 Equilibrium, Thermodynamic and Kinetic Studies for Removal of Copper (II) from Aqueous Solution by Onion and Garlic Skin
Equilibrium, Thermodynamic and Kinetic Studies for Removal of Copper (II) from Aqueous Solution by Onion and Garlic Skin
Avisha Chowdhury1, Avijit Bhowal2*, and Siddhartha Datta3
1Department of Environmental Science, University of Calcutta, Kolkata 700019, India
2School of Advanced Studies in Industrial Pollution Control Engineering, Jadavpur University, Kolkata 700032, India
3Department of Chemical Engineering, Jadavpur University, Kolkata 700032, India
*Correspondence E-mail: avijit_bh@yahoo.co.in
Key Words: Onion skin, Garlic skin, Copper (II), Biosorption, Remediation
Received December 14th, 2011; Accepted April 25th, 2012; Published October 21st, 2012; Available online November 15th, 2012
Summary
Removal of Cu2+ from aqueous solution by onion skin and garlic skin was studied as a function of various experimental parameters. FTIR analysis and Boehm titration were used to identify and estimate the surface functional groups. Point of zero charge for the onion and garlic skin was between 4.25 to 4.45 and 4.55 to 4.75 respectively. Compared to garlic skin (66.7 mg/g), the maximum adsorption capacity of onion skin (76.9 mg/g) was slightly higher (pH = 5.3, T = 303K). The pseudo second order kinetic model fitted the batch data adequately. The rate constant for onion and garlic scale in the absence of diffusional resistance was estimated to be 0.07 and 0.075 mg/g/min respectively at 303 K. The thermodynamic studies showed that the Cu2+ adsorption on the onion and garlic skin was spontaneous and endothermic.
Article Outline
- Introduction
- Materials and Methods
- Results and Discussion
- Conclusions
- References
- Discussion with Reviewers
Introduction
Heavy metals are known to cause toxicological problems to environmental and human health. Copper present in industrial waste water leads to the pollution and contamination of the environment. Industries like metal cleaning, plating and metal processing, mining, refineries, paper and pulp, fertilizers, wood preservatives, etc. are the main sources of this metal emanation to the environment (Aksu & Kutsal 1999, Abu Al-Rub et al. 2006). Copper toxicity leads to serious health hazards like anemia, diarrhea, convulsions, gastro-intestinal malfunction (Kumar et al. 2006). Furthermore, it degrades the natural soil and water physiology that eventually affects the biota.
Researchers have focused upon a sustainable way of development using techniques that are environment friendly as well as cost-effective and practical. This has directed attention to the use of biological wastes. Several biosorbents such as rice husks (Jaman et al. 2009), wheat straws (Dang et al. 2009), tea factory waste (Wasewar et al. 2008), orange peel (Annadurai et al. 2002), fish scale (Espinosa et al. 2001), castor seed hull (Sen et al. 2010), wheat shell (Basci et al. 2009), coffee husk (Oliveira et al. 2008), maple sawdust (Rahaman & Islam 2009) has been investigated for adsorbing the ion from aqueous solutions. Bhatnagar and Sillanpää (2010) reviewed the use of various agro-based waste materials as low-cost adsorbents.
In this study, the effectiveness of onion skin and garlic skin for removal of Cu2+ from aqueous solutions was investigated. The skin is the dried up outer parchment of onion and garlic and originates as kitchen and agricultural waste. Literature survey indicates that there has been no previous study for removal of metal ions by garlic skin. Kumar and Dara (1981) studied the uptake of various metal ions by polymerized onion skin. Equilibrium and batch studies were undertaken to examine the thermodynamic and kinetic aspects of Cu2+ adsorption by these biosorbents. A method was described to determine the reaction rate constant in the absence of diffusional limitation.
Materials and Methods
Biosorbent Preparation
Onion and garlic skin was obtained from kitchen dumping. The skins were rinsed and washed with warm distilled water to remove dirt. The biosorbents were dried overnight in the hot air oven at around 45ºC. The dried skins were stored in the laboratory desiccators to avoid moisture interruption.
