SPECIAL EDITION
High Dilutions
Effects of Highly Diluted Substances on Aquatic Animals: A Review
Nagai MYO1, Von Ancken ACB1, Bonamin LV1 *
1] Graduate Program in Environmental and Experimental Pathology – Universidade Paulista (UNIP). Rua Dr. Bacelar, 1212 – 4th floor. ZIP-CODE: 04026-002 – São Paulo, SP, Brazil.
Authors’ emails: Nagai MYO (mynagai@yahoo.com.br), Von Ancken ACB (acbvonancken@hotmail.com), Bonamin LV * (leoni.bonamin@docente.unip.br; leonibonamin@gmail.com).
Keywords: homeopathy, solvatochromic dyes, fish, bivalve, crustacean, amphibian.
• Received: November 3, 2021
• Revised: February 1, 2022
• Accepted: March 3, 2022
• Published: April 25, 2022
Abstract
Recent studies on the use of highly diluted substances to treat aquatic animals have been raised in literature. These studies are mainly focused on experimental tools to elucidate the mechanisms of high dilutions’ actions on living beings, and that reveal the potential use of these products as a clean and cheap technology to improve animal health. Endler and colleagues carried out the most reproducible experimental model in this field more than 20 years ago – that is, the effects of highly diluted thyroxine on tadpole development, whose papers were published between 1994 and 2015. More recently, certain species of aquatic animals – such as zebrafish and microcrustaceans – have been used as experimental models to evaluate toxicity and bioresilience. Concerning microcrustaceans, a series of studies using Artemia salina have shown interesting results in inducing adaptative processes to hazardous substances. At least partially, these effects seem to be associated with changes in the electric properties of water, as seen by its interaction with solvatochromic dyes.
Introduction
Environmental challenges demand new attitudes and perspectives from humanity concerning its economic relationship with the Earth’s fauna and flora. Finding solutions implies more research on the properties of water; thus, the global scientific community urges new and accurate experimental models to be carried out to strengthen the planet’s bioresilience.
The use of highly diluted substances – including homeopathic preparations – to promote aquatic animal health has been mentioned in recent studies. Some species are used as bioindicators of toxicity (Martins et al. 2007), a fact which motivated our group to conduct experimental research on the microcrustacean Artemia salina. The objectives of this research were to verify the putative protection of highly diluted substances, prepared according to the homeopathic pharmacopeia, to xenobiotics-exposed aquatic organisms (Coimbra Melo, 2020; Nagai, 2021; Pinto, 2021).
The findings indicate that the protection is mostly – but not exclusively – related to both changes in electric properties of water (Bonamin et al. 2020; Cartwright 2016, 2017, 2018, 2020) and hormesis, an adaptative phenomenon of living beings triggered by subtle stimuli (Berry III and López-Martínez, 2020; Bozhkov et al. 2010; Lajqi et al. 2019). The high dilution of toxic substances could hypothetically desensitize intoxicated organisms in a post-hormetic manner, even beyond Avogadro’s number, via specific information processed at a nanoscale level (Calabrese and Giordano, 2021; Ullman, 2021). The results show not only enhancement of aquatic organisms-derived food production (Lima et al. 2015; Mazón-Suástegui et al. 2019), but also the possibility of providing animal welfare (Narita et al. 2021).
Herein, we present an updated overview of the literature on basic and applied research concerning the effects of highly diluted solutions on aquatic organisms, followed by a discussion on further perspectives involving this technical resource as a tool for improvement of water quality and bioresilience.
Search Methods
The same methodology for reviewing indexed articles on the Pubmed® website presented by Bonamin et al. (2015) was used: searching for different experimental studies using aquatic animals published between 2001 and 2021. A thorough investigation was carried out using the following keywords:
• High-dilutions, high dilutions, highly-diluted, highly diluted, or homeopathy / fish;
• High-dilutions, high dilutions, highly-diluted, highly diluted, or homeopathy / bivalve;
• High-dilutions, high dilutions, highly-diluted, highly diluted, or homeopathy / crustacean;
• High-dilutions, high dilutions, highly-diluted, highly diluted, or homeopathy / amphibian;
• High-dilutions, high dilutions, highly-diluted, highly diluted, or homeopathy / zoological.
The inclusion criteria were based on language (only papers written in English) and type of article (only original experimental studies). Thus, the exclusion criteria were papers written in languages other than English, reviews, editorials, opinion articles, single-case reports, and studies focused on non-highly diluted interventions.
