 Aqueous Solutions and other Polar Liquids Perturbed by Serial Dilutions and Vigorous Shaking: Analyses of Their UV Spectra
Aqueous Solutions and other Polar Liquids Perturbed by Serial Dilutions and Vigorous Shaking: Analyses of Their UV Spectra
Yinnon, TA 1,2*
1K. Kalia, D.N. Kikar Jordan 90666, Israel
2Reedmace Lake, Enot Tsukim Nature Reserve at Kalia, Israel.
*Correspondence E-mail: lwcdsrc@kalia.org.il
Keywords: Very dilute aqueous solutions; serially diluted solutions; ultraviolet spectra; water molecule aggregates; ferroelectric orderings; polar liquids.
Received: January 12, 2018; Revised: July 14, 2018; Accepted: July 18, 2018; Published: September 29, 2018; Available Online: September 29, 2018
Abstract
Aqueous solutions and other polar liquids, perturbed by serial dilutions and vigorous shaking, have properties which are of importance for toxicology, medicine and wastewater treatment. Extensive research has shown that for serially diluted polar liquids, e.g., water, alcohol and their solutions, vigorous shaking after each dilution step may alter their structure, physicochemical characteristics and bioactivity. The alterations may occur when the concentrations of the liquids drop below a solute dependent threshold concentration. Typically, the threshold concentrations are in the 10-6 – 10-10 mol / liter range. In the current study, the ultraviolet absorption and emission spectra of such liquids are analyzed. The analyses are carried out within the context of quantum electrodynamics (QED). The analyses show that the spectra can be consistently explained by the QED model for serially diluted vigorously shaken polar liquids, developed by Yinnon and Yinnon [Int J Mod Phys B 25:3707-3743 (2011)].
Introduction
Serial diluting polar liquids, and perturbing these by vigorous shaking after each dilution step, may cause their properties to statistically significantly differ from those of the same liquids which are identically diluted but not vigorously shaken (Elia and Niccoli, 1999, 2000, 2004a; Elia et al., 2004b). During the last three decades, many researchers demonstrated that such perturbations may affect the bioactive, structural and physicochemical properties of serially diluted polar liquids. For example, such effects were demonstrated by Burlakova et al. (1986, 2004, 2005); Davenas et al. (1988), Palmina et al. (1994), Pynzar et al. (1995), Elia and Niccoli (1999, 2000, 2004a), Elia et al., (2004b, 2005, 2008a, 2008b, 2010), Lobyshev et al. (2005), Konovalov et al. (2008), Bhattacharyya et al. (2008), Ryzhkina et al. (2010, 2011a&b,2012a-c, 2013, 2015a&b, 2016, 2017a&b), Belov et al. (2011), Konovalov (2013), Bellavite et al. 2014), Mishina et al. (2015), Voeikov and Yablonskaya (2015), Betti et al. (2017).
Serially diluted polar liquids may be affected by vigorous shaking when these are diluted beyond a threshold concentration (Cthr). Solute type determines Cthr. Typically, Cthr is of the order of 10-6 – 10-10 M. Serially diluted polar solutions of many solutes, but not all kind of solutes, are affected by vigorous shaking. The solutes may include inorganic-, organic- and bio-molecules. Solute attributes required for vigorous shaking to have an effect on the liquids’ properties are not yet clarified (Konovalov, 2013; Ryzhkina et al., 2015a).
On serial diluting vigorously shaken polar liquids below Cthr and up to picomolar, femtomolar concentrations or beyond, their properties may non-monotonically change — for reviews see Konovalov and Ryzhkina (2014) and Elia et al. (2015). Just as in Yinnon and Liu (2015a), I here denote serially diluted vigorously shaken polar liquids as SDVSPL.
SDVSPL typically, are prepared from a stock (“mother”) solution with a concentration (C) in the 4 M – 10-3 M range. The stock solution is repetitively (serially) decimally or centesimally diluted at ambient conditions. After each dilution step, the liquid is vigorously shaken with lab dancer shaker, by vertical vortexing or other methods.
The main aspects of aqueous SDVSPL, measured by independent research groups, were recently summarized in the 2nd section of the paper by Yinnon (2017). Acquaintance with this summary is helpful for appreciating the issues presented in the current article.
Impurities released by containers or other contaminants affect the physicochemical variables of SDVSPL. However, analyses of SDVSPL prepared in glass or plastic vessels show that such impurities cannot account for their typical properties (Elia and Niccoli, 2004a&b; Witt et al., 2006; Ciavatta et al., 2008; Montagnier et al., 2009; Elia and Napoli, 2010; Upadhyay and Nayak, 2011; Demangeat, 2013, 2015; Pershin et al. 2015; Ryzhkina et al., 2015b; Yinnon and Liu, 2015b).
Electro-magnetic (EM) radiation significantly affects the formation of the nano to micron sized molecular associates (ensembles), which are present in bioactive SDVSPL diluted below Cthr (Ryzhkina et al. 2012a-c; Konovalov and Ryzhkina, 2014). These associates are mainly composed of solvent molecules. The pronounced effects of ambient EM radiation on these associates indicate that explaining the properties of SDVSPL necessitates an electro-dynamic theory (Preparata, 1995; Yinnon and Liu, 2015a; Fiorini, 2016). The quantum electro-dynamic (QED) model for SDVSPL proposed by Yinnon and Yinnon (2011) has provided consistent explanations for many of their phenomena (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013; Yinnon and Liu, 2015b,c; Yinnon, 2017).
The goal of this paper is to employ the QED model for clarifying some hitherto unexplained ultra violet (UV) spectral features of SDVSPL reported in the literature. The clarifications are important, because experiments have shown that just as for many other liquids, UV spectra contain information on these liquids’ electronic distributions.a Some reported UV spectral features will not be analyzed. Seemingly not all researchers are familiar with the hysteretic and far-from-equilibrium dissipative system properties of SDVSPL. These properties have implications for assuring that SDVSPL features are significant, as discussed in the introduction section of Yinnon (2017). For example, an implication is that the controls (blanks) should be carefully designed.
The outline of the paper is as follows: In the “Theory Section,” the SDVSPL model is concisely summarized.b In the subsequent section, i.e., the “Analyses of Reported Data Section,” explanations are presented for the UV and visible (vis) radiation absorbance and fluorescence spectra of SDVSPL. Next, the “Discussion Section” focuses on the fact that the experimental data analyzed in this paper, as well as the extensive data analyzed in numerous other papers, did not yet reveal the following important relation: The relation between the characteristics of the solutes in the mother solutions of SDVSPL and the physicochemical, structural and bioactive properties of these liquids after their dilution below Cthr.
No new experimental results are presented in this paper. All the experimental results, which will be analyzed, were obtained in previous studies and reported in the literature. A list with abbreviations is presented at the end of this paper.
a At the time of submission of this paper, an article was published that report UV absorption spectra of aqueous SDVSPL of (S)-lysine and aqueous SDVSPL of (R)-lysine (Ryzhkina et al., 2018). The article reports the variations in the spectra of the SDVSPL of these enantiomers and their relations to the domains and nano-associates present in these liquids. Analyses of the findings reported in this article I intend to carry out in a future publication.
b Elaborate discussions on the various aspects of the model are published in Yinnon and Yinnon (2011), Yinnon and Elia (2013) and Yinnon and Liu (2015b&c). Since QED of aqueous solutions hitherto mainly has been employed for explaining special phenomena, many readers may be unfamiliar with it. Its aspects relevant to this paper’s analyses are concisely summarized in Yinnon and Liu (2015a).
Theory
Customary models of polar liquids predict that serial dilutions, combined with vigorous shaking and exposure to ambient EM radiation, do not affect their characteristics (Horne, 1972; Robinson and Stokes, 2002). In addition, these models show that solvent molecules, with the exception of solvation shells’ molecules, move randomly. Moreover, these models show that solvated solutes distribute homogenously, move independently and randomly. These customary models explicitly describe electro-static forces and assume electro-dynamic ones can be treated perturbatively or often even may be ignored. However, QED models explicitly (non-perturbatively) describing electro-dynamic forces show that these forces may lead to association of the molecules constituting polar liquids (Del Giudice, 1988, 1998, 2000, 2006; Preparata, 1995 chapters 2, 5, 10; Arani et al., 1995; Yinnon and Yinnon, 2012). Interactions between EM radiation and electrolytic solutes, polar solute molecules, the dipole moment or electrons of solvent molecules may lead to formation of various domain types. Here we denote such domains as QED domains. Formation of these domains occurs only in specific concentration ranges, which depend on solute type. These domains may agglomerate into supra-domains. These supra-domains are not ensembles of molecules but agglomerates of domains, like domains in liquid crystals.
A concise review of the QED theory of polar liquids has been presented by Yinnon and Liu (2015a). The various hitherto identified QED domains present in these liquids, their schematic pictures, their properties and the physics underlying the dependence of the domains’ formation on concentration are all summarized in this review. In the review, it is emphasized that the QED theory of water is based on an explicit non-perturbative description of the van der Waals’ dispersion forces. The imperative for adequately describing van der Waals’s dispersion interactions and other electro-dynamic interactions in various condensed matter systems has also recently been pointed out by Ferri et al. (2015), Fiorini (2016) and Ambrosetti et al. (2016).
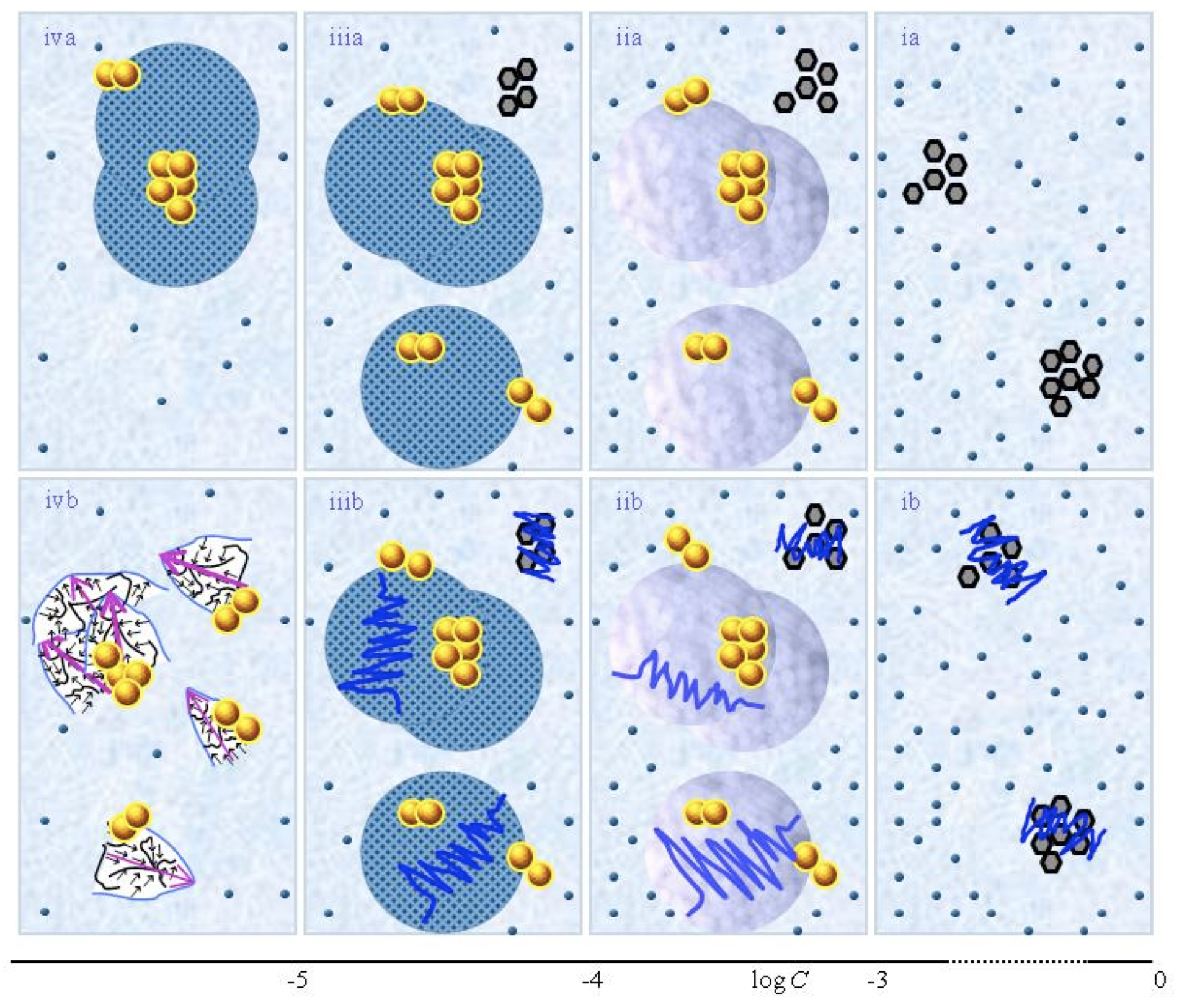
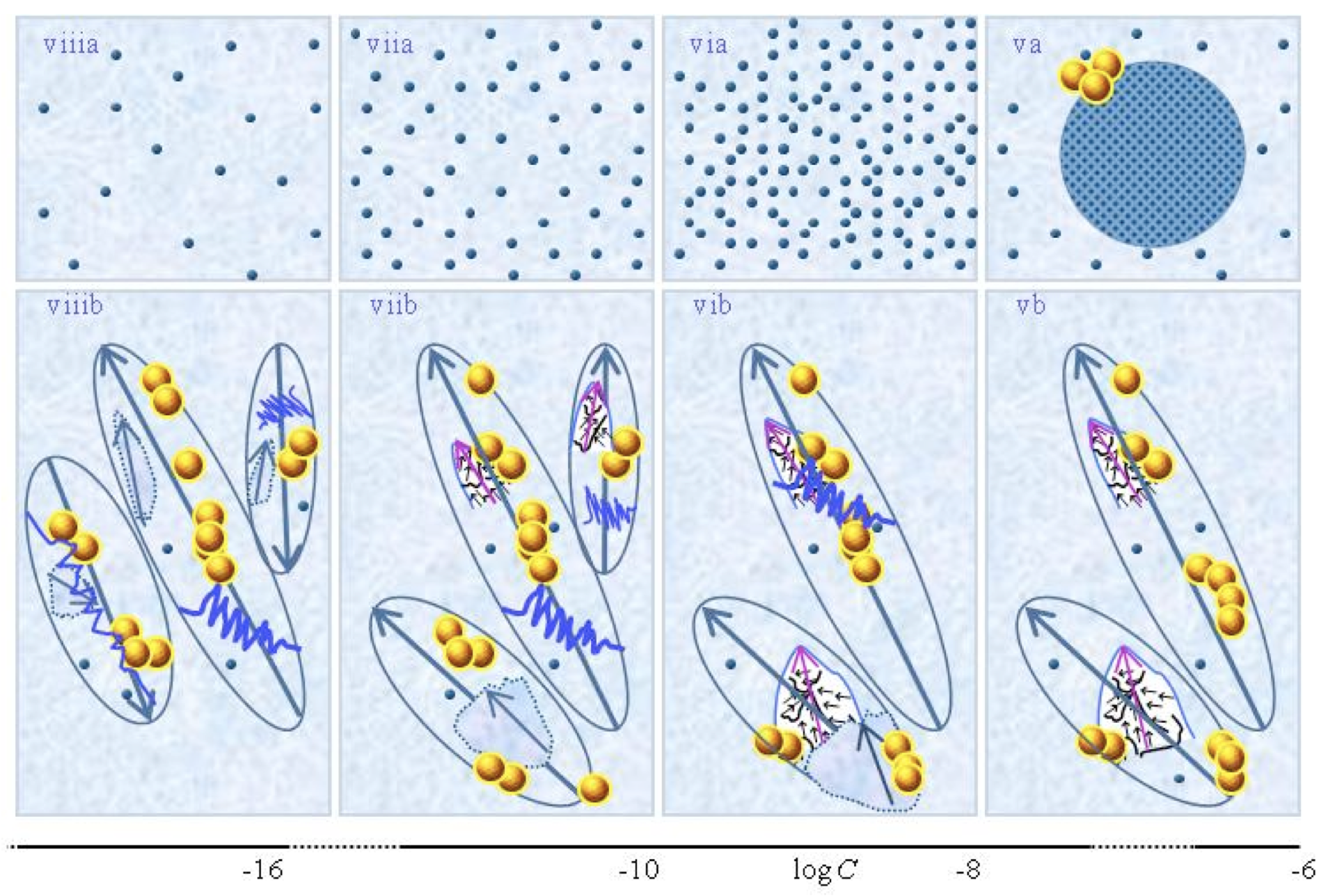
Figure 1. This figure presents a schematic picture of serially diluted solutions of weak electrolytes or non-electrolytic compounds. The top row (a) and bottom row (b) series pertain to solutions which, respectively, were not vigorously shaken and those which were vigorously shaken after each dilution step. Figure ia illustrates that for C larger than a transition concentration ![]() , all solvated solutes move randomly, i.e., do not organize in a QED domain. The tiny blue balls represent randomly moving ~10-9 – 10-8 m solvated solutes. The irregularly shaped bunches of black hexagons represent aggregates of non-solvated solutes. Figure iia illustrates that on dilution below
, all solvated solutes move randomly, i.e., do not organize in a QED domain. The tiny blue balls represent randomly moving ~10-9 – 10-8 m solvated solutes. The irregularly shaped bunches of black hexagons represent aggregates of non-solvated solutes. Figure iia illustrates that on dilution below ![]() , solvated solutes organize in a QED domain type denoted CDplasma (symbolized with purple-blue colored balls). The yellow-brown balls and their agglomerates represent, respectively, ~10-7m sized QED domains denoted CDelec and supra-CDelec, which both are stabilized by CDplasma. Figures iia and iiia illustrate the transformation of CDplasma into another type of QED domain denoted IPDplasm.a. The transformation occurs at the transition concentration
, solvated solutes organize in a QED domain type denoted CDplasma (symbolized with purple-blue colored balls). The yellow-brown balls and their agglomerates represent, respectively, ~10-7m sized QED domains denoted CDelec and supra-CDelec, which both are stabilized by CDplasma. Figures iia and iiia illustrate the transformation of CDplasma into another type of QED domain denoted IPDplasm.a. The transformation occurs at the transition concentration ![]() .