The experimental studies were carried out in duplicate. Point of zero charge (PZC) was determined using the mass titration method described by Valdés et al (2002). Aqueous solutions were prepared of pH 3, 6 and 11. Increasing amount of biosorbents (0.05%, 0.1%, 0.5%, 1.0%, 3.0%, 7.0% and 10.0% w/w) were added in 20 ml of each solution and allowed to equilibrate in a temperature-controlled shaker incubator for 24h in 25ºC and 100 rpm. The equilibrium pH was measured for each solution using a digital pH meter. The pH versus biomass amount curve gave the PZC range.
Fourier transform infrared spectroscopy (FTIR) was used to identify the chemical groups present in the biosorbents. The surface functional groups of both onion and garlic skin was quantified by the method of Boehm titration (Valdés et al. 2002, Goertzen et al. 2010). Solutions of NaHCO3 (0.1M), Na2CO3 (0.05M), NaOH (0.1M ) and HCl (0.1M ) was prepared with deionized water. The biosorbent (1g) were added to a volume of 50 ml of these solutions and shaken at 100 rpm for 24h to reach equilibrium. After filtration, the excess of base or acid was then determined by titration using the NaOH and HCl solutions.
Biosorption Studies
The copper solution was prepared by dissolving its sulfate salt (Merck) in deionized water. Equilibrium sorption studies were performed in 250 ml Erlenmeyer flask in a temperature-controlled shaker incubator at 120 rpm for 24 h. For these studies, skin for onion and garlic (0.2g) was contacted with the desired concentrations of Cu2+ solution at defined initial pH and temperature.
Batch studies were carried out in a vessel of diameter 12 cm with 500 ml of solution and specified amount of biosorbent. A mechanical stirrer (1500 rpm) was used to agitate the system. The concentration of the ions in the solution was measured by Atomic absorption spectrophotometer (Perkin Elmer AAnalyst 200). The amount of metal uptake by the biosorbent was calculated using the following equation:
(1) qt = V(Ci – Ct)/W
where qt (mg/g) and Ct (mg/L) is the amount of metal ion biosorbed per gram of biomass and solution concentration at time t (min), Ci (mg/L) is the initial solution concentration, V (ml) is the volume of metal solution and W (g) is the weight of biosorbent.
Results and Discussion
Characterization of Biosorbent
The infrared (IR) spectrum obtained from FTIR of the onion and garlic skin (Figure 1a and Figure 1b) displayed a number of absorption peaks, indicating the complex nature of the examined biomass. The results revealed biosorbent heterogeneity, evidenced by different characteristic peaks. The infrared absorption wavelengths of each peak and the corresponding functional groups are presented in Table 1a and Table 1b for onion and garlic skin respectively.
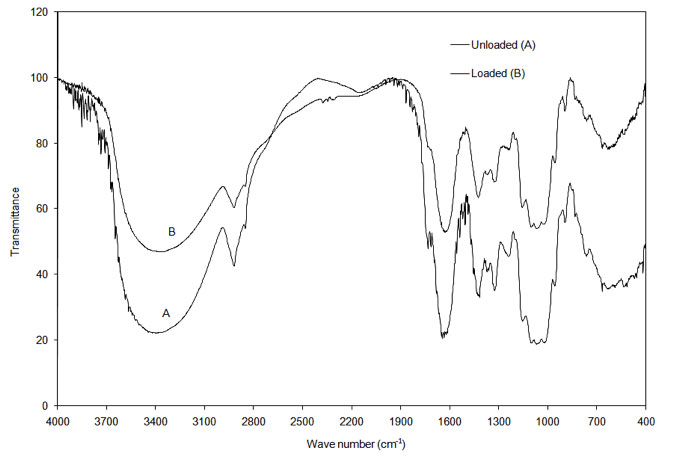
Figure 1a: FTIR analysis of loaded and unloaded onion skin.
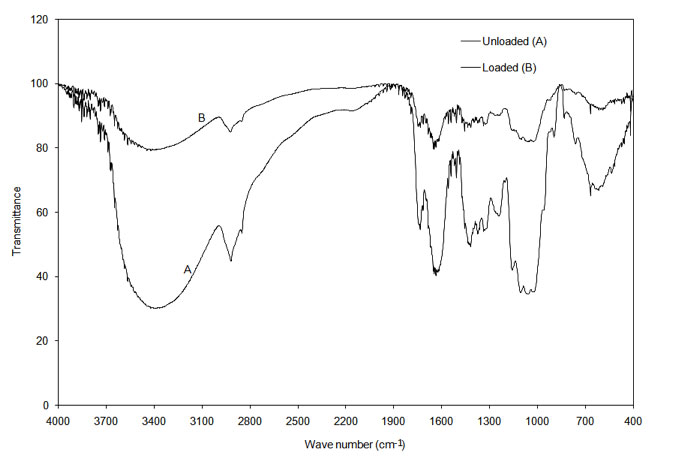
Figure 1b: FTIR analysis of loaded and unloaded garlic skin.
