y Publishing, Las Vegas.
13. Germano R, Del Giudice E, De Ninno A, Elia V, Hison C, Napoli E, Tontodonato V, Tuccinardi FP and Vitiello G. Oxyhydroelectric effect in bi-distilled water. Key Engineering Materials 543: 455-459.
14. Ho MW. (2011). Quantum coherent water & life. Science in Society 51: 26-28.
15. Ho MW (2012a). Living Rainbow H2O, World Scientific and Imperial College Press, Singapore and London.
16. Ho MW (2012b). Super-conducting liquid crystalline water aligned with collagen fibres in the fascia as acupuncture meridians of traditional Chinese Medicine. Forum on Immunopathological Diseases and Therapeutics 3: 221-36.
17. Huang C, Wikfeldt KT, Tokushima T et al. and A. Nilsson (2009). The inhomogeneous structure of water at ambient conditions. PNAS 106: 15214-8.Physics Letters A.
18. Kožišek F, Auerback D, Gast MKH and Lindner K (2013). Comment on: “Evidence for the existence of stable-water-clusters at room temperature and normal pressure” [Phys. Lett. A 373 (2009) 3872]. Physic Letters A 377: 2826-2827.
19. Lo A, Cardarella J, Turner J and Lo SY (2012). A soft matter state of water and the structures it forms. Forum on Immunopathological Disease and Therapeutics 3: 237-52.
20. Soft matter. Wikipedia, 14 August 2013, http://en.wikipedia.org/wiki/Soft_matter
21. Lo S-Y (1996). Anomalous state of ice. Modern Physics Letter B 10: 909-19.
22. Lo S-Y (2013). Reply to the comment by F. Kožišek et al. on “Evidence for the existence of stable-water-clusters at room temperature and normal pressure” [Phys. Lett. A 373 (2009) 3872]. Physic Letters A 377: 2828-2829.
23. Lo S-Y. Survey of IETM clusters. 1998 In Gann DL and Lo SY. In Double-Helix Water, D and Y Publishing, Las Vegas, 2009, pp. 117-159.
24. Lo S-Y (2012). Stable water clusters, meridians, and health. Forum on Immunopathological Diseases and Therapeutics 3: 193-219.
25. Lo S-Y, Lo A, Chong LW, Lin T, Li HH, Geng X (1996). Physical properties of water with IE structures. Modern Physics Letters B 10: 921-30.
26. Lo S-Y, Geng X and Gann D (2009). Evidence for the existence of stable-water-clusters at room temperature and normal pressure. Physics Letter A 373: 3872-6.
27. Upadhyay RP and Nayak C (2011). Homeopathy emerging as nanomedicine. In J High Dilution Res 10: 299-310.
28. Pollack GH. The Fourth Phase of Water, Ebner & Sons Publishers, Seattle, Washington, 2013.
29. Ryzhkina IS, Murtazina LI, Kiseleva YV and Konovalov AI (2009). Properties of supramolecular nanoassociates formed in aqueous solutions of biologically active compounds in low or ultra-low concentrations. Doklady Physical Chemistry 428: 196-200.
30. Ryzhkina IS, Murtazina LI and Konovalov AI (2011).Actioon of the external electromagnetic field is the condition of nanoassociate formation in highly diluted aqueous solutions. Doklady Physical Chemistry 440: 201-204.
31. Ryzhkina IS, Kiseleva YV, Murtazina LI, Mishina OA, Sherman ED and Konovalov A (2012). Comparative study of self-organization and physicochemical properties of highly diluted aqueous solutions of phenol bioantioxidants. Doklady Physical Chemistry 447: 203-206.
32. Velasquez R, Chu H and Lo S-Y (2012). Case study of autistic subjects with stable clusters in Panama. Forum on Immunopathological Diseasea and Therapeutics 3: 267-80.
33. Yinnon TA and Yinnon CA (2011). Electric dipole aggregates in very dilute polar liquids: theory and experimental evidence. Int J Mod Phys B 25: 3707-43.
34. Zhang JM and Pollack GH (2003). Long-range forces extending from polymer gel surfaces. Phys Rev E 68: 314-8.
Web Reference
1. Soft matter. Wikipedia, 14 August 2013, http://en.wikipedia.org/wiki/Soft_matter
Discussion with Reviewers
Anonymous Reviewer: What predictions could be made from your proposal on the structure of the clusters?
M-W. Ho: This is a key question. My proposal on the structure of the clusters depends on the interaction of water with the vacuum field or ambient electromagnetic field (emf), in accordance with the quantum electrodynamical field theory as described by Del Giudice (2007) Del Giudice et al. (1988, 2010a, b) and Del Giudice and Vitiello (2006). Hence the first prediction is that the clusters would fail to form in a container shielded from emf, as already suggested by the finding of Ryzhkina et al. (2011). Additional predictions are that it should be possible to measure distinct frequencies involved in the microwave and radiowave (kHz) regions respectively for the formation of the CDs and the extended coherence among the CDs that lead to clustering, because these frequencies would be emitted by the clusters as well as being absorbed by them. That is essentially why they are stable. Supplying the right frequencies should promote the formation of these clusters. It might even be possible to find frequencies that are especially good for “double helix water” for example.
Reviewer: What further experiments could be done?
Ho: The experiments involved in my answer to Question 1 could be done. But perhaps more importantly, a systematic approach should be adopted in characterizing the clustered water obtained under different conditions. TEM, AFM and EFM should all be performed under the same conditions, as well as other physical measurements. For example, EZ water should be harvested directly and tested to see if they form clusters or something different when dried as described by Lo et al. What is the role of charge (negative vs positive or neutral) in the gel surface? Do clusters compete with the EZ water structure as described in Pollack (2013)? If so, that might explain why EZ water appears less stable near positively charged surfaces (Pollack, personal communication).
Reviewer: What implications are there for homeopathy?
Ho: Lo’s team have not explicitly mentioned homeopathy, although high dilution with succussion is obviously related to homeopathy. It would be very interesting to see if distinct frequencies and nanostructures are associated with different initiating solutes. If so, it would be possible to correlate specific biological effects with distinct frequencies and nanostructures, thereby putting homeopathy on a firm empirical scientific basis.