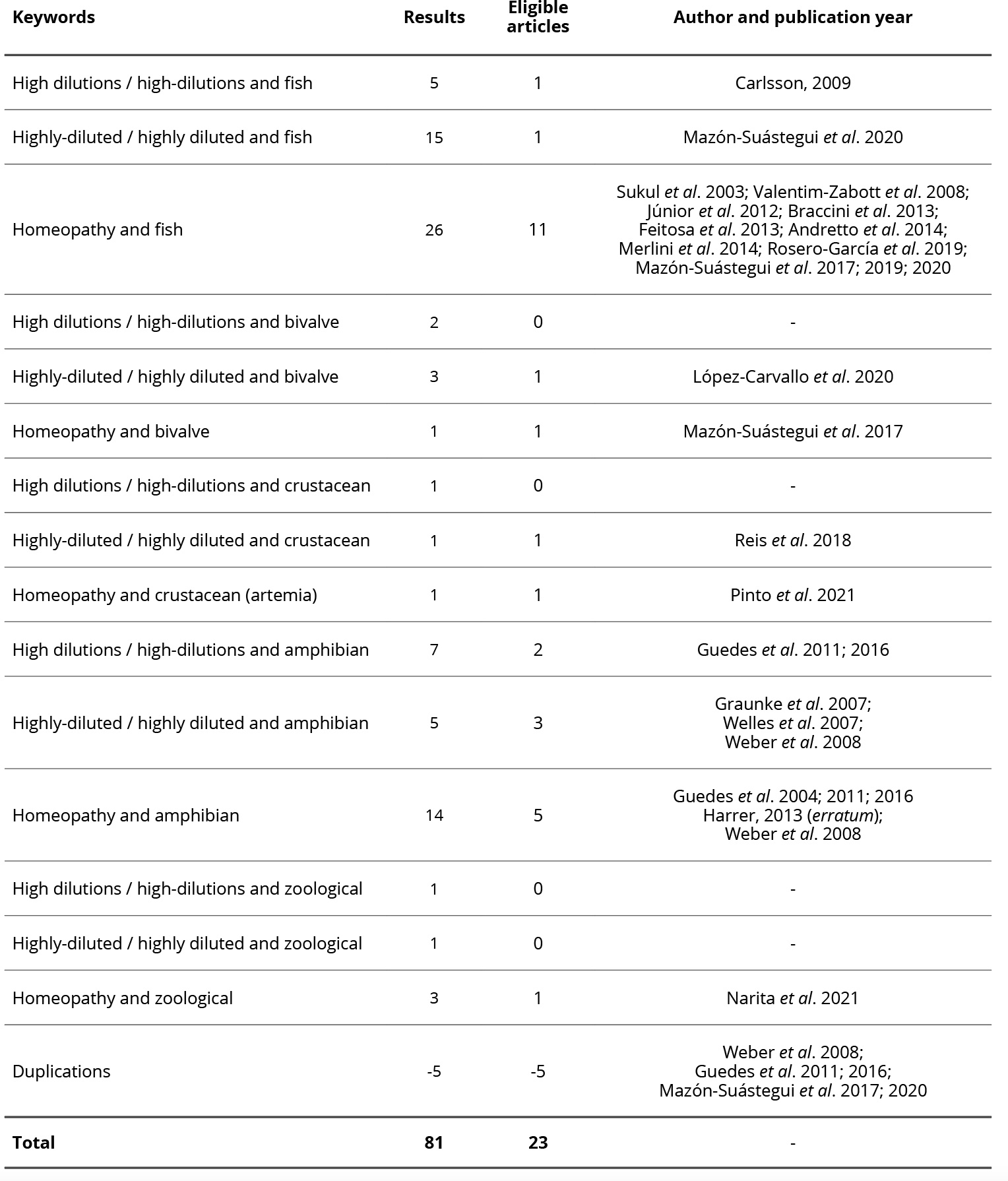
Table 1. Keywords, results, eligible articles, author, and year of publication.
Results and Discussion
Keywords and their respective results are indicated in Table 1. From the general results, eligible articles were chosen according to inclusion and exclusion criteria, resulting in 86 articles, of which 28 were eligible, and five were duplicates. Thus, 23 articles were finely selected for discussion.
The evolution observed over the last few years is noticeable, indicating the topic’s relevance despite scarce literature. The first report contemplating aquatic organisms and high dilution effects was seen in Endler and colleagues’ study, 1994. In this study, highly diluted thyroxine D30 (30 decimal dilution) was described as a factor that delayed metamorphosis in juvenile frogs following its introduction into water. It was the first study of a series from the same and other groups – see Table 1 – (Guedes et al. 2004; 2011; 2016; Graunke et al. 2007; Weber et al. 2008; Welles et al. 2007), and is one of the most reproducible experimental models involving high dilution research (Endler et al. 2010, 2015a,b; Oberbaum, 2013).
The reproducibility of Endler’s amphibian model was successfully tested by Guedes et al. (2004). The study focused on the 5 x 10-24 M (10cH or 10th centesimal Hahnemannian dilution) hormone, triiodothyronine (T3), and its action on tadpole tail explants that first suffered the induction of an apoptotic process by immersion in a 100 nM solution of the same hormone (Guedes et al. 2011). This group also ratified the initial proposal – that the highly diluted solution could have transmitted certain drug information, via water, to the tadpoles. From then on, the group improved its experimental model (randomized and blinded).
The expression of caspase proteins is directly related to the process of cell apoptosis. In another experiment that reproduced their previous results, Guedes et al. (2016) identified lower RNA expression of caspases 3 and 7 in tadpoles stimulated by T3 solution at 100 nM and subsequently treated with the 10cH dilution of this hormone. Again, the ability of high dilutions of thyroid hormones to induce changes in the metamorphosis-related process through the water was seen.
Graunke et al. (2007) observed that the immersion of lowland amphibians in highly diluted thyroxine also had a delaying effect on metamorphosis, preventing many from reaching the four-legged stage of development, even in amphibians that had been pretreated with thyroxin 10-8. On the other hand, Welles et al. (2007) found no direct relationship between the pretreatment of amphibians with thyroxine 10-8 and the subsequent immersion of the animals in a thyroxine 10-30 M solution to reduce metamorphosis. Later, Weber et al. (2008) demonstrated that sources such as microwaves and cellular phones inhibited the transmission of information by dilution. Airport x-ray and barcode scanners did not alter the results of the dilution on amphibians.