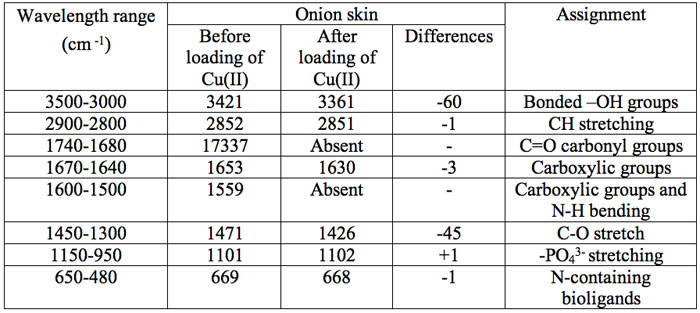
Table 1a: The FTIR spectral characteristics of raw onion skin before and after biosorption of Cu2+.
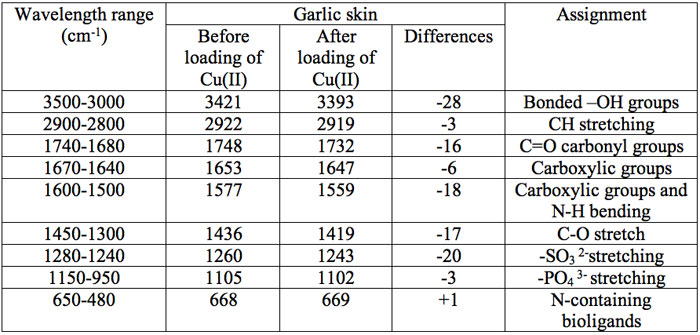
Table 1b: The FTIR spectral characteristics of raw garlic skin before and after biosorption of Cu2+.
To determine the functional groups involved in the adsorption process, FTIR spectra obtained before and after Cu2+ loading were compared. As seen in Table 1a and Table 1b, the major functional groups that took part in adsorption are -OH, C=O, C-O, C-H and -COOH. The functional group -SO32- was additionally involved in adsorption with garlic skin.
Surface functional groups were estimated based on the Boehm titration method and were found predominantly phenolic followed by basic, lactonic and carboxylic groups on both onion and garlic skin. Figure 2 shows the estimated amounts of these groups on both biosorbents. Presence of polyphenolic flavonoid called Quercetin in both onion and garlic attributes to the abundance of the phenolic group on their skin surfaces (Lombard et al. 2005).
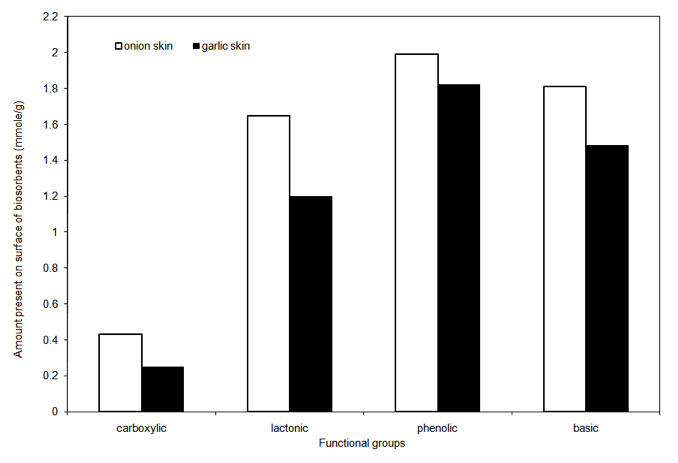
Figure 2: Amount of functional group present on onion and garlic skin.