.
Figures ia-iiia illustrate that on dilution the non-solvated solutes diminish, i.e., solvate. Figures iiia-va illustrate that on dilution the diameter of IPDplasma does not change, but the number of IPDplasma diminishes. Figures via-viiia illustrate that below a certain concentration there are insufficient solutes to form IPDplasma. The concentration, below which no IPDplasma form, has not yet been theoretically derived. Figures via-viiia illustrate that whenever there are too few solutes to form IPDplasma, the solution has the characteristics predicted by the customary models, i.e., all solvated solutes move randomly and their number diminishes on dilution.
In the Figure 1b series, the blue zigzag curves symbolize that shaking excites or cracks domains and aggregates. Figure iib illustrates that excitations or cracking does not significantly alter the internal structure of CDplasma, which just as in Figure 1iia are represented with purple-blue colored balls. Figures iib and iiib illustrate the transition from CDplasma to IPDplasma, with the latter pictured as blue-crystalline balls just as in Figure iiia. Figures iiib and ivb illustrate that shaking excites or breaks up IPDplasma. The excited or broken IPDplasma pieces, which in the text are denoted electric dipole aggregates (EDAIPDplasma), are pictured as irregularly shaped aggregates in Figure ivb. Their aligned black arrows orderings symbolize EDAIPDplasma’s distorted ferroelectric H2O orderings. The purple arrow in the EDAIPDplasma symbolizes these domains’ dipole moments. Figures ivb and vb illustrate that on diluting below a solute type dependent critical concentration (![]() ) a QED domain denoted CDrot becomes stabilized by EDAIPDplasma, i.e., the irregularly shaped EDAIPDplasma are located within the elongated ovals representing CDrot. The dark blue arrows symbolize the dipole moment of CDrot. Figure vib shows that vigorous shaking excites or breaks up CDrot, thus creating entities denoted EDACDrot. The lump outlined with an irregularly shaped broken curve and located at the bottom of one of the left CDrot represents the EDACDrot. Figures vib-viib show that at certain concentrations both EDAIPDplasma and EDACDrot are present within CDrot,, though the sizes of EDAIPDplasma diminish with decreasing concentration. Figure viiib shows that on diluting further, no EDAIPDplasma persist, i.e., there are too few solute particles to sustain EDAIPDplasma. At these concentrations, vigorous shaking just breaks up CDrot and creates new EDACDrot. These in turn stabilize new CDrot, as pictured in Figure viiib. Figures vb-viib illustrate that CDrot may align with their dipole moments parallel. Figure viiib illustrates that at certain concentrations their dipoles may be aligned anti-parallel. (Note that the sizes of the various domains, their broken pieces and the sizes of the solvated solutes with their hydration shells are not presented according to their realistic scale ratios.)
) a QED domain denoted CDrot becomes stabilized by EDAIPDplasma, i.e., the irregularly shaped EDAIPDplasma are located within the elongated ovals representing CDrot. The dark blue arrows symbolize the dipole moment of CDrot. Figure vib shows that vigorous shaking excites or breaks up CDrot, thus creating entities denoted EDACDrot. The lump outlined with an irregularly shaped broken curve and located at the bottom of one of the left CDrot represents the EDACDrot. Figures vib-viib show that at certain concentrations both EDAIPDplasma and EDACDrot are present within CDrot,, though the sizes of EDAIPDplasma diminish with decreasing concentration. Figure viiib shows that on diluting further, no EDAIPDplasma persist, i.e., there are too few solute particles to sustain EDAIPDplasma. At these concentrations, vigorous shaking just breaks up CDrot and creates new EDACDrot. These in turn stabilize new CDrot, as pictured in Figure viiib. Figures vb-viib illustrate that CDrot may align with their dipole moments parallel. Figure viiib illustrates that at certain concentrations their dipoles may be aligned anti-parallel. (Note that the sizes of the various domains, their broken pieces and the sizes of the solvated solutes with their hydration shells are not presented according to their realistic scale ratios.)
SDVSPL Model
Fig.1 presents a schematic picture of the SDVSPL model proposed by Yinnon and Yinnon (2011), i.e., the structure of SDVSPL for different C ranges. The figure is a reprint of Fig.1 in Yinnon and Liu (2015c). The figure pertains to SDVSPL of weak- or non-electrolytic compounds. The schematic picture for the model of SDVSPL of strong electrolytes is very similar. It appeared in Fig. 1 of Yinnon and Liu (2015b). The differences between the models of weak-, non- and strong electrolytes are minor ones, as discussed below in paragraphs I and II. The various aspects of the models are discussed in the following paragraphs I-VI. Experimental results, obtained in independent research groups, are in agreement with these aspects, as shown by Yinnon and Yinnon (2011), Yinnon and Elia (2013),Yinnon and Liu (2015b&c), Yinnon (2017). Subsequently, it will not be mentioned again that the aspects are in agreement with experimental data. Instead, the few aspects which still have to be definitely confirmed by measurements will be pointed out.
- In solutions of weak- or non-electrolytes, for C above a transition concentration
 , only few solute molecules solvate. The solvated solutes move randomly (see Fig.1i). For
, only few solute molecules solvate. The solvated solutes move randomly (see Fig.1i). For  <C<
<C<  , part of the solvated weak electrolytes, or solvated non-electrolytes with a sufficiently large electric dipole moment, aggregate in a QED domain type denoted CDplasma (see Fig.1 ii). Within these domains, the solute molecules together with their solvation shell solvent molecules perform coherent plasma oscillations. The diameter of a CDplasma is of the order of 10-6 m. CDplasma may agglomerate into supra-CDplasma. A schematic picture of CDplasma is presented in Fig. 3a in Yinnon and Liu (2015a). QED theory predicts that the interactions between the solute molecules and Tera Herz (Hz) to mega Hz EM radiation underlie formation of CDplasma, but this has not yet been confirmed by experimental data. The transition concentrations
, part of the solvated weak electrolytes, or solvated non-electrolytes with a sufficiently large electric dipole moment, aggregate in a QED domain type denoted CDplasma (see Fig.1 ii). Within these domains, the solute molecules together with their solvation shell solvent molecules perform coherent plasma oscillations. The diameter of a CDplasma is of the order of 10-6 m. CDplasma may agglomerate into supra-CDplasma. A schematic picture of CDplasma is presented in Fig. 3a in Yinnon and Liu (2015a). QED theory predicts that the interactions between the solute molecules and Tera Herz (Hz) to mega Hz EM radiation underlie formation of CDplasma, but this has not yet been confirmed by experimental data. The transition concentrations  and
and  depend on solute type. Typically, ~10-4 M<
depend on solute type. Typically, ~10-4 M<  < 1M and ~10-6 M<
< 1M and ~10-6 M<  <~10-4 M.
<~10-4 M.
In solutions of strong electrolytes, at any C larger than
 , part of the solvated solutes together with numerous solvent molecules organize in CDplasma. Thus the difference between solutions of weak- or non-electrolytes versus those of strong electrolytes is that in the latter there exists no concentration above which CDplasma do not form, i.e., there is no
, part of the solvated solutes together with numerous solvent molecules organize in CDplasma. Thus the difference between solutions of weak- or non-electrolytes versus those of strong electrolytes is that in the latter there exists no concentration above which CDplasma do not form, i.e., there is no  .
.According to QED theory, CDplasma, supra-CDplasma and aggregates of unsolvated solutes may stabilize a QED domain type denoted CDelec. These domains may aggregate into supra-CDelec. A CDelec is composed of solvent molecules, which coherently oscillate between their electronic ground state and an excited electronic state. Interactions between the solvent molecules and UV radiation underlie formation of CDelec. Only for aqueous solutions, experimental evidence for presence of CDelec has been provided. Since we will mainly analyze aqueous SDVSPL, we provide a special denotation for CDelec composed of H2O, i.e.,
 . The diameter of a CDelec is about 10-7 m. The electron residing in the excited state of a H2O belonging to a
. The diameter of a CDelec is about 10-7 m. The electron residing in the excited state of a H2O belonging to a  is almost free (binding energy ≈0.4 eV). Hence, a
is almost free (binding energy ≈0.4 eV). Hence, a  is a pool of ~106 quasi-free electrons (QFE). cannot contain solutes. A schematic picture of
is a pool of ~106 quasi-free electrons (QFE). cannot contain solutes. A schematic picture of  is presented in Fig. 1 in Yinnon and Liu (2015a).
is presented in Fig. 1 in Yinnon and Liu (2015a).The abovementioned characteristics for C>
 hold independent of the solutions’ preparation procedure. In other words these do not just hold for SDVSPL, but also for solutions prepared without serial dilutions or vigorous shaking. Serial dilutions or vigorous shaking affect CDplasma and CDelec, mainly causing their breakup. However, after perturbations are over, these domains reform, as illustrated in Figs.1 iia&b.
hold independent of the solutions’ preparation procedure. In other words these do not just hold for SDVSPL, but also for solutions prepared without serial dilutions or vigorous shaking. Serial dilutions or vigorous shaking affect CDplasma and CDelec, mainly causing their breakup. However, after perturbations are over, these domains reform, as illustrated in Figs.1 iia&b. - In solutions of strong electrolytes, at
 , CDplasma transform into another type of QED domain, i.e., IPDplasma (see Figs.1ii-iii). IPDplasma, just as CDplasma, are composed of few solvated solutes and numerous solvent molecules. The solvated solutes in IPDplasma are crystalline ordered. The solvated solutes and solvent molecules constituting IPDplasma perform in phase plasma oscillations. A schematic picture of IPDplasma is presented in Fig. 3b in Yinnon and Liu (2015a). The diameter of an IDPplasma is about 10-6 m. IPDplasma may aggregate in supra-IPDplasma. Dilution below
, CDplasma transform into another type of QED domain, i.e., IPDplasma (see Figs.1ii-iii). IPDplasma, just as CDplasma, are composed of few solvated solutes and numerous solvent molecules. The solvated solutes in IPDplasma are crystalline ordered. The solvated solutes and solvent molecules constituting IPDplasma perform in phase plasma oscillations. A schematic picture of IPDplasma is presented in Fig. 3b in Yinnon and Liu (2015a). The diameter of an IDPplasma is about 10-6 m. IPDplasma may aggregate in supra-IPDplasma. Dilution below  diminishes the number of randomly moving solvated solutes as well as the number of solvated solutes incorporated in IPDplasma. At very low concentrations, the number of solutes is too low for formation of IPDplasma. As shown in Fig. 1iiib, IPDplasma may stabilize CDelec and supra-CDelec.
diminishes the number of randomly moving solvated solutes as well as the number of solvated solutes incorporated in IPDplasma. At very low concentrations, the number of solutes is too low for formation of IPDplasma. As shown in Fig. 1iiib, IPDplasma may stabilize CDelec and supra-CDelec.
QED theory predicts that the interactions between the solute molecules and Tera Herz to mega Herz EM radiation underlie formation of IPDplasma, but this has not yet been confirmed by experimental data. The aforesaid holds independent of the solutions’ preparation procedure, i.e., not just for SDVSPL, but also for solutions prepared without serial dilutions or vigorous shaking.
For solutions of weak- or non-electrolytes, for C ≤
 , QED theory also predicts that CDplasma transform into IPDplasma. The differences between the transition concentrations
, QED theory also predicts that CDplasma transform into IPDplasma. The differences between the transition concentrations  and
and  may be tiny. However, definite experimental evidence for these aspects of such solutions has yet to be provided, as discussed by Yinnon and Liu (2015c).
may be tiny. However, definite experimental evidence for these aspects of such solutions has yet to be provided, as discussed by Yinnon and Liu (2015c). - For solutions containing IPDplasma, their agitation by vigorous shaking affects their properties. Vigorous shaking transforms IPDplasma into aggregates with an electric dipole moment (see Fig.1iv). These aggregates are denoted electric dipole aggregates EDAIPDplasma. The sizes of EDAIPDplasma may reach 10-6 m, but may also be much smaller.
The formation of EDAIPDplasma results from agitations exciting or breaking up IPDplasma. A few eV are required for desorption of a molecule from an IPDplasma. Since vigorous shaking provides about 1010 – 1015 eV per (domain) (Yinnon and Liu, 2015b), vigorously shaking of SDVSAS can break up IPDplasma.
The excitations or break up of IPDplasma partly destroy the spherically symmetric alignments of the dipole moments of the numerous H2O in the large solvation shells surrounding its crystalline ordered solvated solutes. The partial destruction of the spherically symmetric alignments endows the disturbed IPDplasma with an electric dipole moment.
Vigorous shaking of SDVSAS containing CDplasma, i.e., at C>
 , does not create electric dipole aggregates. Solutes are randomly distributed in a CDplasma, i.e., are not crystalline ordered. Moreover, only few H2O constitute its solvation shells, i.e., only few H2O are spherically symmetric aligned. Therefore, excitation or break up of CDplasma does not create aggregates with a sizable electric dipole moment.
, does not create electric dipole aggregates. Solutes are randomly distributed in a CDplasma, i.e., are not crystalline ordered. Moreover, only few H2O constitute its solvation shells, i.e., only few H2O are spherically symmetric aligned. Therefore, excitation or break up of CDplasma does not create aggregates with a sizable electric dipole moment. - For aqueous SDVSPL, EDAIPDplasma may stabilize
 and supra-
and supra- (see Fig.1 iv). For non-aqueous SDVSPL, the possibility of EDAIPDplasma stabilizing and affecting CDelec has not yet been investigated.
(see Fig.1 iv). For non-aqueous SDVSPL, the possibility of EDAIPDplasma stabilizing and affecting CDelec has not yet been investigated.
- For C less than the critical concentration
 , yet another type of QED domain may stabilize, i.e., CDrot. This domain is composed of ferroelectrically ordered solvent molecules. A CDrot has an electric dipole moment due to the ferroelectric ordering of its polar molecules. The molecules constituting CDrot coherently oscillate between two of their rotational states. Interactions between the solvent molecules’ electric dipole moments and infra-red (IR) EM radiation underlie formation of CDrot. For a schematic picture of CDrot see Fig. 2 in Yinnon and Liu (2015a).