To reproduce the data obtained in previous research, Endler et al. (2015) proceeded to carry out a study that compiled results – from different years and stages of development – from amphibians treated with the thyroid hormone in the 30cH homeopathic potency. Although external variables – such as season, sample size, and duration of the experiment – could produce minor interferences in the results, there was statistical confirmation on the dilutions’ capacity to delay metamorphosis. Thus, following more than 20 years of refinement, the amphibian model seems to be well recognized now. Its outcomes can be considered a new cornerstone of fundamental research on high dilutions (Oberbaum, 2013). A prospective analysis of the functionality of experimental models was carried out by Endler et al. in 1994 (2015 b). Besides the apparent improvement of techniques and theoretical upgrade, the study found that most models – such as the amphibian model itself – were adequately developed and applied in laboratories.
Sukul et al. (2003) are responsible for the oldest report about high dilution effects on fish. In this study, the authors verified whether ultra-high dilutions of mercury chloride and Nux vomica extract (Mercurius corrosivus 30c and Nux vomica 30c) could change water permeability in fish erythrocytes pretreated with ethanol. Both testing dilutions increased water permeability in these cells, and these effects were related to aquaporin activity.
Between 2008 and 2014, a series of experiments using Nile Tilapia (Oreochromis niloticus) were performed at the State University of Maringá, Paraná, Brazil. They showed the effects of a homeopathic commercial product based on a mixture of highly diluted substances, Homeopatila Complex RS 100™ (Table 2) that, when poured onto food, improved weight gain, sex ratio, and gill and liver histology. In this case, alcohol 30% (negative) and the hormone, 17 alpha-methyltestosterone (positive), were the controls facing the treated group; the assays were performed in quadruplicate. Although Homeopatila Complex RS 100™ treated fish were significantly smaller than other groups, their survival was significantly greater (Valentim-Zabott et al. 2008).

Table 2. Composition of the Homeopatila Complex RS 100 ™
Some years later, Júnior et al. (2012) applied the same homeopathic complex to verify a possible increase in hormone-induced muscle hypertrophy. Environment temperature, water temperature, pH, dissolved oxygen, ammonia, and nitrite contents were measured. Again, although the high dilution treated fish were smaller in size, their survival and muscle fiber hypertrophy had improved. Studies with Nile Tilapia and the homeopathic complex continued in 2013 and 2014. Braccini et al. (2013) evaluated the prevalence of ectoparasites, and the morpho-functional response of the liver and gills of this aquatic organism fed with fishmeal and the Homeopatila Complex RS 100™ at different concentrations. Chemical parameters of water, temperature, pH, dissolved oxygen, and electrical conductivity were also measured three times a week and verified twice a day. The best results in the liver and branchiae occurred in fish receiving Homeopatila Complex RS 100™ at 40 mL/kg. Many parameters were improved, such as hepatocyte cell density, intercellular glycogen content, histological features (hyperplasia, lamella fusion, and telangiectasia), and percentage of mucin-producing cells. One year later, Merlini et al. (2014) studied the effect of Homeopatila Complex RS 100™ in the levels of plasma cortisol under stress conditions. The same treatment enabled Nile Tilapia to cope with stress since there was a decrease in cortisol levels.
Other interesting results concerning the performance of fish meat production using this product were described in Andretto et al. 2015 and Fuzinatto et al. 2015. On the other hand, the same performance of Homeopatila Complex RS 100™ could not be observed in an Amazonian fish, Tambaqui (Colossoma macropomum), as described by Pinheiro et al. (2015).
Concerning marine organisms, Mazón-Suástegui et al. (2017) conducted the first research of another high diluted commercial complex (Passival™) on juvenile scallops, Argopecten ventricosus, challenged with the bacterium, Vibrio alginolyticus. Growth, survival, and immune response were evaluated. The other two products, Phosphoricum acid 30cH and Silicea terra 30cH, were also studied and compared with two antibiotics (Ampicillin and Oxytetracycline). The researchers concluded that homeopathic treatments improved growth and survival against V. alginolyticus infection in juvenile scallops compared to the controls and the antibiotics. The formula of Passival™ was described as Passiflora incarnata 30cH, Valeriana officinalis 30cH, Ignatia amara 30cH, and Zincum valerianicum 30cH. In 2019 and 2020, the same group studied the gene expression of immune response cytokines in Seriola rivoliana juveniles (30- and 60-days post-hatching) treated with a high diluted product made from V. alginolyticus and a complex made by a mixture of Phosphoric acidum and Silicea terra. Fishes challenged with V. alginolyticus and treated with both protocols presented over-expression of IL-1 beta, but different outcomes related to oxidative and digestive enzymatic activity. In general, the complex Phosphoric acidum and Silicea terra improved immune response and food conversion – promising tools for sustainable and clean meat fish production (Mazón-Suástegui et al. 2019; 2020).
Another recent study was carried out by Roséro-Garcia et al. (2019) on intestinal coccidia infection, and cellular immune response of Pargo Rosa (Lutjanus gutattus) after different homeopathic treatment protocols; the products were sprinkled on the food. An increase in the accumulation of metabolic reserves in the liver, and of mucin production in gills, alongside significant maturation of neutrophils and lymphocytes in the blood, revealed a positive effect on fish health.