Effect of pH
The effect of initial pH on adsorption capacity of onion and garlic skin is shown in the Figure 3. Diluted HCl was added to the solutions to get the desired pH values. The uptake increased with change in initial pH from 3 to 5.3 for both the biosorbents. Similar findings were established for several other biosorbents (Abu Al-Rub et al. 2006, Yahaya et al. 2009). Experiments were restricted to pH 5.3 as at higher pH copper precipitates out in the form of insoluble copper hydroxide.
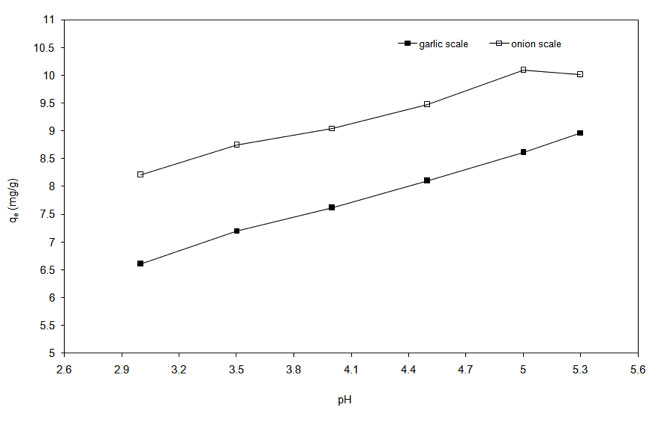
Figure 3: Effect of pH on adsorption process.
An increase in pH results in more negative charges on the biosorbent surface that is likely to attract the positively charged Cu2+ ions to bind to its surface (Wahab et al. 2010) resulting in increased uptake. At low pH, the approach of positively charged Cu2+ cations will be inhibited due to the overall surface positive charge caused by protons that would restrict access to ligands by metal ions as a result of repulsive forces (Hameed & Ahmad 2009, Varshney et al. 2011). It is also likely that these protons will compete with Cu(II) ions for the ligands and thereby decreases the interaction of metal ions. PZC for onion and garlic skin were determined to prove the aforesaid fact. The experimental curves in Figure 4a and Figure 4b show that the PZC values are in the range of 4.55 to 4.75 for garlic skin and 4.25 to 4.45 for onion skin. It clearly interprets that to ensure a completely negatively charged surface on the biosorbents, the pH must be maintained above 4.75 to achieve the maximum adsorption.
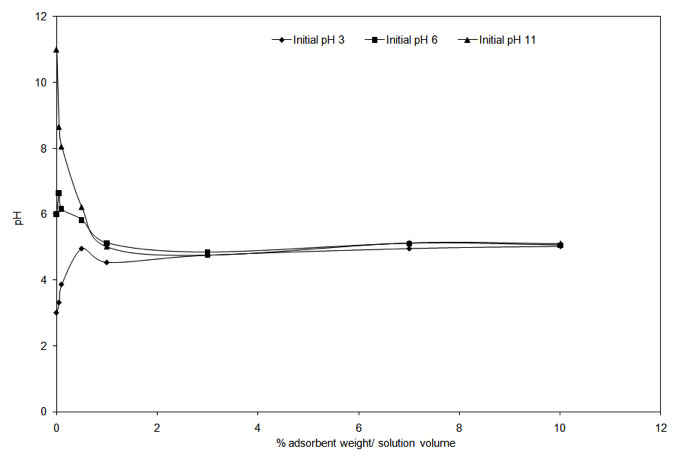
Figure 4a: Experimental curve determining the PZC value for garlic skin.
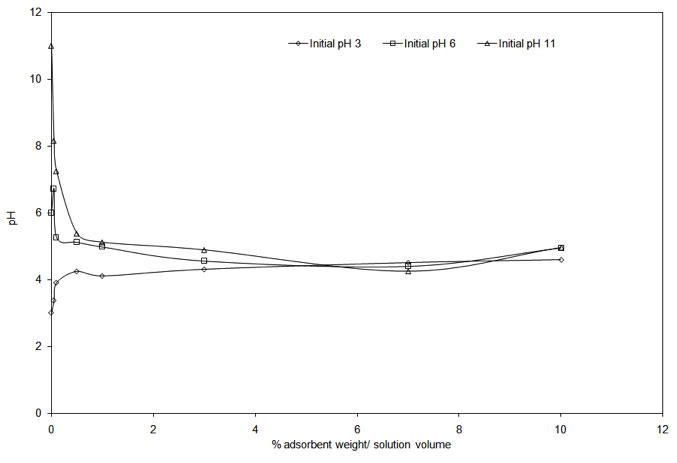
Figure 4b: Experimental curve determining the PZC value for onion skin.