, yet another type of QED domain may stabilize, i.e., CDrot. This domain is composed of ferroelectrically ordered solvent molecules. A CDrot has an electric dipole moment due to the ferroelectric ordering of its polar molecules. The molecules constituting CDrot coherently oscillate between two of their rotational states. Interactions between the solvent molecules’ electric dipole moments and infra-red (IR) EM radiation underlie formation of CDrot. For a schematic picture of CDrot see Fig. 2 in Yinnon and Liu (2015a).
In bulk water and most other polar liquids at ambient conditions, CDrot do not stabilize. In these liquids, the energy gained by a molecule joining a CDrot is of the same order as the energy of the thermal fluctuations. Therefore, thermal aggression prevents stabilization of CDrot in most bulk liquids at ambient conditions. However, objects with sizable asymmetric charge distributions (e.g., macromolecules, hydrophilic membranes or EDAIPDplasma) may stabilize CDrot. Their stabilization causes a permanent time dependent polarization of the liquid. The sizes of CDrot may increase with the passage of time. At ambient conditions, their stabilization, e.g., by hydrophilic surfaces, may require many hours or days. Their sizes may reach 10-4 m.c
Solutes are pulled into CDrot. Few solute particles can locate in CDrot and do not wreck their host. Many solute molecules destroy CDrot. The critical concentration below which CDrot persist (
 ) depends on the solute and solvent. Typically 10-6 M <
) depends on the solute and solvent. Typically 10-6 M < < 10-10 M. Analyses of experimental data of many solutions have shown that
< 10-10 M. Analyses of experimental data of many solutions have shown that  equals Cthr.
equals Cthr.For C<
 , due to the interactions between the electric dipoles of EDAIPDplasma and the electric dipoles of the solvent molecules, EDAIPDplasma stabilize CDrot (see Fig.1vb). In other words, EDAIPDplasma, due to their significant asymmetric charge distributions, stabilize CDrot. Stabilization of CDrot also may be induced by solutes with sufficiently large permanent or induced electric dipoles. Thus even when no IPDplasma and hence EDAplasma form in SDVSPL, solvated solutes with a sufficiently large asymmetric charge distribution still may induce CDrot stabilization for C<
, due to the interactions between the electric dipoles of EDAIPDplasma and the electric dipoles of the solvent molecules, EDAIPDplasma stabilize CDrot (see Fig.1vb). In other words, EDAIPDplasma, due to their significant asymmetric charge distributions, stabilize CDrot. Stabilization of CDrot also may be induced by solutes with sufficiently large permanent or induced electric dipoles. Thus even when no IPDplasma and hence EDAplasma form in SDVSPL, solvated solutes with a sufficiently large asymmetric charge distribution still may induce CDrot stabilization for C<  .
.Vigorous shaking excites or breaks up CDrot (see Fig.1v). Due to the ferroelectric ordering of the molecules constituting CDrot, excited or broken CDrot are also electric dipole aggregates, which we denote as EDACDrot (see Fig.1vi). Due to interactions between the dipoles of EDACDrot and the polar solvent molecules, EDACDrot also stabilize CDrot. Therefore, serial dilutions with vigorous shaking at each dilution step diminish solvated solutes and EDAIPDplasma, but EDACDrot persist. These EDACDrot stabilize CDrot and supra-CDrot too. As a result CDrot persist up to ultra low concentrations and beyond (see Figs.1vib-viiib).
CDrot and supra-CDrot may stabilize
 and supra-
and supra- . Such agglomerates we denote [supra–CDrot <supra-
. Such agglomerates we denote [supra–CDrot <supra- >]. For non-aqueous SDVSPL, the possibility of EDACDrot stabilizing and affecting CDelec has not yet been investigated.
>]. For non-aqueous SDVSPL, the possibility of EDACDrot stabilizing and affecting CDelec has not yet been investigated.CDrot may agglomerate into supra-CDrot with their dipole moments more or less parallel or anti-parallel oriented. The vigorous shaking applied to SDVSPL after each dilution step restructures the orientations of the dipole moments of its supra-CDrot. The restructuring strongly affects the properties of SDVSPL. For instance, it underlies the non-monotonic dependence of its physicochemical variables and the parameters characterizing its associates on C, e.g., the stabilization of
 .
.According to the QED model of SDVSPL, when these liquids are slightly or extremely diluted below
 , the entities underlying their extraordinary physicochemical properties are CDrot, EDACDrot and
, the entities underlying their extraordinary physicochemical properties are CDrot, EDACDrot and  . In addition, it has been hypothesized that for C<
. In addition, it has been hypothesized that for C< =Cthr, CDelec underlie the bioactivity of SDVSPL. Therefore, on screening SDVSPL from the ambient EM radiation that mediates formation of their CDrot and CDelec, these domains disintegrate and the SDVSPL lose their typical characteristics. Placing SDVSPL in Permalloy containers screens the EM radiation mediating formation of their CDrot and CDelec.
=Cthr, CDelec underlie the bioactivity of SDVSPL. Therefore, on screening SDVSPL from the ambient EM radiation that mediates formation of their CDrot and CDelec, these domains disintegrate and the SDVSPL lose their typical characteristics. Placing SDVSPL in Permalloy containers screens the EM radiation mediating formation of their CDrot and CDelec.
In summary, the basic tenets of the QED SDVSPL model are: For solutions of polar liquids, there exist solute type dependent transition concentrations at which specific QED domains may become stabilized; solutes’ characteristics determine the stabilization of the domains; once domains of type CDrot and CDelec become stabilized, serial dilutions in combination with vigorous shaking cause the perpetuation of these domains in the resulting dilutes. In other words, after at C<![]() the domain of type CDrot forms, the solutes are not any longer needed for stabilizing additional CDrot and CDelec in the dilutes.
the domain of type CDrot forms, the solutes are not any longer needed for stabilizing additional CDrot and CDelec in the dilutes.
The QED model of SDVSPL is congruent with recently empirically-based models. The main aspects of the model of Konovalov and Ryzhkina (2014, 2016), which they based on their extensive experimental data, are as follows: During the SDVSPL preparation procedure, for many but not all kinds of solutes, for C less than a solute type dependent Cthr, nano-sized self-organized substrate-induced molecular ensembles (associates) stabilize. The term “substrate” refers to the solute in the stock (mother) solution from which the SDVSPL is prepared. The characteristics of the associates are such that these constitute a phase which is different from the medium, i.e., SDVSPL are nano-heterogeneous. Consequently, these SDVSPL cannot strictly be regarded as solutions in the customary sense. Instead, these are nano-disperse systems. Nano-sized associates composed of polar liquid molecules (the original solvent molecules) constitute the disperse phase. For SDVSPL with C above the Avogadro limit, the experimental data is yet insufficient for determining the distribution of the solute molecules located in the associates versus those located in the dispersion medium (the polar liquid). While SDVSPL are highly diluted, the substrate’s content decreases. Yet, nano-sized associates, solely composed of the polar liquid’s (original solvent) molecules, persist. Thus, on serial diluting a polar liquid’s solution, in combination with vigorous shaking the liquid after each dilution step, a special kind of disperse system emerges — a disperse system of polar liquid associates immerged in a polar liquid (the medium). The electronic structure and the ordering of the polar liquid molecules in the associates are determined by the characteristics of the substrate molecules. Therefore, the associates contain the molecular information of the substrate. However, the specific relation between the characteristics of the substrate and the electronic state and molecular ordering of the associates has not yet been discovered.
The model proposed by Elia et al. (2014, 2015), which is based on their extensive experimental data, also emphasizes the aggregates (molecular ensembles) composed of the polar liquid (solvent) molecules in SDVSPL, described in the previous paragraph. In addition, their model addresses the solid phase which is obtainable by isolating the aggregates. The aggregates can be “extracted” by evaporating SDVSPL in air at ambient temperatures, by evaporation at high temperatures (90o C) or by lyophilizing. The solid, i.e., the isolated aggregates, is soluble in the polar liquid. After dissolving the solid in the pure polar liquid, the liquid’s physicochemical parameters are almost exactly like those of the original SDVSPL from which the solid was extracted. As such the model emphasizes that the aggregates are a phase which differs from the known liquid and ice phases of polar
liquids.
c I thank the anonymous reviewer for bringing to my attention the paper by Elton and Fernandez-Serra (Nature Communications 2016). Its analyses reveal a property of water conforming to an aspect of the dynamics of H2O constituting CDrot . Its analyses of Raman and infrared spectroscopic data indicate the presence of coherent long-range dipole–dipole interactions in water. As to additional experimental evidence for properties of CDrot, analyses of dielectric permittivity measurements of SDVSPL have shed light on their ferroelectric properties (Yinnon and Liu, 2016). Moreover, analyses of thermogravimetric measurements of water perturbed by a Nafion membrane have provided information on the transition concentration below which these domains can become stabilized adjacent to the membrane (Yinnon et al., 2016).
Analyses of Published Experimental Data on UV Radiation’s Absorbance, Fluorescence and Transmission
Aqueous SDVSPL, prepared in glass containers, absorb radiation in the 190 – 325 nm range (Lo 1996; Lobyshev et al., 2005; Wolf et al., 2011; Klein et al., 2013; Ghosh et al. 2015; Elia et al., 2014; Chakraborty et al., 2015). The UV radiation’s absorbance, transmission or fluorescence by aqueous SDVSPL prepared in plastic containers, to the best of our knowledge, has not been reported.
The absorbance spectra of aqueous SDVSPL mainly are typified by three broad absorbance bands (Lo 1996; Lobyshev et al., 2005; Ghosh et al. 2015; Elia et al., 2014; Chakraborty et al., 2015). The wavelengths of the bands’ maxima are in the range of 205 – 210 nm, 260 – 280 nm and 300 – 310 nm. The wavelengths of the maxima slightly non-monotonically depend on the number of dilution steps. The half-widths of the bands are about 20 – 30 nm.
UV-vis radiation fluorescence features of SDVSPL have been reported for aqueous SDVSPL of NaCl and for SDVSPL-of-Distilled-Water (Lobyshev et al., 2005).d These SDVSPL were irradiated with 300 nm radiation. The fluorescence spectra consist of a broad band with maximum at about 385 nm. The half-width of the band is about 100 nm. For SDVSPL-of-Distilled-Water, the fluorescence intensity non-monotonically changes with the number of dilution step (NDS). For aqueous SDVSPL of NaCl, at concentrations below C≈10-7 M≈Cthr,e the fluorescence intensity also non-monotonically changes with NDS. For both these SDVSPL, the intensity non-monotonically varies with the storage time of the samples. The samples were stored in glass bottles closed with lids. The samples were stored in the dark at room temperature.
The transmission of UV radiation by aqueous SDVSPL of NaCl, aqueous SDVSPL of nitric acid (HNO3) and aqueous SDVSPL of sodium hydroxide (NaOH) has been measured by Lo (1996). These SDVSPL were decimally diluted and had concentrations in the range of 10-3 M to 10-13 M. On decreasing the concentration of these SDVSPL from 10-3 M to10-5 M, the transmission increased. However, for concentrations below about 10-5 – 10-7 M, the transmission non-monotonically changed with NDS.
Highly controlled, blinded and randomized experiments of UV radiation transmission by SDVSPL were carried out by Wolf et al. (2011) and Klein et al. (2013). They measured the transmission of UV radiation by aqueous SDVSPL of copper sulfate (CuSO4), aqueous SDVSPL of hypericum and aqueous SDVSPL of sublimed sulfur (S8). These SDVSPL were 6 to 30 times centesimally or decimally diluted. Statistical analyses of the data show that the UV radiation’s transmission of these aqueous SDVSPL significantly differ from that of the controls. The controls were vigorously shaken solvents. These solvents were of the various types of purified waters or the 99% water and 1% ethanol mixture with which the SDVSPL were prepared. The purified waters included distilled water, Quartz distilled water and de-ionized water. Some of the experiments were carried out in a metal-free class 100 High Efficiency Particulate Air (HEPA) filtered clean room. Clean flow boxes had class 5. These experiments showed that the UV radiation’s transmission by aqueous SDVSPL of CuSO4 or aqueous SDVSPL of hypericum is lower than that of the control. However, for aqueous SDVSPL of S8, it is higher than the control.
Transmission of UV radiation measurements also showed that the properties of the residues of evaporated SDVSPL depend on the NDS and the solute used in their preparation (Klein and Wolf, 2016). SDVSPL were poured on sugar globules. The solvent in these SDVSPL was ethanol.
The globules were left to dry in air. Subsequently, the globules together with their residue of the evaporated SDVSPL were dissolved in water. The resultant liquid’s UV radiation’s transmission was measured. For SDVSPL of Aconitum napellus, Atropa belladonna, phosphorus, sulfur, Apis mellifica, quartz, which were 30 and 200 times centesimal diluted, the transmissions statistically significant differed.
d SDVSPL-of-Distilled-Water is prepared by serially diluting distilled water and vigorously shaken the liquid after each dilution step.
e Cthr of aqueous SDVSPL of NaCl have been measured by Ryzhkina et al. (2012c).
Analyses of UV Radiation Absorbance
The UV radiation’s absorbance by all aqueous SDVSPL, including those diluted much below the Avogadro limit, significantly differs from that of pure non-serially diluted non-vigorously shaken water in its various phases, e.g., bulk water, gas phase water, amorphous-, hexagonal- or cubic-ice. A UV absorbance band with a maximum around 270 nm, which exists in the UV spectra of SDVSPL, was also observed in water by Larzul et al. in 1965. However, subsequent extensive research has shown that pure water does not absorb in this range (Quickenden and Irvin, 1980; Mulliken and Ermler, 1981; Segarra-Martí, 2013). Hence, the absorbance band around 270 nm measured by Larzul et al. (1965) had been attributed to impurities. For gas phase H2O, its lowest-energy band of the electronic spectrum covers the 151 – 182 nm range with a maximum at 168 nm. In pure bulk liquid water, this band is broader and blue-shifted. Its maximum is at 151 nm and its absorbance falls off monotonically by about ten orders of magnitude in the 151 – 400 nm range (it is only about 0.0001 cm-1 at 320 nm). Amorphous, hexagonal or cubic ice has absorbance features similar to those detailed in the previous sentence (Quickenden and Irvin, 1980; Cabral do Couto et al., 2012; Segarra-Martí et al., 2013).
The UV radiation’s absorbance features of aqueous SDVSPL resemble those of structured waters. For example, a UV absorbance band in the 225 – 325 nm range, with a maximum around 260 – 280 nm, is typical for structured waters (Segarra-Martí et al., 2013). Such a band has been observed for example for the following waters:
- the ordered water adjacent to hydrophilic membranes like Nafion [so called exclusion zone (EZ) water] (Zeng et al., 2006);
- water perturbed with a Nafion membrane (Elia et al., 2013b, 2014b);
- water perturbed with cellulose (Elia et al., 2018);
- water-ethanol mixtures (Liu et al., 2007);
- iterative filtered water (Elia et al., 2014c);
- aqueous solutions of non-luminescence compounds (e.g. aqueous glycylasparagine at 10-9 M<C<10-3 M, aqueous alkali chlorides at ~1 M< C< ~ 5 M, aqueous L-lysine monohydrochloride at 0.1 M< C<1 M, D-alanine, or aqueous D-glucose at 0.1 M< C<1 M) (Lobyshev et al., 1999; Chai et al., 2008).
As to the absorbance band with maxima in the range of 205 – 210 nm observed for aqueous SDVSPL, such a band has also been measured for the following structured waters: for EZ water (Zheng et al., 2006), for water perturbed with a Nafion membrane (Elia et al., 2013a) and for water perturbed with cellulose (Elia et al., 2018). A similar band has been observed for magnetized water (Pang, 2014).