The most relevant paper published by this group (López-Carvallo et al. 2020) shows a transcriptome analysis of Catarina Scallop (Argopecten ventricosus) juveniles treated with high dilutions of three immunostimulatory agents: Phosphoricum acidum with Silicea terra; lysate obtained from Vibrio parahaemolyticus, and scorpion venom. A combination of gene expression and silencing was seen among the tissue samples for the different treatments. The increment in specific genes’ expression related to different molecular functions (ion binding, oxireductase activity, and transmembrane transporter activity) and metabolic pathways (enzymes related to food assimilation, biosynthesis of secondary metabolites, thermogenesis, hemocyte count, phagosomes, endocytosis, and NF-ĸB signal) highlighted A. ventricosus juvenile skills to cope with pathogen infections, also reducing proliferation. In sum, the authors state that transcriptome analysis allowed for comprehensive understanding of highly diluted treatments related to the immune response, reducing parasite proliferation, and allowing survival despite infection challenges.
Less frequent studies about the toxicology of highly diluted substances have also been reported. However, most of the studies in this field are related to the toxicology of nanoparticles, low doses, and low concentrations of active substances (Mirshafiee et al. 2017; Pulido-Reyes et al. 2017; Syberg et al. 2015), representing a higher level of active substances in comparison with ultra-dilutions. Thus, these studies are not in the scope of this issue.
Regarding the safety of high diluted products, Gupta et al. (2016) observed the presence of nanoparticles in highly diluted solutions related to patterns of tissue health in zebrafish embryos (Danio rerio) using electron microscopy technique. No toxicity was identified for the embryos (studied by the incidence of malformation, edema, necrotic or apoptotic cell processes, and death), even at different times of exposure.
From another point of view, due to the need to control the impact of water pollution on living beings, efficient models have been developed to study bioresilience. The discharge of untreated industrial effluents favors the accumulation of heavy metals in water bodies that, in turn, have a great capacity to generate alterations in animal behavior, such as the establishment of anxiety-like behavior. Carlsson (2009) described that very high levels of a range of pharmaceuticals were found in an effluent that receives garbage from approximately 90 manufacturers of bulk drugs. Despite their high toxicity to microorganisms, the effect of these drugs is unknown for aquatic animals due to the lack of studies involving chronic toxicity in these species. Milligram-per-liter levels of fluoroquinolones, for example, can be conserved across various organisms and impact wildlife, like the metamorphosis of tadpoles and zebrafish behavior. Coelho et al. (2018) demonstrated the anxiolytic capacity of Arsenicum album 6 cH by reversing the effects of intoxication induced by sodium arsenate added to water containing a zebrafish (Danio rerio) population.
Microcrustaceans can also perform different bioresilience mechanisms when facing various sources of stress. Knowing the impact of metals such as uranium on aquatic ecosystems is critical to ensure their long-term health and sustainability. Reis et al. (2018) have shown that exposing Daphnia magna to a 2% uranium solution permitted a transgenerational recovery auto-capacity under intermittent stress. Metal-induced DNA damage is no longer detected after the third generation, although more research, especially considering mutations, is still required.
Between 2019 and 2020, the Artemia salina model was inserted into the routine of our research group. There were three different works; each one focused on a specific intoxication challenge: mercury chloride, sodium arsenate, and glyphosate; thus, treatments based on homeopathic highly diluted solutions of the respective intoxicant were applied (Coimbra Melo, 2020; Nagai, 2021; Pinto, 2021).
Coimbra Melo (2020) observed that the hatching rate of Artemia salina cysts was higher following exposure to sodium arsenate in a concentration-dependent manner, and the effects were modified according to the moon phase. Furthermore, all testing with highly diluted sodium arsenate (6cH, 30cH, and 200cH corresponding to 3.2 x 10-14 M; 3.2 x 10-62 M and 3.2 x 10-402 M, respectively) reduced the hatching rate and improved the survival of the born nauplii; the crescent moon was established as standard. Curiously, the treatment of water containing sodium arsenate at the highest concentration (6.0 mg/ml) and the co-treatment with sodium arsenate 30cH resulted in greater arsenic precipitation and probably less bioavailability.
Nagai (2021) demonstrated that the treatment of glyphosate exposed Artemia salina cysts (at EC10 or 10% efficacy concentration) with glyphosate 6cH (5.9 x 10-13 M) reduced cysts’ hatching rates, leading to a selection of nauplii presenting more vitality and less defective morphology – an apparent effect of bioresilience. The post-treatment delay in the hatching rate was even more significant in 80% salinity water. The water analysis by the interaction of solvatochromic dyes revealed coumarin as the most specific physicochemical marker of samples treated with glyphosate 6cH, whatever the degree of water salinity. However, the statistical significance was higher in water containing glyphosate EC10 and 80% salinity. The results suggest that, besides the expected hormesis effect, the participation of the electrical properties of water after adding glyphosate 6cH could be at least part of the adaptative process of Artemia salina to glyphosate exposition, following its clear effects on solvatochromic dyes.
Pinto et al. (2021) subjected Artemia salina cysts to mercury chloride exposure at a concentration of 5.0 μg/ml (EC10), and treated them with Mercurius corrosivus at 6cH, 30cH, and 200cH dilutions (or mercury chloride 3.68 x 10-14 M; 3.68 x 10-62 M; 3.68 x 10-402 M respectively). Compared to control groups, the results showed a statistically significant delay in cyst hatching following treatment with Mercurius corrosivus 30cH. This outcome was associated with an increase in the total soluble mercury concentration in water, and an increase in the chlorine/oxygen ratio in microaggregates found in suspension, again suggesting some reduction in mercury bioavailability. In this case, there was a particular interaction of MC 30cH with the solvatochromic dye ET33.