Biosorption Isotherms
The adsorption isotherm for garlic and onion skin at temperatures of 293 K, 303 K and 313 K are shown in Figure 5a and Figure 5b.
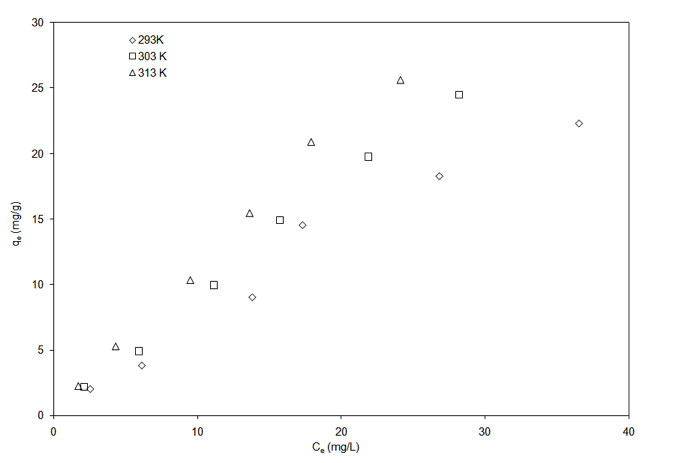
Figure 5a: Adsorption isotherm of Cu2+ onto onion skin (pH = 5.3).
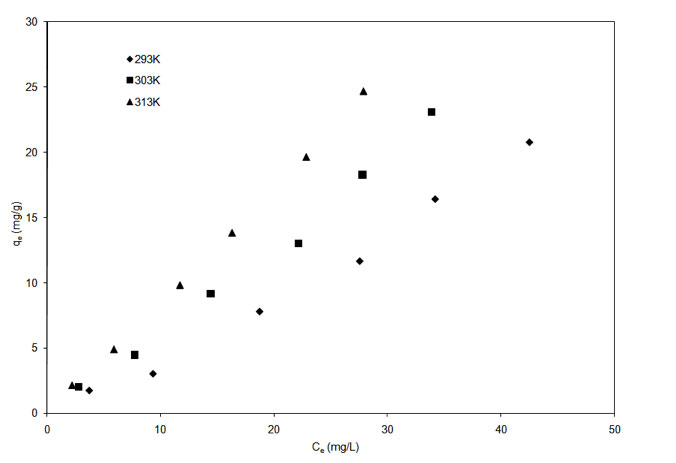
Figure 5b: Adsorption isotherm of Cu2+ onto garlic skin (pH = 5.3).
The Langmuir, Freundlich, Temkin and D-R isotherm models were employed to describe the uptake of Cu2+ by onion and garlic skins.
Langmuir
(2) qe /qmax = KLCe /(1+KLCe)
Freundlich
(3) qe= KFCe1/n
Temkin
(4) ![]()
Dubinin-Radushkevich
(5) ![]()
In the above equations qe (mg/g) and Ce (mg/L) is the amount of the metal adsorbed per unit mass of adsorbent, and equilibrium metal concentration in the solution at equilibrium respectively, qmax (mg/g) is the Langmuir constant related the maximum monolayer adsorption capacity and KL (L/mg) is the Langmuir constant related to the affinity of the binding sites. The terms KF and n in Equation (3) are related to binding energy and adsorption capacity and to the intensity of adsorption respectively. In Equation (4), B = (RT/b) is related to the heat of the adsorption process. A and B are Temkin constants. For D-R model in Equation (5), β is a coefficient related to the mean free energy of adsorption (mol2 J-2), and e = RT ln (1+1 / Ce ) is the Polanyi potential (J mol-1).
The estimated value of these constants obtained by fitting the model expressions to the experimental data along with R2 are listed in Table 2. Out of the 4 isotherm models studied, the higher values of R2 obtained from fitting to Langmuir, Freundlich and D-R indicated that these give good fit to the experimental data over the entire concentration range studied.