The resemblances between the UV radiation’s absorbance features of aqueous SDVSPL and those of the above listed structured waters suggest that some information on the structuring of H2O in the former can be obtained from analyses of the latter. From the above list of structured waters, that of water perturbed with a Nafion membrane has recently been extensively analyzed with many state-of-the-art techniques (Elia et al., 2013a, 2013b, 2014c, 2015, 2017; Capolupo et al., 2014; Yinnon et al., 2016). The analyses show that its UV absorbance and fluorescence is due to its H2O aggregates. It contains 10-4 mol / liter of micron-sized H2O aggregates. Its chemical analyses showed that it contains 10-6 mol / liter non-H2O compounds, i.e., fluorine (F–) and sulfate (HSO4–) ions released by the Nafion membrane. The very low concentration of these non-H2O compounds implies that these cannot underlie formation of the H2O aggregates or the significant UV absorbance. Instead, the various analyses of the data obtained for water perturbed by a Nafion membrane indicate that its aggregates are composed of ![]() and CDrot (Capolupo et al., 2014; Elia et al, 2015, 2017; Yinnon et al., 2016). The analyses also indicate that this water’s UV radiation’s absorbance features can be attributed to
and CDrot (Capolupo et al., 2014; Elia et al, 2015, 2017; Yinnon et al., 2016). The analyses also indicate that this water’s UV radiation’s absorbance features can be attributed to ![]() . Also the more restricted analyses of the H2O aggregates constituting EZ water, the molecular associates in aqueous solutions and the aggregates in water perturbed with cellulose indicate that these include
. Also the more restricted analyses of the H2O aggregates constituting EZ water, the molecular associates in aqueous solutions and the aggregates in water perturbed with cellulose indicate that these include ![]() (Del Giudice et al., 2013; Yinnon and Yinnon, 2009; Yinnon et al., 2016; Elia et al., 2018). Accordingly, because of the resemblance of the UV radiation’s absorbance features of aqueous SDVSPL and water perturbed with a Nafion membrane or cellulose, EZ water and aqueous solutions, also for aqueous SDVSPL these features are attributable to
(Del Giudice et al., 2013; Yinnon and Yinnon, 2009; Yinnon et al., 2016; Elia et al., 2018). Accordingly, because of the resemblance of the UV radiation’s absorbance features of aqueous SDVSPL and water perturbed with a Nafion membrane or cellulose, EZ water and aqueous solutions, also for aqueous SDVSPL these features are attributable to ![]() . Indeed the QED of SDVSPL model and the analyses of many other measured physicochemical properties of aqueous SDVSPL indicate that these liquids contain
. Indeed the QED of SDVSPL model and the analyses of many other measured physicochemical properties of aqueous SDVSPL indicate that these liquids contain ![]() (see paragraphs I, II, IV and V, and Yinnon and Liu, 2015b&c). The analyses of the UV-vis radiation’s fluorescence of aqueous SDVSPL, presented in the next sub-section, provide additional evidence that
(see paragraphs I, II, IV and V, and Yinnon and Liu, 2015b&c). The analyses of the UV-vis radiation’s fluorescence of aqueous SDVSPL, presented in the next sub-section, provide additional evidence that ![]() underlie the UV radiation’s absorbance features.
underlie the UV radiation’s absorbance features.
Analyses of UV-vis Fluorescence
The above-cited fluorescence features of aqueous SDVSPL resemble those of the structured waters listed in the previous sub-section, i.e., when these structured waters were excited with radiation with wavelengths in the 225 – 325 nm range. The fluorescence spectra of all these waters consist of a rather featureless broad band (Lobyshev et al., 1999, 2005; Liu et al., 2007; Chai et al., 2008; Elia et al., 2017). The wavelengths of the band and of its maximum depend on the method with which the water was perturbed. It also depends on the type of solutes. Moreover, for aqueous solutions of glycylasparagine, Lobyshev et al. (1999) showed that that just as for aqueous SDVSPL, their fluorescence intensity may increase during their storage in a dark place.
The absence of any sharp peaks in the fluorescence spectra of the above listed structured waters indicates that excimers are presentf (Liu et al., 2007, 2012; Segarra-Martí et al., 2013, 2014). In particular, the excimers underlying the structure of EZ water have been in depth analyzed. The analyses have been carried out with high-level computations, i.e., with the well established quantum mechanical ab initio derived Complete-Active-Space Self-Consistent-Field second-order perturbation theory (CASPT2) (Segarra-Martí et al., 2013, 2014). The analyses indicate the following: EZ waters contain aggregates which are composed of excimers forming networks of multilayer honeycomb ice-like layers (see Fig. 1 and 6 in Segarra-Martí et al., 2014). The formula of the excimers is H38O20. The formula of the two monomers constituting the excimer is H19O10. The H2O forming the monomer are organized in two fused hexagons. The electronic properties of the H38O20 excimer is a function of its inter-monomer distance, i.e., its structural relaxation.
The quantum mechanics excimer-based model of EZ water has important features in common with the quantum dynamic QED model of EZ water developed by Del Giudice et al. (2013). Elia et al. (2017) first pointed out the similarities between these models. In the H38O20 excimer, each of the two central (fused) H2O of one monomer attracts its opposite central (fused) H2O of the other monomer (See Fig. 6 in Segarra-Martí et al., 2014). The attractive interaction is due to π-stacking. In the H38O20 networks, the π-stacked H2O resonate between their ground and an excited electronic state, i.e., about 10 percent of the H2O constituting the network are simultaneously electronically excited. According to QED, in a ![]() , the H2O also resonate between their ground and an excited electronic state. About 10 percent of the H2O constituting the
, the H2O also resonate between their ground and an excited electronic state. About 10 percent of the H2O constituting the ![]() are simultaneously electronically excited. One of the differences between the excimer and QED models is that the former predicts that the π-stacked H2O resonate between two of their electronic states, while the latter predicts that all of the about 106 H2O composing a
are simultaneously electronically excited. One of the differences between the excimer and QED models is that the former predicts that the π-stacked H2O resonate between two of their electronic states, while the latter predicts that all of the about 106 H2O composing a ![]() resonate between two of their electronic states. For many body condensed systems, quantum electro-dynamics is a more accurate theory than quantum mechanics (Preparata, 1995).
resonate between two of their electronic states. For many body condensed systems, quantum electro-dynamics is a more accurate theory than quantum mechanics (Preparata, 1995).
Analyses of experimental data have indicated that aggregates with properties of ![]() or of excimers are present in the following structured waters: in non-vigorously shaken aqueous solutions of alkali halides or glucose with C in the 1 – 10-3 M range, water-alcohol mixtures, aqueous deoxyribonucleic acid (DNA) with C in the range 2.5 – 0.25 g dL-1 (Liu et al., 2007; Yinnon and Yinnon, 2009; Segarra-Martí et al., 2013); in EZ water (Del Giudice et al., 2013; Segarra-Martí et al., 2013, 2014; Yinnon et al., 2016); and in water perturbed with a Nafion membrane or cellulose (Elia et al., 2015, 2017; Yinnon et al., 2016). Hence, the UV-vis fluorescence of structured waters is attributable to associates of water molecules, wherein part of the H2O transit between their ground and an excited electronic state, i.e., aggregates composed of excimers or their quantum dynamics analogous QED
or of excimers are present in the following structured waters: in non-vigorously shaken aqueous solutions of alkali halides or glucose with C in the 1 – 10-3 M range, water-alcohol mixtures, aqueous deoxyribonucleic acid (DNA) with C in the range 2.5 – 0.25 g dL-1 (Liu et al., 2007; Yinnon and Yinnon, 2009; Segarra-Martí et al., 2013); in EZ water (Del Giudice et al., 2013; Segarra-Martí et al., 2013, 2014; Yinnon et al., 2016); and in water perturbed with a Nafion membrane or cellulose (Elia et al., 2015, 2017; Yinnon et al., 2016). Hence, the UV-vis fluorescence of structured waters is attributable to associates of water molecules, wherein part of the H2O transit between their ground and an excited electronic state, i.e., aggregates composed of excimers or their quantum dynamics analogous QED ![]() .
.
The resemblance between the UV-vis fluorescence features of aqueous SDVSPL and those of the above cited structured waters evokes that the fluorescence of aqueous SDVSPL also is due to ![]() . Or alternatively, these features may be due to the quantum mechanic aggregates composed of excimers. To corroborate this evocation, in the next two sub-sections are represented, respectively, for aqueous SDVSPL of NaCl and SDVSPL-of-Distilled-Water, the fine-details of their fluorescence after their irradiation with 300 nm radiation. Subsequently, the details are analyzed within the context of QED.
. Or alternatively, these features may be due to the quantum mechanic aggregates composed of excimers. To corroborate this evocation, in the next two sub-sections are represented, respectively, for aqueous SDVSPL of NaCl and SDVSPL-of-Distilled-Water, the fine-details of their fluorescence after their irradiation with 300 nm radiation. Subsequently, the details are analyzed within the context of QED.
f The characteristics of excimers, the mechanisms underlying their formation and their UV-vis emission features are summarized in the Appendix of the paper by Elia et al. (2017).
Details of the UV-vis Fluorescence Features of SDVSPL of NaCl and Their Explanation
The details, which are summarized in Fig. 1 and in the table in Lobyshev et al. (2005) are:
- The fluorescence intensity of samples with C=2 M, one day after their preparation, is about seven times larger than that of distilled water. For 2×10-3 M<C <2 M, the fluorescence intensity monotonically drops by about a factor of five. For C<2×10-3 M, the fluorescence intensity non-monotonically changes with C. For C in the range of 2×10-4 M – 2×10-24 M, the values of the intensity vary between that of distilled water and values twice or even four times larger than that of distilled water. Pronounced maxima in the intensity are observed for C = 2×10-1 M, C = 2×10-8 M, C=2×10-12 M, C=2×10-19 M and for a 28 times decimal diluted solution. The strongest maxima are the ones at C = 2×10-1 M and C=2×10-12 M. Both maxima are about 250 percent higher than the other maxima.
- The fluorescence intensity non-monotonically varies with the storage time of the samples. During the six weeks after their preparation, the intensity may decrease or increase by as much as 50 – 80 percent.
- The fluorescence intensity is negatively correlated with the motility of the infusorium Spirostoma ambiguum in the SDVSPL.
Explanations for the above presented UV-vis fluorescence features of aqueous SDVSPL of NaCl are feasible within the context of the results of the extensive studies of aqueous NaCl and of aqueous SDVSPL of NaCl which were carried out during the last two decades. In the following, I cite the relevant data and with the QED model of SDVSPL I explain the UV-vis fluorescence features of aqueous SDVSPL of NaCl obtained by Lobyshev et al. (2005).
◉ For 0.68 M< C< 5.32 M, in non-vigorously shaken aqueous NaCl, dynamic light scattering (DLS) revealed the presence of domains with diameters in the 102 – 103 nm range and small clusters with sizes of 0.1 – 0.4 nm (Georgalis et al., 2000; Samal and Geckeler, 2001; Sedlak 2006). The clusters are hydrated ions. The domains are not nano-bubbles (Sedlak and Rak, 2013). In aqueous SDVSPL of NaCl, for 0.15 M<C<1.8 M, DLS also revealed hydrated ions and 102 – 103 nm sized domains (Ryzhkina et al., 2012c). For C<0.15 M, DLS cannot clearly distinguish domains, except for ~220 nm sized domains at C=2×10-10 M (Ryzhkina et al., 2012c). Yet, also for C<0.15 M, electrical conductivity and UV absorbance measurements have indicated that molecular associates are present in these solutions (Lo, 1996, 2013; Lo et al., 2009; Elia and Niccoli, 2004a; Ryzhkina et al., 2012c).
Analyses of the characteristics of the domains and their impact on the physicochemical properties of the solutions, for C>0.15 M, showed that the domains include the 103 nm sized CDplasma and supra-CDplasma, which stabilized the 102 nm sized ![]() and supra-
and supra-![]() (Yinnon and Yinnon, 2009, 2012; Yinnon and Liu, 2015b). As such the analyses confirmed the characteristic of the QED model of SDVSPL detailed in paragraph I.
(Yinnon and Yinnon, 2009, 2012; Yinnon and Liu, 2015b). As such the analyses confirmed the characteristic of the QED model of SDVSPL detailed in paragraph I.
The presence of ![]() with their pool of quasi free electrons (QFE) in aqueous NaCl solutions at the aforementioned concentrations (or their analogous quantum mechanics agglomerates composed of excimers), explain the strong UV absorbance and fluorescence of these solutions observed by Lobyshev et al. (2005). In other words, just as for EZ water or water perturbed with a Nafion membrane or with cellulose, for which similar UV phenomena were observed and shown to be attributable to
with their pool of quasi free electrons (QFE) in aqueous NaCl solutions at the aforementioned concentrations (or their analogous quantum mechanics agglomerates composed of excimers), explain the strong UV absorbance and fluorescence of these solutions observed by Lobyshev et al. (2005). In other words, just as for EZ water or water perturbed with a Nafion membrane or with cellulose, for which similar UV phenomena were observed and shown to be attributable to ![]() or excimers (Chai et al., 2008; Elia et al., 2013a, 2017, 2018; Yinnon et al., 2016), also the UV phenomena of aqueous NaCl are attributable to
or excimers (Chai et al., 2008; Elia et al., 2013a, 2017, 2018; Yinnon et al., 2016), also the UV phenomena of aqueous NaCl are attributable to ![]() . As to the decrease in the fluorescence observed on dilution from C=2 M to C=2×10-3 M, it is attributable to the diminishment of the number of solutes affecting CDplasma and thus reducing the stabilization of
. As to the decrease in the fluorescence observed on dilution from C=2 M to C=2×10-3 M, it is attributable to the diminishment of the number of solutes affecting CDplasma and thus reducing the stabilization of ![]() .
.
◉ On reducing C form ~10-3 M to ~10-6 M, for aqueous SDVSPL of NaCl, Lo (1996) observed a fall off in the UV absorbance in the190 – 250 nm range, while Ryzhkina et al. (2012c) observed a fall off in χ. The fall offs were shown to be ascribable to the reduction in the number of IPDplasma and EDAIPDplasma, which caused a diminishment in the number of stabilized ![]() (Yinnon and Liu, 2015b). The diminishment explains the fall offs in the UV fluorescence intensity observed by Lobyshev et al. (2005).
(Yinnon and Liu, 2015b). The diminishment explains the fall offs in the UV fluorescence intensity observed by Lobyshev et al. (2005).
◉ At C< ~10-6 M, for aqueous SDVSPL of NaCl, their observed physicochemical variables (e.g., dielectric constant, UV absorbance in the190 -250 nm range, χ, heat of mixing and pH), all non-monotonically vary with the number of dilution steps (Lo, 1996; Lo et al., 1996; Elia and Niccoli, 2004a; Ryzhkina et al., 2012c). QED analyses have shown that the non-monotonic variation is attributable to the realignments of CDrot. For C below the critical concentration ![]() , CDrot become stabilized by EDAIPDplasma (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013; Yinnon and Liu, 2015b&c). For aqueous SDVSPL of NaCl,
, CDrot become stabilized by EDAIPDplasma (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013; Yinnon and Liu, 2015b&c). For aqueous SDVSPL of NaCl, ![]() is about 10-7 M (Yinnon and Liu, 2015b). In addition, the QED analyses have indicated that the vigorous shaking of the SDVSPL after each dilutions step breaks up the CDrot. Due to the ferroelectric ordering of the H2O constituting the CDrot, these domains’ broken pieces also have a net electric dipole moment, i.e., these are the electric dipole aggregates EDACDrot. These aggregates stabilize new CDrot. The dilution, shaking and resulting break up of CDrot cause realignments of these domains’ electric dipole moments, i.e., affect the polarization of the liquid.
is about 10-7 M (Yinnon and Liu, 2015b). In addition, the QED analyses have indicated that the vigorous shaking of the SDVSPL after each dilutions step breaks up the CDrot. Due to the ferroelectric ordering of the H2O constituting the CDrot, these domains’ broken pieces also have a net electric dipole moment, i.e., these are the electric dipole aggregates EDACDrot. These aggregates stabilize new CDrot. The dilution, shaking and resulting break up of CDrot cause realignments of these domains’ electric dipole moments, i.e., affect the polarization of the liquid.