Finally, the effect of high dilution was also described in marine birds. Narita et al. (2021) reported a series of clinical cases about treating acute and chronic pododermatitis in Magellanic penguins (Spheniscus magellanicus) with Calcarea carbonica 12cH and Arnica montana 6cH. The better rate of improvement was related to the early treatment. Animals presented regression of edema and infection, and no side effects.
Final Considerations
As seen above, there are a reasonable number of studies about highly diluted products concerning their effects on fish and other aquatic animals in both clinical and experimental approaches. These studies have two main foci of interest: the development of sustainable fish or bivalve meat production and laboratory models to elucidate the biological mechanisms of high dilution. An improvement of adaptative processes is seen, such as changes in metamorphosis or cysts’ hatching rates following the addition of highly diluted substances into water. These adaptative changes are related to the improvement of natural bioresilience processes that can vary according to species and environmental context, but in all cases can be facilitated by high dilution. Similar adaptative mechanisms on immune and physiological parameters to stressful situations were also reported by other authors (Dias-Neto et al. 2017). The set of findings present an interesting approach that contributes with the one-health concept – in which human, animal and environmental health must be considered as a whole – to provide modern and efficient solutions to contemporary problems, such as microorganism resistance to antimicrobials.
Nevertheless, a series of new studies points to the possibility of electric water changes being somehow involved in the bioresilience process. Although these involved mechanisms are not entirely understood, certain changes seem to be related to hormesis, since they are associated, at least in part, to the regulatory process of gene expression and protein production (Berry III and López-Martínez, 2020; Calabrese, 2018; Calabrese and Giordano, 2021; De Pádua et al. 2021; López-Carvallo et al. 2020). From the method developed by Steven Cartwright, variations on dipole moment of high dilutions submitted to succussion have been well established in a series of studies (Cartwright, 2016. 2017, 2018, 2019) in which a broad panel of dyes was tested. Additional studies performed on both cell cultures (Bonamin et al. 2020) and Artemia salina hatching cysts (Nagai, 2021; Pinto et al. 2021) have shown the capacity of the culture medium to interact with the same solvatochromic dyes that were reactive with the respective homeopathic products used as treatment. Moreover, the treatment of water reservoirs with homeopathic products has been prospected, opening up a new technical perspective to improve adaptative processes in the natural environment through water (Aparicio et al. 2020).
Conflict of interest
There is no conflict of interest referring to this article.
Discussion With the Reviewers (DWR)
Reviewer: Would there be a hypothesis for the result of Pinheiro et al. 2015?
Authors: The authors declared that it might be due to differences in fish metabolism, since Homeopatila Complex RS 100™ was designed to improve weight gain in Nile tilapia but not in Tambaqui fish. A certain species specificity was then observed.
Reviewer: According to the presented review, “high dilutions” have an essential role within the “One Health” perspective – so discussed nowadays. I would like to know the authors’ views on the subject. Moreover, how can they be used to demonstrate this role to the scientific community?
Authors: The One Health concept doubtless perfectly matches new possibilities to warrant potable water by means of innovative useful techniques of treating water. Social venues in parks with lakes, lagoons, and rivers could be an initial focus to proceed with scientifically established methodologies regarding water clean-up and increase of bioresilience in degraded fields through ultra-diluted aqueous solutions. Water physical and physicochemical analysis has been carried out over the last two years, resulting in outcomes that are related to the respective mechanisms (Aparicio et al. 2020; Bonamin et al. 2020; Cartwright 2018; Cartwright 2020; Yinnon and Liu, 2015; Yinnon, 2020). The reunion of all results could afford critical importance to homeopathy and its possibilities for human, animal, and environmental health.
References
Andretto AP, Fuzinatto MM, Bonafe EG, Braccini GL, Mori RH, Pereira RR, Oliveira CA, Visentainer JV, Vargas L (2014). Effect of a homeopathic complex on fatty acids in muscle and performance of the Nile tilapia (Oreochromis niloticus). Homeopathy 103(3):178-85. doi: 10.1016/j.homp.2014.02.001.
Andretto AP, Lösch JA, Gonçalves GA, Fuzinatto MM, Lima DP, Braccini GL, Filho LA, Canan C, Peralta RM, Vargas L (2015). Assessment of the oxidative state, related parameters and quality of muscle tissue in Nile tilapia with the application of homeopathic product Homeopatila 100 ® in high-density cages. African Journal of Pharmacy and Pharmacology 9(9): 279–286. doi:10.5897/ajpp2014.4256.
Aparicio ACC, de Oliveira LHS, Silva JS, Coelho CP, Pinheiro SR, Souza MF, Suffredini IB, Cartwright SJ, Bonamin LV (2020). Interaction between Solvatochromic Dyes and Water Sampled from a Natural Source Treated with High Dilutions of Phosphorus. Homeopathy 109(3):126-132. doi: 10.1055/s-0039-3400255.
Berry R 3rd, López-Martínez G (2020). A dose of experimental hormesis: When mild stress protects and improves animal performance. Comp Biochem Physiol A Mol Integr Physiol. 242:110658. doi: 10.1016/j.cbpa.2020.110658.
Bonamin LV, Cardoso TN, de Carvalho AC, Amaral JG (2015). The use of animal models in homeopathic research–a review of 2010-2014 PubMed indexed papers. Homeopathy 104(4):283-91. doi: 10.1016/j.homp.2015.06.002.