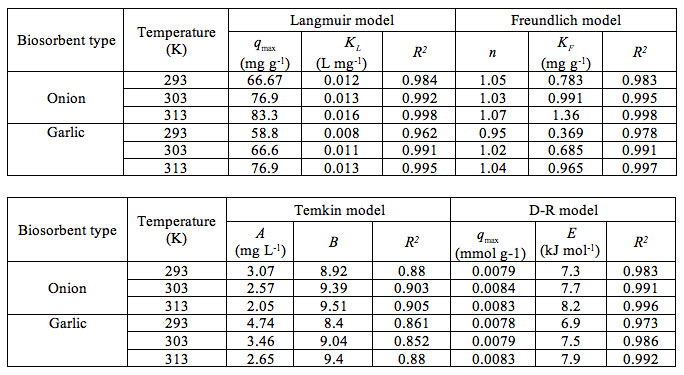
Table 2: Adsorption isotherm constants for onion and garlic skin.
Table 3 compares the values of qmax obtained in this study with that of other biosorbents reported in the literature. It is seen that the adsorption capacity of the skin of onion and garlic was higher than that of many other biosorbents for this metal ion.
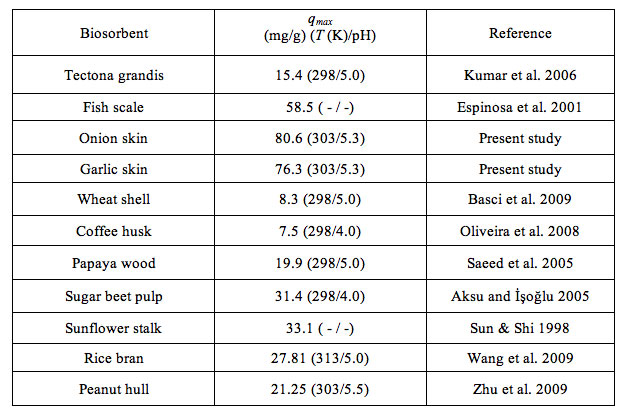
Table 3: Comparison of Langmuir based maximum adsorption capacity of several biosorbents for Cu2+ adsorption.
Biosorption Kinetics Studies
Experiments were carried out to determine the variation of the uptake of the metal ion with time at 303 K with biosorbent amount and initial metal concentration. The results for the different biomass dosage are depicted in Figure 6. These data were used for elucidating the mass transfer rate controlling mechanism and the reaction rate constant.
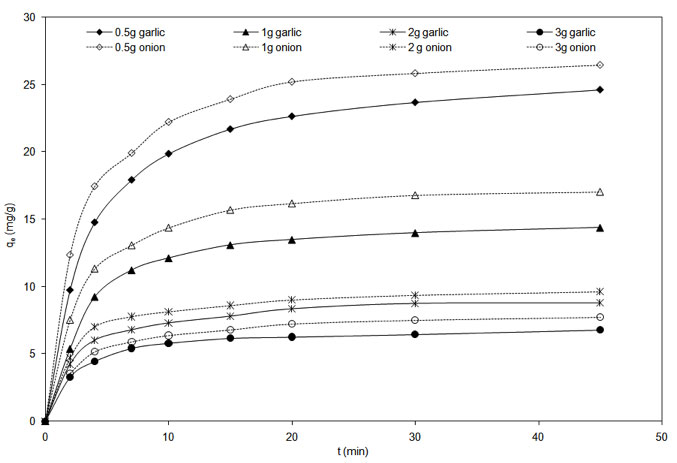
Figure 6: Effect of biosorbent dosage concentration on Cu2+ biosorption (pH = 5.3, Temperature = 303 K).
Intrinsic Rate Constant
The kinetic data for the studied biosorption process was analyzed by fitting pseudo first-order (Lagergren 1898) and pseudo second-order (McKay & Ho 1999) kinetic model to the experimental data. Better fit was obtained with the pseudo second-order model. The linear form of the pseudo second order model expression is:
(6)
where K2 is the rate constant of the pseudo second-order equation.