QED analyses of experimental data on aqueous SDVSPL have indicated that CDrot may stabilize ![]() (Yinnon and Liu, 2015c). The stabilization is influenced by the polarization of the liquid. Since after each dilution and vigorous shaking step, the polarization changes, the prevalence of
(Yinnon and Liu, 2015c). The stabilization is influenced by the polarization of the liquid. Since after each dilution and vigorous shaking step, the polarization changes, the prevalence of ![]() non-monotonically varies with the number of dilutions steps.
non-monotonically varies with the number of dilutions steps.
Based on the findings of the QED analyses pointed out above, it is possible to account for the non-monotonic dependence on the number of dilution steps of the fluorescence of aqueous SDVSPL of NaCl for C< ~10-6 M, observed by Lobyshev et al. (2005). The variation is attributable to the non-monotonic dependence on the number of dilution steps of the prevalence of ![]() stabilized by CDrot in this concentration range.
stabilized by CDrot in this concentration range.
◉ A high maximum in the fluorescence intensity of aqueous SDVSPL of NaCl for C< ![]() , i.e., the one at C=2×10-12 M, is a feature which also has been observed for other physicochemical variables of SDVSPL (Miranda et al., 2011; Ryzhkina et al., 2012c; Nain et al., 2015). However, the number of dilutions steps at which the maximum appeared is not the same for SDVSPL investigated by independent research groups. It should be stressed that even just the distilled water with which the SDVSPL are produced affects the maximum — distilled waters produced in different companies affect the maximum in different ways. The hysteretic and far-our-of-equilibrium properties of SDVSPL explain the variance of the concentration at which the maximum appears. These properties imply that each experimental set up greatly affects the alignment of CDrot and hence the prevalence of
, i.e., the one at C=2×10-12 M, is a feature which also has been observed for other physicochemical variables of SDVSPL (Miranda et al., 2011; Ryzhkina et al., 2012c; Nain et al., 2015). However, the number of dilutions steps at which the maximum appeared is not the same for SDVSPL investigated by independent research groups. It should be stressed that even just the distilled water with which the SDVSPL are produced affects the maximum — distilled waters produced in different companies affect the maximum in different ways. The hysteretic and far-our-of-equilibrium properties of SDVSPL explain the variance of the concentration at which the maximum appears. These properties imply that each experimental set up greatly affects the alignment of CDrot and hence the prevalence of ![]() .
.
It should be emphasized that the prevalence of ![]() is not solely affected by the alignment of CDrot. In the presence of ions, external alternating magnetic fields may enhance the stabilization of
is not solely affected by the alignment of CDrot. In the presence of ions, external alternating magnetic fields may enhance the stabilization of ![]() (Del Giudice et al., 2002; Yinnon, 2017). For example, the ambient Schumann resonances may enhance the stabilization. The effect of the alternating magnetic field depends on the concentration and other characteristics of the ions (Montagnier et al., 2011, 2015). Hence, maxima in the prevalence of
(Del Giudice et al., 2002; Yinnon, 2017). For example, the ambient Schumann resonances may enhance the stabilization. The effect of the alternating magnetic field depends on the concentration and other characteristics of the ions (Montagnier et al., 2011, 2015). Hence, maxima in the prevalence of ![]() should depend also on the concentration of the SDVSPL and the characteristics of its original solute.
should depend also on the concentration of the SDVSPL and the characteristics of its original solute.
◉ The storage time dependence of the non-monotonic variation of the fluorescence intensity of aqueous SDVSPL of NaCl, observed by Lobyshev et al. (2005), is a phenomenon these liquids have in common with other measured physicochemical variables of numerous types of SDVSPL and other structured waters (Elia et al., 2004a, 2005, 2008a&b; Belon et al., 2008, Elia et al., 2013b, 2015). QED analyses showed that the variation is due to these liquids residing in a far-out-of-equilibrium state (Yinnon and Elia, 2013; Yinnon et al., 2016). CDrot continuously realign, agglomerate into supra- CDrot and alter the stabilization of ![]() and supra-
and supra-![]() . The peculiar quasi-periodic dynamics of these domains have been analyzed by Yinnon and Elia (2013).
. The peculiar quasi-periodic dynamics of these domains have been analyzed by Yinnon and Elia (2013).
◉ The fluorescence intensity of the SDVSPL of NaCl, which is negatively correlated with the motility activity of the infusorium Spirostoma ambiguum in this media (Lobyshev et al., 2005) is yet another example of the relation between the physicochemical properties of numerous SDVSPL and their bio-activity (Konovalov and Ryzhkina, 2014). Elucidating the effects of SDVSPL on biosystems is hampered by the many unsolved puzzles pertaining to biochemical reactions. Yet the discussion by Yinnon and Liu (2015c) and Yinnon (2017) hints that ![]() play a role. The observed relation between the fluorescence intensity and the motility activity therefore is not surprising.
play a role. The observed relation between the fluorescence intensity and the motility activity therefore is not surprising.
SDVSPL-of-Distilled-Water Samples’ UV-vis Fluorescence Features and Their Explanations
In order to differentiate between the impact of the solute (NaCl) on the properties of the fluorescence of aqueous SDVSPL of NaCl, as well as the impact of the serial dilutions and vigorous shaking, Lobyshev et al. (2005) also measured the fluorescence of SDVSPL-of-Distilled-Water. On irradiating such perturbed water samples with 300 nm radiation, they mainly observed the following fluorescence spectral features [see Fig. 2 in Lobyshev et al. (2005)]:
★ The spectra are similar to those of aqueous SDVSPL of NaCl.
★ For SDVSPL-of-Distilled-Water which was 1 to 12 times decimally diluted, the luminescence intensity is about three to five times larger than that of “normal” distilled water. Here “normal” distilled water refers to water which was not vigorously shaken and not serially diluted.
★ For SDVSPL-of-Distilled-Water which was 13 to 30 times decimal diluted, the fluorescence intensity values vary between that of “normal” distilled water or are about twice as high.
★ The fluorescence intensities of SDVSPL-of-Distilled-Water, just as for aqueous SDVSPL of NaCl, non-monotonically depend on the time of its storage.
Explanations for these spectral features are feasible within the context of the findings of the extensive studies of SDVSPL-of-Distilled-Water or SDVSPL-of-ethanol. These studies were carried out by independent researchers during the last decade (Elia and Niccoli, 2004a, 2005; Miranda et al, 2011; Upadhyay and Nayak, 2011; Nain et al., 2015). These studies have shown that the values of the physicochemical variables (e.g., χ, Q, pH, viscosities, densities, ultrasonic speeds or refraction index) of SDVSPL-of-Distilled-Water or SDVSPL-of-ethanol significantly differ, respectively, from those of “normal” distilled water or “normal” ethanol.
The differences between SDVSPL-of-Distilled-Water and “normal” distilled water are not directly attributable to compounds released by the glass container or other impurities, as evidenced by the carefully controlled experiments carried out by Elia and Niccoli (2004a, 2005). They showed that the vigorous shaking indeed released compounds from the glass, e.g., Na+ or SiO2. They showed that the concentration of these compounds in SDVSPL-of-Distilled-Water was of the order of 10-5 M or below. However, these compounds could not account for the physicochemical properties of the SDVSPL-of-Distilled-Water. They showed that for the control, i.e., “normal” distilled water with the same chemical composition as SDVSPL-of-Distilled-Water, the values of its physicochemical variables significantly differ from those of SDVSPL-of-Distilled-Water. Here “normal” distilled water “with the same chemical composition as SDVSPL-of-Distilled-Water” refers to “normal” distilled water containing the same compounds released by the glass, which are present at the same concentrations.
Elia and Niccoli (2004a, 2005) showed that vigorous shaking of the SDVSPL-of-Distilled-Water altered the ordering of its H2O. They showed that on omitting the vigorous shaking, the physicochemical variables of serial diluted liquids and “normal” liquids were the same. They also showed that the SDVSPL-of-Distilled-Water is a far-out-of equilibrium system. Changes in its H2O orderings continuously occur for many months after its preparation. They observed that on aging of the liquid, the electric conductivity, heat of mixing and pH values vary non-monotonically (Elia et al., 2000, 2008a&b; Belon et al., 2008).
In regard of the findings by Elia and Niccoli (2004a, 2005, 2008a&b) and those of the other groups which investigated SDVSPL-of-Distilled-Water, it is not surprising that the UV-vis fluorescence features of SDVSPL-of-Distilled-Water observed by Lobyshev et al. (2005) also differ from those of “normal” distilled water. Moreover, it is not surprising that the fluorescence intensity of SDVSPL-of-Distilled-Water varied non-monotonically with the storage time of these liquids.
Based on the experimental data and theoretical developments that accumulated during the last decade, the following two complementary mechanisms can explain the differences between the ordering of the solvent molecules in SDVSPL-of-polar liquids and “normal” polar liquids.
A. “Activation” of the ordering of the H2O in SDVSPL-of-Distilled-Water by compounds released by the glass containers has been conjectured by Elia and Niccoli (2004a). It should be emphasized that Elia and Niccoli (2004a) conjectured that the changes are activated by the compounds, but not simply due to the presence of the compounds.
The conjecture of Elia and Niccoli (2004a) agrees with the QED model of SDVSPL. As noted in paragraphs II-V: At concentrations of about ![]() and about two orders of magnitude lower, ions and polar solvent molecules form IPDplasma. Typically, ~10-6 M <
and about two orders of magnitude lower, ions and polar solvent molecules form IPDplasma. Typically, ~10-6 M < ![]() <~10-4 M. For aqueous Na+,
<~10-4 M. For aqueous Na+, ![]() =2×10-4 M (Yinnon and Yinnon, 2012). Vigorous shaking of solutions containing IPDplasma results in formation of EDAIPDplasma. For C <
=2×10-4 M (Yinnon and Yinnon, 2012). Vigorous shaking of solutions containing IPDplasma results in formation of EDAIPDplasma. For C < ![]() , EDAIPDplasma can stabilize CDrot. Typically, 10-6 M <
, EDAIPDplasma can stabilize CDrot. Typically, 10-6 M < ![]() < 10-10 M. So indeed according to QED, in SDVSPL-of-polar liquids, the presence of impurities released by the container might induce stabilization of CDrot. Since dilution, shaking and aging processes alter the alignment of the electric dipoles of CDrot, these processes affect the stabilization and prevalence of CDelec. For the UV-vis fluorescence of SDVSPL-of-Distilled-Water, the aforementioned implies that its intensity should vary with each dilution step and with the storage time, just as observed by Lobyshev et al. (2005).
< 10-10 M. So indeed according to QED, in SDVSPL-of-polar liquids, the presence of impurities released by the container might induce stabilization of CDrot. Since dilution, shaking and aging processes alter the alignment of the electric dipoles of CDrot, these processes affect the stabilization and prevalence of CDelec. For the UV-vis fluorescence of SDVSPL-of-Distilled-Water, the aforementioned implies that its intensity should vary with each dilution step and with the storage time, just as observed by Lobyshev et al. (2005).
B. Recent developments enable pointing to another source of CDrot and CDelec in SDVSPL. In water and other polar liquids, adjacent to the wall of vessel made of glass or other hydrophilic materials, an exclusion zone (EZ) has been shown to form (Zheng et al., 2006; Chai and Pollack, 2010). The analyses by Del Giudice et al. (2013) and Yinnon et al. (2016) have indicated that the EZ contains CDelec and CDrot. Just gently stirring of the water adjacent to a hydrophilic surface has been shown to release clumps (aggregates) of ordered H2O from the EZ (Elia et al., 2013a&b, 2014a, 2015, 2017, 2018; Yinnon et al., 2016). Analyses of the characteristics of these aggregates and their impact on the physicochemical and thermodynamic properties of the liquid have shown that these aggregates have the typical features of supra-CDrot containing supra-![]() (Yinnon et al., 2016).
(Yinnon et al., 2016).
The current available experimental data are insufficient for delineating the relative contribution of the two mechanisms to the prevalence of CDrot and CDelec in SDVSPL. The hysteretic far-out-of-equilibrium dissipative properties of SDVSPL imply that SDVSPL’s phenomena are repeatable but not quantitatively reproducible. Hence, even the major differences between the UV-vis fluorescence intensity of the first 13 decimally diluted SDVSPL-of-Distilled-Water and aqueous SDVSPL of NaCl, observed by Lobyshev et al. (2005), cannot be simply attributed to the dominance of one of the above described mechanisms. For example, just a difference in the storage time of polar liquids affects the sizes of their EZ adjacent to the wall of the vessel. The accumulation of polar solvent molecules in an EZ is a rather slow process (Zheng et al., 2006; Chai and Pollack, 2010). Typically, after a few seconds, the EZ becomes observable. It grows to a significant size (hundreds of microns) during the first few minutes after the liquid gets into contact with the hydrophilic surface. However, about a day is required for its width to expand to its maximum size. Accordingly, the age of the distilled polar liquid employed for preparing SDVSPL affect their properties. Hence, the prevalence of CDrot and CDelec differs even in onetime decimal diluted SDVSPL samples.
Clearance of the abovementioned fuzziness concerning the relative contribution of the two mechanisms seems possible by repeating the UV spectral analyses of Lobyshev et al. (2005) for SDVSPL of NaCl and SDVSPL-of-Distilled-Water prepared and kept in plastic vessels, which do not release impurities and which are closed by plastic lids. Thus, such experiments are called for.
Analyses of UV Radiation Transmission
As shown in the previous paragraphs and previous publications (Lo, 1996; Ryzhkina et al., 2012c; Lobyshev et al. 2005), many investigations of the physicochemical properties of SDVSPL have focussed on aqueous SDVSPL of NaCl. Analyses of the data obtained in these investigations confirmed the various aspects of the QED model of SDVSPL (Yinnon and Liu, 2015b). In particular, the analyses of the UV radiation’s fluorescence by aqueous SDVSPL of NaCl, presented above, indicate that the fluorescence intensity is a function of the prevalence of ![]() . For aqueous SDVSPL of NaCl, the drop in the fluorescence intensity on diluting from 2 M to 2×10-3 M, we attributed to the decrease in the prevalence of
. For aqueous SDVSPL of NaCl, the drop in the fluorescence intensity on diluting from 2 M to 2×10-3 M, we attributed to the decrease in the prevalence of ![]() . This decrease is ascribable to the diminishment in the prevalence of the domains composed of Na+ and Cl– ions and H2O, i.e., the CDplasma. These domains stabilize
. This decrease is ascribable to the diminishment in the prevalence of the domains composed of Na+ and Cl– ions and H2O, i.e., the CDplasma. These domains stabilize ![]() . Moreover, the non-monotonic dependence of the fluorescence intensity on the number of dilution steps for concentrations below
. Moreover, the non-monotonic dependence of the fluorescence intensity on the number of dilution steps for concentrations below ![]() ≈2×10-4 M, we attributed to the presence of EDAIPDplasma which stabilize
≈2×10-4 M, we attributed to the presence of EDAIPDplasma which stabilize ![]() . For concentrations below
. For concentrations below ![]() ≈10-7 M, these EDAIPDplasma stabilize CDrot, which in turn stabilize
≈10-7 M, these EDAIPDplasma stabilize CDrot, which in turn stabilize ![]() . As noted above, the polarization of the liquid caused by CDrot changes with the number of dilution steps. The polarization affects the stabilization of
. As noted above, the polarization of the liquid caused by CDrot changes with the number of dilution steps. The polarization affects the stabilization of ![]() . Therefore, also the prevalence of
. Therefore, also the prevalence of ![]() changes with the number of dilution steps. According to the aforementioned, the UV radiation’s transmission should increase on diluting SDVSPL of NaCl until C≈10-4 M. Moreover, it should change non-monotonically with the number of dilution steps for C<10-4 M. This indeed was observed by Lo (1996). Since the
changes with the number of dilution steps. According to the aforementioned, the UV radiation’s transmission should increase on diluting SDVSPL of NaCl until C≈10-4 M. Moreover, it should change non-monotonically with the number of dilution steps for C<10-4 M. This indeed was observed by Lo (1996). Since the ![]() of aqueous solutions of HNO3 and NaOH are the same as those of NaCl (Yinnon and Yinnon, 2012), the transmission of UV radiation by aqueous SDVSPL of these liquids should be similar to those of that of aqueous SDVSPL of NaCl. Indeed Lo (1996) observed such a similarity.
of aqueous solutions of HNO3 and NaOH are the same as those of NaCl (Yinnon and Yinnon, 2012), the transmission of UV radiation by aqueous SDVSPL of these liquids should be similar to those of that of aqueous SDVSPL of NaCl. Indeed Lo (1996) observed such a similarity.