Bonamin LV, Pedro RRP, Mota HMG, Aguiar MSC, Pinto SAG, de Souza J, de Oliveira LHS, Aparicio AC, Peres GB, Suffredini I, Dutra-Correa M, Cartwright SJ (2020). Characterization of Antimonium crudum Activity Using Solvatochromic Dyes. Homeopathy 109(2):79-86. doi: 10.1055/s-0039-1697000.
Bozhkov A, Padalko V, Dlubovskaya V, Menzianova N (2010). Resistance to heavy metal toxicity in organisms under chronic exposure. Indian J Exp Biol. 48(7):679-96.
Braccini GL, Natali MR, Ribeiro RP, Mori RH, Riggo R, Oliveira CA, Hildebrandt JF, Vargas L (2013). Morpho-functional response of Nile tilapia (Oreochromis niloticus) to a homeopathic complex. Homeopathy 102(4):233-41. doi: 10.1016/j.homp.2013.06.002.
Calabrese EJ, Giordano J (2021). Ultra Low doses and biological amplification: Approaching Avogadros’s number. Pharmacological Research 170: 105738.
Calabrese EJ (2018). Hormesis: Path and Progression to Significance. Int J Mol Sci. 19(10):2871. doi: 10.3390/ijms19102871.
Carlsson G, Orn S, Larsson DG (2009). Effluent from bulk drug production is toxic to aquatic vertebrates. Environ Toxicol Chem. 28(12):2656-62. doi: 10.1897/08-524.1.
Cartwright SJ (2016). Solvatochromic dyes detect the presence of homeopathic potencies. Homeopathy 105(1):55-65. doi: 10.1016/j.homp.2015.08.002.
Cartwright SJ (2017). Interaction of homeopathic potencies with the water soluble solvatochromic dye bis-dimethylaminofuchsone. Part 1: pH studies. Homeopathy 106(1):37-46. doi: 10.1016/j.homp.2017.01.001.
Cartwright SJ (2018). Degree of Response to Homeopathic Potencies Correlates with Dipole Moment Size in Molecular Detectors: Implications for Understanding the Fundamental Nature of Serially Diluted and Succussed Solutions. Homeopathy 107(1):19-31. doi: 10.1055/s-0037-1617448.
Cartwright SJ (2020). Homeopathic Potencies May Possess an Electric Field(-like) Component: Evidence from the Use of Encapsulated Solvatochromic Dyes. Homeopathy 109(1):14-22. doi: 10.1055/s-0039-1693985.
Coelho CP, Chaulet VRL, Cappelli KLT, Santos RS, Vieira HC, Bernardi MM (2018). Neurobehavioral assessment of Danio rerio intoxicated by sodium arsenate and the use of Arsenicum Album to reverse the condition of anxiety. Int J High Dilution Res. 17(2):05-06. doi: 10.51910/ijhdr.v17i2.934.
Coimbra Melo EN (2020). Protective effect of isotherapeutic on the outbreak of cysts Artemia salina intoxicated with sodium arsenate. PhD Thesis, Postgraduate Program in Environmental and Experimental Pathology, Universidade Paulista – UNIP, São Paulo, 2020. Available at: https://repositorio.unip.br/dissertacoes-teses-programa-de-pos-graduacao-stricto-sensu-em-patologia-ambiental-e-experimental/efeito-protetor-do-isoterapico-sobre-a-eclosao-de-cistos-de-artemia-salina-intoxicadas-com-arseniato-de-sodio/
De Pádua SMF, Botter-Carvalho ML, Gomes PB, de Oliveira CS, Dos Santos JCP, Pérez CD (2021). The alien octocoral Carijoa riisei is a biogenic substrate multiplier in artificial Brazilian shipwrecks. Aquat Ecol. 2021: 1-18. doi: 10.1007/s10452-021-09908-8. Online ahead of print.
Dias-Neto J, Valladão GMR, Viadanna PHO, Pilarski F (2017). Homeopathic complex increases survival without affecting the performance of Nile tilapia during masculinization. Journal of Applied Aquaculture 29(1): 33-45. doi: 10.1080/10454438.2016.1274705.
Endler PC, Pongratz W, Kastberger G, Wiegant FA, Schulte J (1994). The effect of highly diluted agitated thyroxine on the climbing activity of frogs. Vet Hum Toxicol. 36(1):56-9.
Endler P, Thieves K, Reich C, Matthiessen P, Bonamin L, Scherr C, Baumgartner S (2010). Repetitions of fundamental research models for homeopathically prepared dilutions beyond 10(-23): a bibliometric study. Homeopathy 99(1):25-36. doi: 10.1016/j.homp.2009.11.008.
Endler PC, Scherer-Pongratz W, Harrer B, Lingg G, Lothaller H (2015). Amphibians and ultra high diluted thyroxine – further experiments and re-analysis of data. Homeopathy 104(4):250-6. doi: 10.1016/j.homp.2015.10.001.(a)
Endler PC, Schulte J, Stock-Schroeer B, Stephen S (2015). “Ultra High Dilution 1994” revisited 2015–the state of follow-up research. Homeopathy 104(4):223-6. doi: 10.1016/j.homp.2015.07.005. (b)
Feitosa KC, Povh JA, by Abreu JS (2013). Physiological responses of pacu (Piaractus mesopotamicus) treated with homeopathic product and submitted to transport stress. Homeopathy 102(4):268-73. doi: 10.1016/j.homp.2013.07.004.