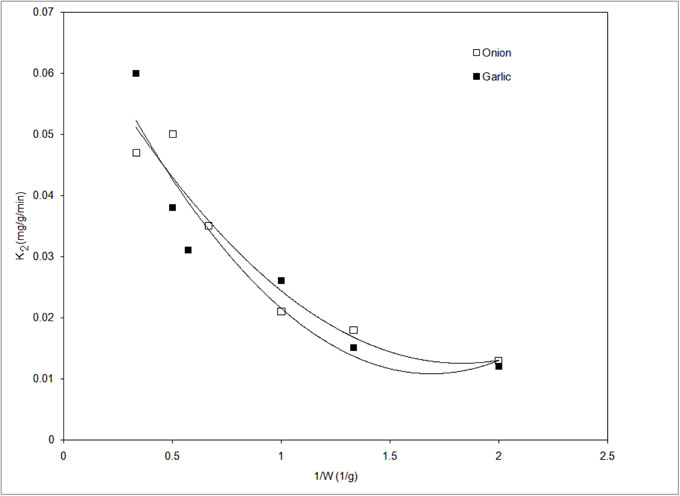
Figure 7: Variation of pseudo second order rate constant with 1/weight of biomass.
The values of the model constants for different operating parameters are shown in Table 4. The value of the experimental equilibrium adsorption (qe)exp was determined by the intercept of adsorption isotherm and the overall mass balance equation. The model parameters of Equation (6) were determined using linear regression technique.
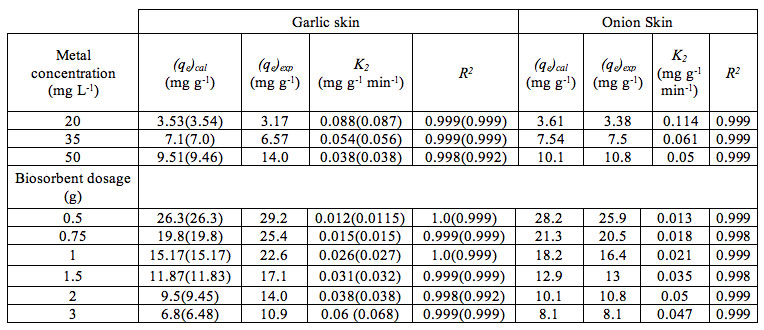
Table 4: Pseudo-second-order Kinetic rate constants related to the biosorption of Cu2+ biosorption onto onion and garlic skin.
Various studies (Chowdhury and Saha (2010, 2011) have drawn attention to the drawbacks of utilizing least square regression technique with the linearly transformed pseudo second order kinetic model for determining the model constants. Non linear regression of the original form of the pseudo second order expression:
(7) 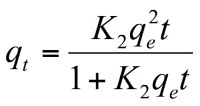
as heat, mass and momentum transfer. The change in the value of K2 with variation of the biomass amount and initial feed concentration suggests that it is not the intrinsic reaction rate. Qualitatively, increase of metal loading in biomass (Figure 6) implies greater penetration of the sorbate into the adsorbent thereby increasing the intra particle mass transfer resistance.
The weight of the biomass gives good representation of the surface area. As W tends to infinity, the product of bulk phase mass transfer coefficient and surface area also tends towards infinite and the diffusional resistance for transport of metal ion from the bulk solution to the biosorbent-solution interface towards zero. Further, the intra particle mass transfer resistance can be neglected as the reaction can be assumed to occur at the surface of the biosorbent itself. The value of K2 in the absence of mass transfer resistances was obtained from the intercept of the curve shown in Fig. 7 on extrapolation to 1/W → 0 (W is infinite). The desired value of K2 for onion and garlic scale are 0.07 mg/g/min (R2 = 0.94) and 0.075 mg/g/min (R2 = 0.89) respectively.
Thermodynamic Parameters
The chemical equilibrium constant of a reversible reaction can be related to temperature by the following expression:
(9) ![]()
where Ka is the equilibrium constant, ∆G0 is the change in standard Gibbs free energy, ∆S0 is the standard entropy change, ∆H0 is the standard enthalpy change, T is the temperature and R is the universal gas constant. The free energy change for the process was determined by using the equilibrium constants obtained from Langmuir isotherm model. Thermodynamic parameters of ∆H0 and ∆S0 were obtained from the slope and intercept of the plot between ln Ka versus 1/T (Figure 8).