As noted above, the UV radiation transmission by aqueous SDVSPL of CuSO4 or aqueous SDVSPL of hypericum, prepared under the same experimental conditions, is lower than that of the control. In contrast, for aqueous SDVSPL of sublimed S8, the UV radiation transmission is higher than that of the control. The controls were vigorously shaken, but not diluted water. Since the UV spectral features of SDVSPL have been attributed to the presence of ![]() , the UV transmission data indicate that the prevalence of
, the UV transmission data indicate that the prevalence of ![]() is less in SDVSPL of S8 than in SDVSPL of CuSO4 or hypericum. This result implies that the solute affects the
is less in SDVSPL of S8 than in SDVSPL of CuSO4 or hypericum. This result implies that the solute affects the ![]() . It is plausible that the solute directly impact on the stabilization of
. It is plausible that the solute directly impact on the stabilization of ![]() .
.
However, it is also possible that the solute affects the stabilization of CDrot, which in turn affects the prevalence of ![]() .
.
Discussion and Conclusions
The above analyses show that the QED model of SDVSPL consistently explains the UV spectral data. As such, the analyses complement explanations for many types of other physiochemical and structural data which consistently were explained with the model (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013; Yinnon and Liu, 2015b,c; Yinnon, 2017).
The analyses of the absorbance or fluorescence UV spectra do not enable to unambiguously differentiate between the spectra of highly dilute (C<Cthr) SDVSPL prepared with different substrates (i.e., solutes present in the mother solution). The spectra consist of broad featureless bands. The spectra resemble those of various types of structured polar liquids. These structured liquids, just as SDVSPL with C<Cthr, contain nano to micron sized associates mainly composed of the polar liquid molecules. Therefore, studying the absorbance or fluorescence UV spectra of SDVSPL does not facilitate extracting information on the imprint of the properties of the substrate on the electronic structure and molecular organization of the associates present in SDVSPL diluted below Cthr.
UV transmission intensities, in contrast to UV absorbance or fluorescence spectral peaks, do reveal statistically significant differences between extremely diluted SDVSPL prepared with different substrates. Highly controlled experiments showed that there is a difference between the transmission intensities of SDVSPL of copper sulfate (CuSO4), aqueous SDVSPL of hypericum and aqueous SDVSPL of sublimed sulfur (S8), which were up to 30 times centesimally diluted (Klein et al., 2013). However, the available transmission data do not reveal the relation between the characteristics of the substrate and the electronic structure and/or molecular organization of the associates present in extremely diluted SDVSPL.
Discovering relations between characteristics of the substrate and properties of their extremely diluted SDVSPL is required for studying the active principle underlying these liquids’ bioactivity. Konovalov and Ryzhkina (2014) showed that for SDVSPL with C<Cthr, their bioactivity is related to their 102 nm associates, which are mainly composed of solvent molecules. On screening SDVSPL from ambient EM fields by placing these liquids in Permalloy containers, their 102 nm associates disintegrate and the liquids’ bioactivity is destroyed. Montagnier et al. (2009, 2011, 2015, 2017a &b) carried out experiments and analyses that provide some additional insights into aspects of the bioactive principle. Firstly, they demonstrated that aqueous SDVSPL of some types of DNA may emit ultra low frequency (ULF) (500-3000 Hz) EM radiation. Such types include DNA from Borrelia burgdorferi or the LTR of HIV-1. The number of dilutions steps required for the SDVSPL to emit the radiation depends on the type of DNA. Typically, at least six decimal dilutions of a few nano gram (ng) of DNA are required for the emission to be detectable. The SDVSPL only emit ULF radiation when these liquids are stimulated by background extremely low frequency (ELF) radiation. The source of the ELF radiation may be the natural Schumann resonances of the geomagnetic field or artificial ones.g Secondly, they amplified the emitted ULF, digitally recorded it and sent it to a distant laboratory, where they transformed into an analog form. They sent the electric vector of the analog signal to a solenoid, which generated a magnetic field in a test tube of water. They added enzymatic proteins to the water, so as to allow polymerase chain reaction (PCR) processes to occur. They observed that the PCR processes produced and amplified DNA molecules identical to the original DNA (the substrate of the SDVSPL). In other words, the electromagnetic image of the substrate in the SDVSPL is “read” by Taq polymerase in the presence of primers and nucleotide triphosphates. Thirdly, they exposed a flask of living cultured cells to the aforementioned magnetic field. The cells originated from malignant tumors or from isolated in vitro as continuous “immortalized” cell lines. After several days of exposure, the cells synthesized the SDVSPL’s DNA substrate. The synthesis was detected by common PCR procedures. At the same time, the growth of the exposed cells was inhibited and finally they died. Fourthly, they explained their results with the QED model of aqueous systems. They employed the formulism developed by Kurian et al. (2018). They analyzed the electromagnetic image of DNA templated in these molecules’ surrounding water.h They investigated the spatiotemporal distribution of interaction couplings, frequencies, amplitudes and phase modulations comprising a pattern of fields, such as of the net polarization of water in the vicinity of the DNA. (This net polarization is analogous to the stabilization of CDrot). They studied the effects of this polarization on dipole modes that can be recognized by Taq polymerase.
So far, to the best of my knowledge, no experimental techniques have been identified which can reveal the relation between substrates’ characteristics and the physicochemical and structural properties of extremely diluted SDVSPL. This, in spite of the fact that extensive and many different types of measurements have been carried out, e.g., electric conductivity, heat of mixing with acids or bases, pH, dielectric permittivity, DLS, nanoparticle tracking analyses, surface tension, impedance, Landau-Placzek ratio, Raman scattering, acoustic, volumetric, viscometric, refractive index, atomic force microscopy, scanning electron microscopy, transmission electron microscopy, fluorescence microscopy, UV, IR, ultra low frequency or nuclear magnetic resonance spectral measurements.
Part of the challenge of uncovering the relation between substrates’ characteristics and the physicochemical and structural properties of extremely diluted SDVSPL is that it has not been derived within the context of QED. This paper’s analyses and previous ones have shown that the QED model of SDVSPL adequately describes many aspects of these liquids; therefore future research should be directed at deriving this relation.
In conclusion, during the last decades and also in the current paper, ample and unambiguous evidence has been presented that SDVSPL diluted below Cthr may have properties which statistically significantly differ from those of the same liquids which are identically diluted but not vigorously shaken after each dilution step. The QED model of SDVSPL explains this phenomenon. Yet the vast existing experimental data and the theory leave us in the dark as to the relations between the substrates’ characteristics and the bioactive, physicochemical and structural properties of SDVSPL diluted below Cthr. Because of the importance of SDVSPL for pharmacology and agriculture, major theoretical and experimental efforts for elucidating the relations are called for. This requires shifting the last decades’ extensive research emphasis. Instead of demonstrating that the characteristics of SDVSPL may differ from those of the same liquids which are identically diluted but not vigorously shaken, the focus should be the aforementioned relations.
g The Schumann resonances have frequencies, for example, at 7.83, 14.3, 20.8, 27.3, and 33.8 Hz (Nickolaenko and Hayakawa, 2002).
h DNA templating at least some of its properties in its surrounding water has recently been observed by McDermott et al. (2017). They showed that under physiological conditions DNA imprints its chirality on its vicinal water.
Acknowledgements
I express my gratitude to Prof. A. M. Yinnon for his continuous support, encouragement and manuscript review. I also express my appreciation and thankfulness to Jesús Aguilar for his careful literature search.
References
Ambrosetti A, Ferri N, DiStasio RA Jr., Tkatchenko A (2016). Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science 351:1171-1176.
Arani R, Bono I, Del Giudice E; Preparata G (1995). QED coherence and the thermodynamics of water. Int J Mod Phys B 9:1813-1841.
Bellavite P, Marzotto M, Olioso D, Moratti E, Conforti A (2014). Review: High dilution effects revisited 1. Physicochemical aspects. Homeopathy 103: 4-21.
Belon P, Elia V, Elia L, Montanino M, Napoli E, Niccoli M (2008). Conductometric and calorimetric studies of the diluted and agitated solutions. On the combined anomalous effect of time and volume parameters. J Therm Anal Calorimetry 93:459-469.
Belov VV, Mal’tseva EL, Pal’mina NP (2011). Modification of the structure of plasmatic membranes of the liver by the action of α-tocopherol in vitro. Biophysics 56:323-330.
Betti L, Trebbi G, Kokornaczyk MO, Nani D, Peruzzi M, Dinelli G, Bellavite P, Brizzi M (2017). Number of succussion strokes affects effectiveness of ultra-high-diluted arsenic on in vitro wheat germination and polycrystalline structures obtained by droplet evaporation method. Homeopathy 106:47–54.
Bhattacharyya SS, Mandal SK, Biswas R, Paul S, Pathak S, Boujedaini N, Belon P, Khuda-Bukhsh AR (2008). In vitro studies demonstrate anticancer activity of an alkaloid of the plant Gelsemium sempervirens. Exp Biol Med (Maywood) 233:1591-601.
Buch V, Tarbuck T, Richmond GL, Groenzin H, Li I, Shultz MJ (2007) Sum frequency generation surface spectra of ice, water, and acid solution investigated by an exciton model. J Chem Phys 127: 204710.
Burlakova EB, Grechenko TN, Sokolov EN, Terekhova SF (1986). Effect of inhibitors of radical reactions of lipid oxidation on electrical activity of the isolated snail neuron. Biofizika 31:921-923.
Burlakova EB, Konradov AA, Maltseva EL (2004). Effect of extremely weak chemical and physical stimuli on biological systems. Biophysica (Moscow) 49:522-534.
Burlakova EB (2005). Bioantioxidants: yesterday, today, tomorrow. Pages 18-27 in Chemical and biological kinetics – New Horizons. Vol. 2. Biological kinetics. Eds. Burlakova EB, Shilov AE, Varfolomeev SD, Zaikov G. Koninklijke Brill NV, Leiden, The Netherlands.
Cabral do Couto P, Chipman DM (2012). Insights into the ultraviolet spectrum of liquid water from model calculations: The different roles of donor and acceptor hydrogen bonds in water pentamers. J Chem Phys 137:18430.
Capolupo A, Del Giudice E, Elia V, Germano R, Napoli E, Niccoli M, Tedeschi A, Vitiello G (2014). Self-similarity properties of Nafionized and filtered water and deformed coherent states. Int J Mo. Phys. B 28:1450007.
Chakraborty M, Ghosh S, Das S, Basu R, Nandy P (2015). Effect of Different Potencies of Nanomedicine Aconitum Napelles on Its Spectral and Antibacterial Properties. Int J Innov Res Sci Eng Technol 4:6861-6867.
Chai B-H, Zheng J-M, Zhao Q, Pollack GH (2008). Spectroscopic studies of solutes in aqueous solution. J Phys Chem A 112:2242-2247.
Chai B-H, Yoo H, Pollack GH (2009) Effect of Radiant Energy on Near-Surface Water. J Phys Chem B 113: 13953-13958.
Chai B-H, Pollack GH (2010). Solute-Free Interfacial Zones in Polar Liquids. J Phys Chem B 114:5371-5375.
Ciavatta L, Elia V, Napoli E, Niccoli M (2008). New physico-chemical properties of extremely diluted solutions. Electromotive force measurements of Galvanic cells sensible to the activity of NaCl at 25 oC. J Solution Chem 37:1037-1049.
Davenas E, Beavais F, Amara J, Oberbaum M, Robinzon B, Miadonna A, Tedeschi A, Pomeranz B, Fortner P, Belon P, Sainte-Laudy J, Poitevin B, Benveniste J (1988). Human basophil degranulation triggered by very dilute antiserum against IgE. Nature 333:816-818.
Del Giudice E, Preparata, G, Vitiello G (1988). Water as a free electric dipole laser. Phys Rev Lett 61: 1085-1088.
Del Giudice E, Preparata G (1998). “A new QED picture of water”, in Macroscopic Quantum Coherence, eds. Sassaroli E, Srivastava YN, Swain J, Widom A (World Scientific, Singapore).
Del Giudice E, Preparata G, Fleischmann M (2000). QED coherence and electrolyte solutions. J Electroanal Chem 482:110–116.
Del Giudice E, Fleischmann M, Preparata G and Talpo G (2002). On the “unreasonable” effects of ELF magnetic fields upon a system of ions. Bioelectromagn. 23: 522–530.
Del Giudice E, Vitiello G. (2006). Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transitions. Phys Rev A 74: 022105-1-022105-9
Del Giudice E, Tedeschi A, Vitiello G, Voeikov V (2013). Coherent structures in liquid water close to hydrophilic surfaces. J Phys:Conf Ser 442:012028.
Demangeat JL (2013). Nanosized solvent superstructures in ultramolecular aqueous dilutions: twenty years’ research using water proton relaxation. Homoeopathy 102:87-105.
Demangeat JL (2015). Gas nanobubbles and aqueous nanostructures:The crucial role of dynamization. Homeopathy 104:101-115.
Elia V, Niccoli M (1999). Thermodynamics of Extremely Diluted Aqueous solutions. Ann New York Acad Sci 879:241-248.
Elia V, Niccoli M (2000). New Physico-chemical properties of water induced by mechanical treatments: A calorimetric study at 25oC. J Therm Anal Cal 61:527-537.
Elia V, Niccoli M (2004a). New Physico-chemical properties of extremely diluted aqueous solutions. J Therm Anal Cal 75:815-836.
Elia V, Napoli E, Niccoli M, Nonatelli L, Ramaglia A, Ventimiglia E (2004b). New Physico-chemical properties of extremely diluted aqueous solutions. A calorimetric and conductivity study at 25°C. J Therm Anal Cal 78:331–342.
Elia, V, Marchese M, Montanino M, Napoli E, Niccoli M, Nonatelli L, Ramaglia A (2005). Hydrohysteretic phenomena of extremely diluted solutions induced by mechanical treatments: A calorimetric and conductometric study at 25°C. J. Solut. Chem. 34:947–960.
Elia V, Napoli E, Niccoli M, Marchettini N, Tiezzi E (2008a). New physico-chemical properties of extremely dilute solutions. A conductivity study at 25°C in relation to ageing. J Solution Chem 37: 85-96.
Elia V, Elia L, Marchettini N, Napoli E, Niccoli M,Tiezzi E (2008b). Physico-chemical properties of aqueous extremely diluted solutions in relation to ageing. J Therm Anal Calorimetry 93:1003-1011.
Elia V, Napoli E. (2010). Dissipative structures in extremely diluted solutions of homeopathic medicines. A molecular model based on physico-chemical and gravimetric evidences. Int J Des Nat 5: 39-48.
Elia, V, Marrari LA, Napoli E. (2012). Aqueous nanostructures in water induced by electromagnetic fields emitted by EDS. J Therm Anal Calorim 107:843–851.
Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M (2013a). Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with a Nafion® polymer membrane. Water 5:16-26.
Elia V, Elia L, Napoli E, Niccoli M (2013b). Physical-chemical study of water in contact with a hydrophilic polymer: Nafion. J Therm Anal Calorim 112:937-944.
Elia V, Ausanio G, Gentile F, Germano R, Napoli E, Niccoli M (2014a). Experimental evidence of stable water nanostructures in extremely dilute solutions, at standard pressure and temperature. Homeopathy 103:44-50.
Elia V, Lista L, Napoli E, Niccoli M (2014c). A Thermodynamic Characterization of Aqueous Nanostructures of Water Molecules Formed by Prolonged Contact with the Hydrophilic Polymer Nafion. J Therm Anal Calorim 115:1841-1849.
Elia V, Ausanio G, De Ninno A, Germano R, Napoli E, Niccoli M (2014b). Experimental Evidences of Stable Water Nanostructures at Standard Pressure and Temperature Obtained by Iterative Filtration. Water 5:121-130.
Elia V, Germano R, Napoli E (2015). Permanent Dissipative Structures in Water: The Matrix of Life? Experimental Evidences and their Quantum Origin. Curr Top Med Chem 15:559-571.