Fuzinatto MM, Lima DP, Andretto AP, Menezes LA, Souza AHP, Franco MLS, Steinmacher NC, Mendonça SNTG, Vargas L (2015). Influence of a homeopathic product on performance and on quality flour and cookie (Grissini) of Nile tilapia. African Journal of Pharmacy and Pharmacology 9(27): 675-683. doi: 10.5897/AJPP2014. 4308.
Graunke H, Endler PC, Scherer-Pongratz W, Spranger H, Frass M, Lothaller H (2007). Treatment of lowland frogs from the spawn stage with homeopathically prepared thyroxin (10(-30)). Scientific World Journal 7:1697-702. doi: 10.1100/tsw.2007.220.
Guedes JR, Ferreira CM, Guimarães HM, Saldiva PH, Capelozzi VL (2004). Homeopathically prepared dilution of Rana catesbeiana thyroid glands modifies its rate of metamorphosis. Homeopathy 93(3):132-7. doi: 10.1016/j.homp.2004.04.006.
Guedes JR, Carrasco S, Ferreira CM, Bonamin LV, Souza W, Goldenstein-Schainberg C, Parra ER, Capelozzi VL (2011). Ultra high dilution of triiodothyronine modifies cellular apoptosis in Rana catesbeiana tadpole tail in vitro. Homeopathy 100(4):220-7. doi: 10.1016/j.homp.2011.05.007.
Guedes JRP, Carrasco S, Ferreira CM, Bonamin LV, Goldenstein-Schainberg C, Martins V, Capelozzi VL (2016). A morphometric and molecular study of the apoptosis observed on tadpoles’ tail explants under the exposition of triiodothyronine in different homeopathic dilutions. Homeopathy 105(3):250-256. doi: 10.1016/j.homp.2016.04.001.
Gupta HR, Patil Y, Singh D, Thakur M (2016). Embryonic Zebrafish Model – A Well-Established Method for Rapidly Assessing the Toxicity of Homeopathic Drugs: – Toxicity Evaluation of Homeopathic Drugs Using Zebrafish Embryo Model. J Pharmacopuncture 19(4):319-328. doi: 10.3831/KPI.2016.19.033.
Harrer B (2013). Replication of an experiment on extremely diluted thyroxine and highland amphibians. Homeopathy 102(1):25-30. doi: 10.1016/j.homp.2012.09.003.
Júnior RP, Vargas L, Valentim-Zabott M, Ribeiro RP, da Silva AV, Otutumi LK (2012). Morphometry of white muscle fibers and performance of Nile tilapia (Oreochromis niloticus) fingerlings treated with methyltestosterone or a homeopathic complex. Homeopathy 101(3):154-8. doi: 10.1016/j.homp.2012.05.005.
Lajqi T, Stojiljkovic M, Wetzker R (2019). Toxin-induced hormesis may restrain aging. Biogerontology 20(4):571-581. doi: 10.1007/s10522-019-09806-5.
Lima DP, Fuzinatto MM, Andretto AP, Braccini GL, Mori RH, Canan, C, Mendonça SNTG, Oliveira CAL, Pereira RR, Vargas, L (2014). Mechanically separated fillet and meat nuggets of Nile tilapia treated with homeopathic product. African Journal of Pharmacy and Pharmacology 9(6): 182-189. doi.org/10.5897/AJPP2014. 4173.
López-Carvallo JA, Mazón-Suástegui JM, Hernández-Oñate MÁ, Tovar-Ramírez D, Abasolo-Pacheco F, Morelos-Castro RM, Arcos-Ortega GF (2020). Transcriptome analysis of Catarina scallop (Argopecten ventricosus) juveniles treated with highly-diluted immunomodulatory compounds reveals activation of non-self-recognition system. PLoS One 15(5):e0233064. doi: 10.1371/journal.pone.0233064.
Martins J, Oliva Teles L, Vasconcelos V (2007). Assays with Daphnia magna and Danio rerio as alert systems in aquatic toxicology. Environment International 33(3): 414–425. doi:10.1016/j.envint.2006.12.06.
Mazón-Suástegui JM, García-Bernal M, Saucedo PE, Campa-Córdova Á, Abasolo-Pacheco F (2017). Homeopathy outperforms antibiotics treatment in juvenile scallop Argopecten ventricosus: effects on growth, survival, and immune response. Homeopathy 106(1):18-26. doi: 10.1016/j.homp.2016.12.002.
Mazón-Suástegui JM, Salas-Leiva J, Teles A, Tovar-Ramírez D (2019). Immune and Antioxidant Enzyme Response of Longfin Yellowtail (Seriola rivoliana) Juveniles to Ultra-diluted Substances Derived from Phosphorus, Silica and Pathogenic Vibrio. Homeopathy 108(1):43-53. doi: 10.1055/s-0038-1672197.
Mazón-Suástegui JM, Salas-Leiva J, Teles A, Tovar-Ramírez D (2020). Evaluation of Homeopathic Phosphoric Acid, Silica and Pathogenic Vibrio on Digestive Enzyme Activity of Longfin Yellowtail Fish (Seriola rivoliana). Homeopathy 109(1):3-13. doi: 10.1055/s-0039-1692998.
Merlini LS, Vargas L, Piau R Jr, Ribeiro RP, Merlini NB (2014). Effects of a homeopathic complex on the performance and cortisol levels in Nile tilapia (Oreochromis niloticus). Homeopathy 103(2):139-42. doi: 10.1016/j.homp.2013.08.005.
Mirshafiee V, Jiang W, Sun B, Wang X, Xia T (2017). Facilitating Translational Nanomedicine via Predictive Safety Assessment. Mol. Ther. 25(7):1522-1530. doi: 10.1016/j.ymthe.2017.03.011.
Nagai, MYDO (2021). Remediation of the effects of glyphosate on brine shrimp by isotherapy. PhD Thesis, Postgraduate Program in Environmental and Experimental Pathology, Universidade Paulista – UNIP. São Paulo, p.93. 2021. Available at: https://www.unip.br/eceeic/admin/Anexos/Conteudo/C2020/ C8/file_2508202011043733.pdf
Narita FB, Scardoeli B, Gallo Neto H, Coelho CP (2021). Homeopathic Treatment of Pododermatitis in Magellanic Penguins (Spheniscus magellanicus). Homeopathy 110(1):62-66. doi: 10.1055/s-0040-1716392.
Oberbaum M (2013). Highland amphibians and high potencies: a 20-year metamorphosis. Homeopathy 102(1):1-2. doi: 10.1016/j.homp.2012.10.001.
Pinheiro DA, Cavero BAS, Vargas L, Braccini GL, Yoshioka ETB, Oliveira MSB, Dias MT (2015). Performance, parasitic infections, hematology, and hepatic histology of Colossoma macropomum (tambaqui) fed on homeopathic product. African Journal of Pharmacy and Pharmacology 9 (4): 82-90, 29. doi: 10.5897/AJPP2014. 4194.
Pinto AAG, Nagai MYO, Coimbra EN, Mohammad SN, Silva JS, Von Ancken A, Pinto SAG, Aguiar MS, Dutra-Correa M, Hortellani MA, Miranda A, Sarkis JES, Suffredini IB, Peres GB, Bernardi MM, Cartwright SJ, Bonamin LV (2021). Bioresilience to Mercury Chloride of the Brine Shrimp Artemia Salina after Treatment with Homeopathic Mercurius Corrosivus. Homeopathy 2021. doi: 10.1055/s-0041-1729562. Online ahead of print.
Pulido-Reyes G, Leganes F, Fernández-Piñas F, Rosal R (2017). Bio-nano interface and environment: A critical review. Environ Toxicol Chem. 36(12):3181-3193. doi: 10.1002/etc.3924.
Reis P, Pereira R, Carvalho FP, Oliveira J, Malta M, Mendo S, Lourenço J (2018). Life history traits and genotoxic effects on Daphnia magna exposed to waterborne uranium and to a uranium mine effluent – A transgenerational study. Aquat Toxicol. 202:16-25. doi: 10.1016/j.aquatox.2018.06.009.
Rosero-García ADP, Mazón-Suástegui JM, Dumas S, Chávez-Sánchez MC, Avilés-Quevedo A, Rodríguez-Jaramillo C (2019). Effect of Homeopathic Medicines on Intestinal Coccidia and Immune Response Cells in Spotted Rose Snapper (Lutjanus guttatus). Homeopathy 108(3):201-213. doi: 10.1055/s-0039-1681062.
Sukul NC, De A, Sinhababu SP, Sukul A (2003). Potentized Mercuric chloride and Nux vomica facilitate water permeability in erythrocytes of a fresh-water catfish Clarius batrachus under acute ethanol intoxication. J Altern Complement Med. 9(5):719-25. doi: 10.1089/107555303322524562.
Syberg K, Khan FR, Selck H, Palmqvist A, Banta GT, Daley J, Sano L, Duhaime MB (2015). Microplastics: addressing ecological risk through lessons learned. Environ. Toxicol. Chem. 34(5):945-53. doi: 10.1002/etc.2914.
Ullman D (2021). Exploring Possible Mechanism of Hormesis and Homeopathy in the Light of Nanopharmacology and Ultra-High Dilutions. Dose-response. Available at https://doi.org/10.1177/15593258211022983.
Valentim-Zabott M, Vargas L, Ribeiro RP, Piau R Jr, Torres MB, Rönnau M, Souza JC (2008). Effects of a homeopathic complex in Nile tilapia (Oreochromis niloticus L.) on performance, sexual proportion and histology. Homeopathy 97(4):190-5. doi: 10.1016/j.homp.2008.08.007.
Weber S, Endler PC, Welles SU, Suanjak-Traidl E, Scherer-Pongratz W, Frass M, Spranger H, Peithner G, Lothaller H (2008). The effect of homeopathically prepared thyroxine on highland frogs: influence of electromagnetic fields. Homeopathy 97(1):3-9. doi: 10.1016/j.homp.2007.11.002.
Welles SU, Endler PC, Scherer-Pongratz W, Suanjak-Traidl E, Weber S, Spranger H, Frass M, Lothaller H (2007). Pretreatment with thyroxin 10(-8) and the effect of homeopathically prepared thyroxin 10(-30) on highland frogs–a multi-researcher study. Forsch Komplementmed. 14(6):353-7. doi: 10.1159/000111540.
Yinnon TA, Liu ZQ (2015). Domains formation mediated by electromagnetic fields in very dilute-aqueous solutions 1, 2, 3. Water 5: 33-95.
Yinnon TA (2020). Liquids Prepared by Serially Diluting and Vigorously Shaking of Aqueous Solutions: Unveiling Effects of the Solute on their Properties. Water 10: 115-134.