Elia V, Yinnon TA, Oliva R, Napoli E, Germano R, Bobba F, Amoresano A (2017). Chiral micron-sized H2O aggregates in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water. Water 8:1-29.
Elia V, Oliva R, Napoli E, Germano R, Pinto G, Lista L, Niccoli M, Toso D, Vitiello G, Trifuoggi M, Giarra A, Yinnon TA (2018). Experimental study of physicochemical changes in water by iterative contact with hydrophilic polymers: A comparison between Cellulose and Nafion. J Mol Liq 268: 598-609.
Elton DC, Fernandez-Serra M (2016). The hydrogen-bond network of water supports propagating optical phonon-like modes. Nat Commun 7:10193.
Ferri N, DiStasio RA, Ambrosetti A, Car R, Tkatchenko A (2015). Electronic properties of molecules and surfaces with a self-consistent interatomic van der Waals density functional. Phys Rev Lett 114:176802.
Fiorini RA (2016). Arbitrary multi-scale (AMS) systems biology and biomedical engineering effective modeling and simulation. International Journal of Biology and Biomedical Engineering (BIBIEN)10:61-71.
Georgalis G, Kierzek AM, Saenger W (2000). Cluster Formation in Aqueous Electrolyte Solutions Observed by Dynamic Light Scattering. J Phys Chem B 104:3405-3406.
Ghosh S, Chakraborty M, Das S, Basu R, Nandy P (2015). Effect of Different Potencies of Nanomedicine Cuprum metallicum on Membrane Fluidity – a Biophysical Study. AJHM 107:161-169.
Horne RA (1972). Water and Aqueous Solutions: Structure, Thermodynamic and Transport Processes. Wiley-Interscience New York. Chapters 8-12.
Klein SD, Sandig A, Baumgartner S, Wolf U (2013). Differences in median ultraviolet light transmissions of serial homeopathic dilutions of Copper Sulfate, Hypericum perforatum and Sulfur Evid Based Complement Alternat Med., Vol 2013, Article ID 370609, 11 pages, 2013.
Klein SD, Wolf U (2016). Comparison of homeopathic globules prepared from high and ultra-high dilutions of various starting materials by ultraviolet light spectroscopy. Complement Ther Med 24:111-117.
Konovalov AI, Ryzhkina IS, Murtazina LI, Timosheva AP, Shagidullin RR, Chernova AV, Avvakumova LV, Fattakhov SG (2008). Supramolecular systems based on the melamine salt of bis(hydroxymethyl)phosphinic acid (melafen) dihydrate and surfactants 1. Structure and self-association in water and chloroform. Izv Akad Nauk Ser Khim1207–1214; Russ Chem Bull Int Ed. 57: 1231-1238.
Konovalov AI (2013). The formation of nanosized molecular ensembles in highly dilute aqueous solutions. Herald of the Russian Academy of Sciences 83:513–519.
Konovalov AI, Ryzhkina IS (2014). Reviews: Formation of nanoassociates as a key to understanding of physicochemical and biological properties of highly dilute aqueous solutions. Russ Chem Bull Int Ed 63:1-14.
Konovalov AI, Ryzhkina IS (2016) Nanoassociates as a possible basic element of scientific foundation of homeopathy. 1st Eurasian Congress on homeopathic medicine and 25th Congress of Asian Homeopathic Medical League, Moscow 14-15 October 2016.
Kurian P, Capolupo A, Craddock TJA,Vitiello G (2018). Water-mediated correlations in DNA-enzyme interactions. Phys Lett A 382: 33–43.
Larzul H, Gelebart F, Joahnin-Gilles A (1965). Sur le spectre d’absorptionde l’eau et de l’eau lourde dans l’ultraviolet. Compt Rend 261:4701-4704.
Liu Y, Song C-Y, Luo X-S, Lu J, Ni X-W (2007). Fluorescence spectrum characteristic of ethanol–water excimer and mechanism of resonance energy transfer. Chin Phys Soc 16:1300-1306.
Liu Y-J, Roca-Sanjuán D, Lindh R (2012) Computational photochemistry and photophysics: the state of the art. Photochemistry 40:42-72.
Lo S-Y (1996). Anomalous state of ice. Mod Phys Lett 10:909-919.
Lo S-Y, Lo A, Chong LW, Tianzhang L, Hua LH, Geng X, (1996). Physical properties of water with IE structures. Mod.Phys.Lett. B10: 921-930.
Lo S-Y, Geng XU, Gann, D (2009). Evidence of stable-water-clusters at room temperature and normal pressure. Phys Lett A 373: 3872 -3876.
Lobyshev VI, Shikhlinskaya RE, Ryzhikov BD (1999). Experimental evidence for intrinsic luminescence of water. J Mol Liq 82:73-81.
Lobyshev VI, Tomkevich MS, Petrushanko I Yu (2005). Experimental Study of Potentiated Aqueous Solutions. Biophys 50:416-420.
McDermott ML, Vanselous H, Corcelli SA, Petersen PB (2017). DNA’s chiral spine of hydration. ACS Cent Sci 3: 708-714.
Miranda AR, Vannucci A, Pontuschka WM (2011). Impedance spectroscopy of water in comparison with high dilutions of lithium chloride. Mater Res Innov 15:302-309.
Mishina OA, Murtazina LI, Ryzhkina IS, Konovalov AI (2015). The relationship between self-organization, physicochemical properties, and biological activity of low-concentration solutions of p-aminobenzoic acid. Russ Chem Bull Int Ed 64:590-596.
Montagnier L, Aïssa J, Ferris S, Montagnier JL, Lavallee C (2009). Electromagnetic signals are produced by aqueous nanostructures derived from bacterial DNA sequences. Interdiscip Sci Comput Life Sci 1:81–90.
Montagnier L, Del Giudice E, Aïssa J, Lavallee C, Motschwiller S, Capolupo A, Polcari A, Romano P, Tedeschi A, Vitiello G (2015). Transduction of DNA information through water and electromagnetic waves. Electromagn Biol Med 34:106–112.
Montagnier L, Aïssa J, Capolupo A, Craddock TJA., Kurian P, Lavallee C, Polcari A, Romano P, Tedeschi A and Vitiello G (2017a). Water Bridging Dynamics of Polymerase Chain Reaction in the Gauge Theory Paradigm of Quantum Fields Water 9:339-358.
Addendum: L Montagnier L, Aïssa J, Capolupo A, Craddock TJA., Kurian P, Lavallee C, Polcari A, Romano P, Tedeschi A and Vitiello G (2017b). Water Bridging Dynamics of Polymerase Chain Reaction in the Gauge Theory Paradigm of Quantum Fields. Water 9: 436.
Mulliken RS, Ermler WC (1981). Polyatomic molecules, Academic Press, New York, Chapter II.
Nain AK, Droliya P, Manchanda RJ, Khurana A, Nayak D (2015). Physicochemical studies of extremely diluted solutions (homoeopathic formulations) of sulphur in ethanol by using volumetric, acoustic, viscometric and refractive index measurements at different temperatures. J Mol Liq 211:1082–1094.
Nickolaenko AP and Hayakawa M (2002). Resonances in the Earth-ionosphere cavity. Kluwer Academic Publishers, Dordrecht-Boston-London.
Nilsson A, Pettersson LGM (2015) The structural origin of anomalous properties of liquid water. Nat Commun 6:8998.
Noguchi H, Hiroshi M, Tominaga T, Gong JP, Osada Y, Uosaki K (2008) Interfacial water structure at polymer gel/quartz interfaces investigated by sum frequency generation spectroscopy. Phys Chem Chem Phys10: 4987-4493.
Pal’mina NP, Mal’tseva EL, Kurnakova NV, Burlakova EB (1994). Effect of α-Tocopherol over a wide concentration range (10-17-10-2M) on Protein Kinase C. Biochimiya (Russian) 59:193-200.
Pang X-F (2014). Water: Molecular Structure and Properties (World Scientific Publishing, Singapore), Ch. 3.
Pershin SM, Bunkin AF, Grishin MYa, Davydov MA, Lednev VN, Palmina NP, Fedorov AN (2015). Correlation of Optical Activity and Light Scattering in Ultra-Low-Concentrated Phenosan–Potassium Aqueous Solutions. Dokl Phys 60:114–117.
Preparata G (1995). QED Coherence in Matter. World Scientific, Singapore, New Jersey, London, Hong Kong.
Pynzar EI, Bogdanova NG, Palmina NP (1995). Effects of Phorbol Esters on lipid peroxidation in endoplasmic reticulum membranes from mouse liver. Membr and Cell Biol 9:279-288.
Quickenden TI, Irvin JA (1980). The ultraviolet absorption spectrum of liquid water. J Chem Phys 72:4416-4428.
Robinson RA, Stokes RH (2002) Electrolyte Solutions (Dover Publications Inc., Mineola, New York. Chapters 1-3.
Ryzhkina IS, Murtazina LI, Kiseleva YuV, Konovalov AI (2009a). Properties of Supramolecular nanoassociates formed in aqueous solutions of biologically active compounds in low or ultra-low concentrations. Dokl Phys Chem 428: 196–200.
Ryzhkina IS, Murtazina LI, Kiseleva YuV, Konovalov AI (2009b). Supramolecular Systems Based on Amphiphilic Derivatives of Biological Active Phenols: Self-Assembly and Reactivity over a Broad Concentration Range. Dokl Phys Chem 428: 201-205.
Ryzhkina IS, Murtazina LI, Sherman ED, Valitova YuN, Kataev EA, Konovalov AI (2010). Low-Concentration Aqueous Solutions of Macrocyclic Pyridine-Pyrrole Compound: Relationship between the Parameters, Physicochemical Properties, and Physiological Activity of Supramolecular Nanosized Associates. Dokl Phys Chem 443:142-146.
Ryzhkina IS, Murtazina LI, Academician Konovalov AI (2011a). Action of the external electromagnetic Field is the condition of nanoassociate formation in highly diluted aqueous solutions. Dokl Phys Chem 440:201–204.
Ryzhkina IS, Kiseleva YuV, Murtazina LI, Pal’mina NP, Belov VV, Mal’tseva EL, Sherman ED, Timosheva AP, Academician Konovalov AI (2011b). Effect of α-Tocopherol concentrations on the self-organization, physicochemical properties of solutions, and the structure of biological membranes. Dokl Phys Chem 438:109–113.
Ryzhkina IS, Kiseleva YuV, Murtazina LI, Academician Konovalov AI (2012a) Effect of ultralow concentrations and electromagnetic fields. Dokl Phys Chem 446: 153–157.
Ryzhkina IS, Kiseleva YuV, Timosheva AP, Safiullin RA, Kadirov MK, Valitova YuN, Academician Konovalov AI (2012b). Low-Concentration Aqueous Solutions of an Amphiphilic Calix[4]resorcinarene Derivative: Self-Organization, Physicochemical Properties, and Biological Activity under Common and Hypoelectromagnetic Conditions. Dokl Phys Chem 447:193-199.
Ryzhkina IS, Murtazina LI, Masagutova EM, Mishina OA, Pavlova TP, Fridland SV, Academician Konovalov AI (2012c). Self-Organization of Sodium Chloride Solutions in the Absence and Presence of a Biologically Active Substance of Low Concentration under Common and Hypoelectromagnetic Conditions. Dokl Phys Chem 446:184-189.
Ryzhkina IS, Kiseleva YV, Mishina OA, Timosheva AP, Segevaa SY, Kravchenko AN, Academician Konovalov AI (2013). Correlations between the self-organization, physicochemical properties and biological activity of Mebicar in dilute aqueous solutions. Mendeleev. Commun 23: 262-264.
Ryzhkina IS, Kiseleva YV, Murtazina LI, Mishina OA, Timosheva AP, Sergeeva SY, Baranov VV, Kravchenko AN, Konovalov AI (2015a). Self-organization and chirality in the high dilution solutions of glycoluril enantiomers with (R)- and (S)-methionine moieties. Mendeleev Commun 25:72–74.
Ryzhkina IS, Murtazina LI, Kiseleva Yu V,Academician Konovalov AI (2015b). Self-Organization and Physicochemical Properties of Aqueous Solutions of the Antibodies to Interferon Gamma at Ultrahigh Dilution Dokl Phys Chem 462:110–114.
Ryzhkina IS, Sergeeva S Yu, Safiullin RA, Ryzhkin SA, Margulis AB, Murtazina LI, Timosheva AP, Chernova AV, Kadirov MK, Konovalov AI (2016) Specifics of self-organization and properties of highly dilute aqueous solutions of polyoxidonium. Russ Chem Bull, Int Ed 65: 1505-1513.
Ryzhkina IS, Kiselev Yu V, Murtazina LI, Solovieva SE, Manin NG, Konovalov AI (2017a) Disperse Systems Based on a Dodecyl Derivative of p-Sulfo-natocalix[6]arene: Self-Organization and Physico-chemical Properties in a Wide Range of Concentrations and Temperatures. Macroheterocycles 10: 190-195.
Ryzhkina IS, Sergeev SY, Murtazina LI, Sabirzyanova LR, Kuznetsova TV, Zainulgabidinov ER, Knyazev IV, Petrov AM, Konovalov AI (2017b) Aqueous Systems Based on Metaphos in Low Concentration: The Relationship between Self-Assembly,Physico-Chemical, and Biological Properties. Russ J Gen Chem 87:2838–2845.
Ryzhkina IS, Sergeeva S Yu. Kiseleva YV, Timosheva AP, Salakhutdinova OA, Shevelev MD, Konovalov AI (2018) Self-organization and properties of dispersed systems based on dilute aqueous solutions of (S)- and (R)-lysine. Mendeleev Commun 28:66-69.
Samal S, Geckeler KE (2001). Unexpected solute aggregation in water on dilution. Chem Commun 2224–2225.
Sedlak M (2006) Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: Light scattering characterization. J Phys Chem B 110: 4329-4338; 4339-4345;13976-13984.
Sedlak M, Rak D (2013) Large-scale inhomogeneities in solutions of low molar mass compounds and mixtures of liquids: Supramolecular structures or nanobubbles? J Phys Chem B 117: 2495-2504.
Segarra-Martí J, Coto PB, Rubio M, Roca-Sanjuán D, Merchán M (2013). Towards the understanding at the molecular level of the structured-water absorption and fluorescence spectra: A fingerprint of π-stacked water. Mol Phys 111:1308–1315.
Segarra-Martí J, Roca-Sanjuán D, Merchán M (2014). Can the hexagonal ice-like model render the spectroscopic fingerprints of structured water? Feedback from quantum-chemical computations. Entropy 16:4101-4120.
Upadhyay RP, Nayak C (2011). Homeopathy emerging as nanomedicine. Int J High Dilution Res 10: 299-310.
Voeikov VL, Yablonskaya OI (2015). Stabilizing effects of hydrated fullerenes C60 in a wide range of concentrations on luciferase, alkaline phosphatase, and peroxidase in vitro. Electromagn Biol Med 34: 160–166.
Witt CM, Lüdtke R, Weisshuhn TE, Quint P, Willich SN (2006). The role of trace elements in homeopathic preparations and the influence of container material, storage duration, and potentisation. Forsch Komplementmed 13:15-21.
Wolf U, Wolf M, Heusser P, Thurneysen A, Baumgartner S (2011). Homeopathic Preparations of Quartz, Sulfur and Copper Sulfate Assessed by UV-Spectroscopy Evid Based Complement Alternat Med. Vol. 2011:692798. doi: 10.1093/ecam/nep036.
Yinnon CA, Yinnon TA (2009). Domains in aqueous solutions: theory and experimental evidence. Mod Phys Lett 23:1959-1973.
Yinnon TA, Yinnon CA (2011). Electric dipole aggregates in very dilute polar liquids: Theory and experimental evidence. Int J Mod Phys B 25: 3707-3743.
Yinnon TA, Yinnon CA (2012). Domains of solvated ions in aqueous solutions, their characteristics and impact on electric conductivity: theory and experimental evidence. Mod Phys Lett 26: 1150006-1 to 1150006-14.
Yinnon TA, Elia V (2013). Dynamics in perturbed very dilute aqueous solutions: theory and experimental evidence. Int J Mod Phys B 27:1350005-1 to 1350005-35.
Yinnon TA, Liu Z-Q (2015a). Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 1. Quantum Electrodynamic Aspects. Water 7:33-47.
Yinnon TA, Liu Z-Q (2015b). Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 2. Quantum Electrodynamic Analyses of Experimental Data on Strong Electrolyte Solutions. Water 7:48-69.
Yinnon TA, Liu Z-Q (2015c). Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 3. Quantum Electrodynamic Analyses of Experimental Data on Solutions of Weak Electrolytes and Non-electrolytes. Water 7: 70-95.
Yinnon TA, Elia V, Napoli E, Germano R, Liu ZQ (2016). Water ordering induced by interfaces: an experimental and theoretical study. Water 7:96-128.
Yinnon TA (2017). Very Dilute Aqueous Solutions — Structural and Electromagnetic Phenomena.
Water 9:28-66.
Zheng JM, Chin WC, Khijniak E, Khijniak E Jr., Pollack GH (2006). Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv Colloid Interface Sci 127:19–27.
Table 1: List of abbreviations in alphabetic order, followed by Greek symbols abbreviations
Abbrev. / Explanation
C – Concentration
Ccrit – Critical concentration
![]() – Critical concentration below which CDrot may form
– Critical concentration below which CDrot may form
![]() – Transition concentration for CDplasma formation
– Transition concentration for CDplasma formation
![]() – Transition concentration for IPDplasma formation
– Transition concentration for IPDplasma formation
Cthr – Threshold concentration below which no domains are present in SDVSPL samples screened by Permalloy
CD – Coherence domain
![]() – Coherence domain composed of coherent electronically excited water molecules
– Coherence domain composed of coherent electronically excited water molecules
CDplasma – Coherence domain composed of few solvated solutes performing coherent plasma oscillation and numerous polar solvent molecules
CDrot – Coherence domains of ferroelectric ordered polar solvent molecules
DLS – Dynamic light scattering
EDACDrot – Excited or broken CDrot piece, which has an electric dipole moment and therefore is denoted electric dipole aggregate (EDA)
EDAIPDplasma – Excited or broken IPDplasma piece, which has an electric dipole moment and therefore is denoted electric dipole aggregate (EDA)
EM – Electro-magnetic
ELF – Extremely low frequency
eV – Electron Volt
H2O – Water molecule
Hz – Herz
IPDplasma – In phase domains composed of few solvated solutes and numerous solvent molecules performing in phase plasma oscillation.
kB – Boltzmann constant
M – Molarity in mole per liter
m – Meter
mol / l – Mole per Liter
NaCl – Sodium Chloride
nm – Nano-meter
QED – Quantum electro-dynamics
QFE – Quasi free electrons
SDVSPL – Serially diluted vigorously shaken polar liquid
Supra-CD – Agglomerate of coherence domains
ULF – Ultra low frequency
UV – Ultra-violet
Vis – Visible
Discussion with Reviewers
Reviewer: How is the general frame presented by the author related to the EZ phenomenology of water in the presence of a Nafion film?
Author: The general frame employed by Yinnon and Yinnon (2011) for developing their model of SDVSPL is that of QED. The QED models of aqueous systems and of other polar liquids unambiguously describe electrodynamic forces. These forces span 10-7 – 10-4 m. These forces result from the interactions between the EM field and the matter quantum wave field. In contrast, the customary models of polar liquids explicitly describe electro-static forces, but assume that electro-dynamic ones can be treated perturbatively or often even may be ignored. Electrostatic interactions in liquids typically span nm. These interactions result from Coulombic forces between solvent and solute particles.
The need for a QED formulism for developing a model for SDVSPL was recognized by Yinnon and Yinnon (2011). In these liquids up to hundreds of microns sized molecular orderings have been observed (Lo, 1996; Konovalov et al., 2008; Lo et al., 2009; Ryzhkina et al., 2009a&b). The interactions described by the customary models of polar liquids cannot lead to such large orderings (Preparata, 1995). Indeed, the group of Konovalov has shown that interactions between the liquid’s molecules and ambient EM fields are required for formation of the nano- and micron- sized molecular orderings in SDVSPL diluted below the transition concentration Ctrh (Ryzhkina et al., 2011a, 2012a). Typically, Cthr is of the order of 10-6 – 10-10 M.
The main breakthroughs in the attempts to develop a QED model for polar liquids in general and for water in particular were obtained by Del Giudice, Preparata and Vitiello (1988) and Arani, Bono, Del Giudice and Preparata (1995). These scientists showed that two types of coherent domains may be stabilized in polar liquids. In the main text of this paper, I denoted these domains as CDrot and CDelec.
The energy H2O gain by their inclusion in CDrot is of the order of the energies of the thermal fluctuations of water at ambient conditions. Therefore, CDrot do not stabilize in such water. The energy H2O gain by inclusion in CDelec is about an order of magnitude larger than the energy of the aforementioned fluctuations. Accordingly, CDelec are meta-stable in bulk water at ambient conditions.
One of the challenges Yinnon and Yinnon (2011) faced in their pursuit of a QED model of SDVSPL, which is capable of explaining its physicochemical and structural properties, was the following: They had to identify the mechanisms by which CDrot and CDelec are stabilized during these liquids preparation procedure. For aqueous solutions of strong or weak electrolytes, they showed that serially diluting up to a transition concentration (![]() ) leads to formation of a QED domain, denoted as IPDplasma in the main text. They showed that shaking causes IPDplasma to transform into aggregates with an electric dipole moment (EDA). On diluting solutions containing EDA below a critical concentration
) leads to formation of a QED domain, denoted as IPDplasma in the main text. They showed that shaking causes IPDplasma to transform into aggregates with an electric dipole moment (EDA). On diluting solutions containing EDA below a critical concentration ![]() , the EDA stabilize CDrot and CDelec. For solutions of non-electrolytic solutes with a sufficiently strong electric dipole, dilution below
, the EDA stabilize CDrot and CDelec. For solutions of non-electrolytic solutes with a sufficiently strong electric dipole, dilution below ![]() also stabilizes CDrot (Del Giudice et al. 1988; Del Giudice and Vitiello, 2006). These CDrot can stabilize CDelec. These domains underlie these liquids’ physicochemical properties (see main text).
also stabilizes CDrot (Del Giudice et al. 1988; Del Giudice and Vitiello, 2006). These CDrot can stabilize CDelec. These domains underlie these liquids’ physicochemical properties (see main text).
The EZ phenomenology of water in the presence of a Nafion film is the formation of a hundreds of micron wide interfacial water zone. The zone excludes particles such as: medium sized molecules (dyes, acid-base indicators), large molecules (proteins), bacteria, positively or negatively charged colloidal microspheres with diameters of 0.5 – 2.0 µm. Accordingly, it has been denoted Exclusion Zone (EZ). Such a zone forms adjacent to all hydrophilic membranes, as first demonstrated by the group of Pollack (Zheng et al., 2006).
The EZ phenomenology is not explainable with the customary models of aqueous systems. Molecular dynamics simulations solely including electrostatic interactions have been carried out in tandem with experiments. The simulations show H2O ordering due to their polarization by polar surface groups occurs only up to few nm from hydrophilic surfaces (Buch et al., 2007; Noguchi et al., 2008). EM phenomena pertaining to EZ water entail that modeling effects of interfaces on H2O ordering warrants inclusion of electrodynamic interactions. Examples of EM phenomena of EZ water include: widening of the zone on irradiation and its narrowing on onset of darkness (Chai et al. 2009). These phenomena are not attributable to temperature changes. Del Giudice et al. (2013) and Yinnon et al. (2016), by employing the QED model of aqueous systems succeeded to explain many physical and structural properties of EZ water. They showed that interactions between H2O and a Nafion film (or any other hydrophilic membrane) may stabilize CDrot and CDelec, i.e., the EZ consists of these domains.
In summary, EZ water and SDVSPL are systems wherein electrodynamic forces play major roles. Therefore, electrodynamic models are required for their description. In both these systems CDrot and CDelec are stabilized. Albeit, the mechanisms by which these domains are stabilized differ.
Reviewer: Please, clarify a little more how and if the coherent domains coexist with un-organized (bulk) water.
Author: The energy a H2O gains on its inclusion in ![]() is about 0.17 eV at a temperature of 273 K and pressure of 1Atm (Bono et al., 2012). Chemical potentials determine the fraction of H2O included within
is about 0.17 eV at a temperature of 273 K and pressure of 1Atm (Bono et al., 2012). Chemical potentials determine the fraction of H2O included within ![]() . In bulk water at T=298 K and pressure of 1Atm, this fraction is about 20 percent. The fraction decreases with increasing temperatures. H2O, which are not included in
. In bulk water at T=298 K and pressure of 1Atm, this fraction is about 20 percent. The fraction decreases with increasing temperatures. H2O, which are not included in![]() , move randomly in between
, move randomly in between ![]() or evaporate. Continually some of the random moving H2O adsorb on
or evaporate. Continually some of the random moving H2O adsorb on ![]() , while simultaneously others desorb from these domains. These adsorption and desorption process cause a ~10-14 s timescale flickering landscape. In other words,
, while simultaneously others desorb from these domains. These adsorption and desorption process cause a ~10-14 s timescale flickering landscape. In other words, ![]() are meta-stable. Hence, observation of
are meta-stable. Hence, observation of ![]() requires fast resolution probes.
requires fast resolution probes. ![]() agglomerate in supra-domains. According to the ab initio derived QED model of water, the meta-stable supra-
agglomerate in supra-domains. According to the ab initio derived QED model of water, the meta-stable supra-![]() constitute the purely empirically-only based hydrogen-bond network of water (Arani et al., 1995). H2O are tetrahedrally ordered in
constitute the purely empirically-only based hydrogen-bond network of water (Arani et al., 1995). H2O are tetrahedrally ordered in ![]() . The density of a
. The density of a ![]() equals 0.92 g/cm3. The presence of
equals 0.92 g/cm3. The presence of ![]() and randomly moving H2O in bulk water means that water consists of two phases. The phase consisting of
and randomly moving H2O in bulk water means that water consists of two phases. The phase consisting of ![]() constitutes the low density phase.
constitutes the low density phase.
Analyses of vast amounts of recent experimental data indicate that bulk water consists of two phases – a high and a low density phase (Nilsson and Pettersson, 2015). However, hitherto, no widely accepted explanations for the molecular orderings in the phases exist. Analyses of the recent data within the context of the QED approach are called for.
The energy a H2O gains on its inclusion in CDrot is ~0.025 eV (Del Giudice et al., 2013). For bulk water at ambient conditions kBT is of this order. Therefore, thermal aggression prevents auto-organization of CDrot in bulk water. However as noted in the main text, during preparation of an aqueous SDVSPL, CDrot may stabilize. On continuing serial diluting combined with vigorous shaking of aqueous SDVSPL containing CDrot, after the Avogadro limit is crossed, the liquid can be considered as bulk water containing CDrot. Continually, H2O adsorb and desorb from these CDrot. Moreover, these CDrot agglomerate into supra-domains. The CDrot may organize into supra- CDrot with their electric dipoles aligned parallel, anti-parallel or oriented in varying directions. Stabilization of CDrot, the adsorption and desorption of H2O of these domains, and their agglomeration into supra- CDrot are very slow processes. Electric conductivity measurements have shown that these processes last at least months or several years (Elia et al., 2008b; Yinnon and Elia, 2013). Supra-CDrot may stabilize supra-![]() . The latter are encapsulated in the former.
. The latter are encapsulated in the former.
Reviewer: Does the shaking procedure affect the size of the coherent domains?
Author: On shaking 1 liter of SDVSPL for one minute, the energy available for excitation or break-up of aggregates has been estimated to be of the order of 1018 – 1019 eV (Yinnon and Liu, 2015b). Gravimetric data indicate that about 1 to 10 percent of the molecules in SDVSPL, which were 45 times decimally diluted, are organized in aggregates (Elia and Napoli, 2010). This implies that the number of associated molecules in one liter of such liquids is of the order of about 1022 – 1023. A 10-5 – 10-4 m sized CDrot contains about 1015 – 1018 H2O. Accordingly, one liter of aqueous SDVSPL might contain about 104 – 108 CDrot. Thus 1018 – 1019 eV implies 1010 – 1015 eV per aggregate. As noted in the previous paragraphs, the energy for desorption of a H2O from a CDrot is ~0.025 eV, while the energy of desorption of a H2O from ![]() is ~0.17 eV. The aforementioned numerical analysis indicates that vigorous shaking may tear up CDrot, supra- CDrot and
is ~0.17 eV. The aforementioned numerical analysis indicates that vigorous shaking may tear up CDrot, supra- CDrot and ![]() . Thus shaking initially reduces the sizes of these domains. However, the H2O in CDrot are ferroelectrically ordered. Therefore, broken CDrot pieces have electric dipole moments, i.e., are electric dipole aggregates (EDA). These enable stabilization of new CDrot and their agglomeration into supra-CDrot. These domains may stabilize new
. Thus shaking initially reduces the sizes of these domains. However, the H2O in CDrot are ferroelectrically ordered. Therefore, broken CDrot pieces have electric dipole moments, i.e., are electric dipole aggregates (EDA). These enable stabilization of new CDrot and their agglomeration into supra-CDrot. These domains may stabilize new ![]() .
.
Reviewer: Explain the relation between the shaking and coherent domain formation.
Author: Several mechanisms, which are triggered by shaking, may be involved in the stabilization of coherent domains in SDVSPL with concentrations below Ctrh= ![]() . As noted above in the answer to the first question of the reviewer: For aqueous solutions of strong or weak electrolytes, serial diluting up to a transition concentration (
. As noted above in the answer to the first question of the reviewer: For aqueous solutions of strong or weak electrolytes, serial diluting up to a transition concentration (![]() ) leads to formation of IPDplasma. Shaking causes the IPDplasma domains to transform into aggregates with an electric dipole moment (EDAIPDplasma). On diluting the shaken solutions below a critical concentration
) leads to formation of IPDplasma. Shaking causes the IPDplasma domains to transform into aggregates with an electric dipole moment (EDAIPDplasma). On diluting the shaken solutions below a critical concentration ![]() , the EDAIPDplasma stabilize CDrot. The physics underlying entities with a sufficiently strong electric dipole to stabilize CDrot has been described by Del Giudice et al., (1988) and Del Giudice and Vitiello (2006). For non-electrolytes, it is not yet known if these can organize into IPDplasma. However, for solutions of non-electrolytic solutes with a sufficiently strong electric dipole, dilution below
, the EDAIPDplasma stabilize CDrot. The physics underlying entities with a sufficiently strong electric dipole to stabilize CDrot has been described by Del Giudice et al., (1988) and Del Giudice and Vitiello (2006). For non-electrolytes, it is not yet known if these can organize into IPDplasma. However, for solutions of non-electrolytic solutes with a sufficiently strong electric dipole, dilution below ![]() also stabilizes CDrot. No shaking is required for stabilization of CDrot in solutions of electrolytes or non-electrolytes with a sufficiently strong electric dipole when these are diluted below
also stabilizes CDrot. No shaking is required for stabilization of CDrot in solutions of electrolytes or non-electrolytes with a sufficiently strong electric dipole when these are diluted below ![]() . However, when SDVSPL containing CDrot are diluted below
. However, when SDVSPL containing CDrot are diluted below ![]() without shaking, the concentration of CDrot diminishes. After many dilution steps, no CDrot will be present. Rather, for CDrot to persist in SDVSPL with concentrations far below
without shaking, the concentration of CDrot diminishes. After many dilution steps, no CDrot will be present. Rather, for CDrot to persist in SDVSPL with concentrations far below ![]() or beyond the Avogadro limit, shaking is required. As noted above, shaking tears CDrot. Broken CDrot pieces have electric dipole moments, i.e., are electric dipole aggregates (EDA). These enable stabilization of new CDrot and their agglomeration into supra-CDrot.
or beyond the Avogadro limit, shaking is required. As noted above, shaking tears CDrot. Broken CDrot pieces have electric dipole moments, i.e., are electric dipole aggregates (EDA). These enable stabilization of new CDrot and their agglomeration into supra-CDrot.