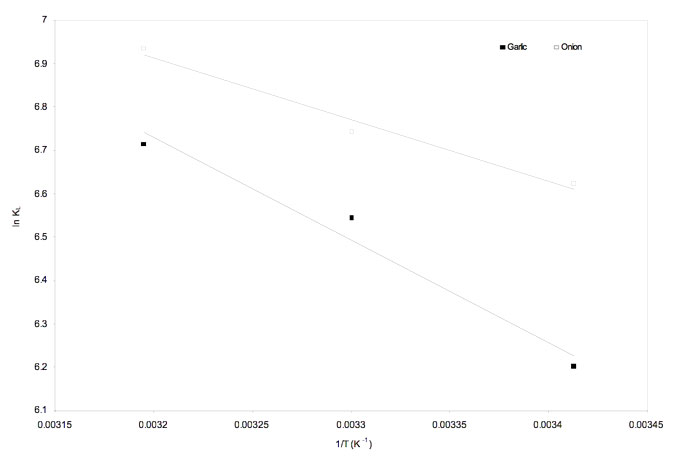
Figure 8: Plot of ln KL versus 1/T for the estimation of thermodynamic parameters.
The negative values of Gibbs-free energy (~ -16.0 kJ/mol) indicated the feasibility of the process. Standard enthalpy change for onion and garlic skin was estimated to be 11.8 kJ/mol and 19.6 kJ/mol respectively. The corresponding standard change in entropy was 0.095 kJ/mol/K and 0.12 kJ/mol/K respectively. The positive values of ∆H0 for Cu2+ removal indicated that the metal adsorption process was endothermic in nature. The positive values of ∆S0 for Cu2+ with both biosorbent showed an increased randomness at solid solutions interface during the adsorption of Cu2+ on the waste biomass (Iftikhara et al. 2009, Özer et al. 2004).
Conclusions
The study showed that both onion and garlic skin are efficient adsorbents for removal of Cu2+ from the aqueous stream. The high adsorptive capacity of these biosorbents implies that it can be looked as an alternative to costly adsorbents like activated carbon, resins, etc. The proposed new technique for estimation of reaction rate constant in the absence of mass transfer resistances provides a means of delineating the influence of reaction kinetics and diffusional resistances on the uptake rate and result in improving contactor design.
References
Abu Al-Rub FA, El-Naas MH, Ashour I, Al-Marzouqi M (2006). Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Proc. Biochem. 41: 457–464.
Aksu Z, İşoğlu İA (2005). Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Proc. Biochem. 40: 3031–3044.
Aksu Z, Kutsal T (1999). Determination of kinetic parameters in the biosorption of copper(II) on Cladophora sp., in a packed bed column reactor. Proc. Biochem. 33: 1-3.
Annadurai JFA, Juang RS, Lee DJ (2002). Adsorption of heavy metals from water using banana and orange peels. Wat. Sci. Technol. 47: 185–190.
Basci N, Kocadagistan E, Kocadagistan B. (2004) Biosorption of copper (II) from aqueous solutions by wheat shell. Desalination 164: 135-140.
Bhatnagar A, Sillpanää M (2010). Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 157: 277-296.
Chowdhury S, Saha P (2010). Biosorption of Methylene Blue onto Tamarind Fruit Shell: Comparison of Linear and Nonlinear Methods. Bioremediation J. 14: 196–207.
Chowdhury S, Saha P. (2011) Adsorption Kinetic Modeling of Safranin onto Rice Husk Biomatrix Using Pseudo-first- and Pseudosecond-order Kinetic Models: Comparison of Linear and Non-linear Methods. Clean 39: 274-282.
Dang VBH, Doan HD, Dang-Vu T, Lohi A (2009). Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 100: 211-219.
Espinosa V, Esparza MH, Ruiz-Treviňo FA (2001). Adsorptive properties of fish scales of Oreochromis Niloticus (Mojarra Tilapia) for metallic ion removal from waste water. Ind. Eng. Chem. Res. 40: 3563-3569.
Goertzen SL, Thériault KD, Oickle AM, Tarasuk AC, Andreas HA (2010). Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 48: 1252 –1261.
Hameed BH, Ahmad AA (2009). Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 164: 870–875.
Iftikhara AR, Bhattia HN, Hanifa MA, Nadeema R (2009). Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. J. Hazard. Mater. 161: 941–947.
Jaman H, Chakraborty D, Saha P (2009). A Study of the Thermodynamics and Kinetics of Copper Adsorption Using Chemically Modified Rice Husk. Clean. 37: 704-711.
Kumar P, Dara SS (1981). Binding metal ions with polymerized onion skin. J. Poly. Sci. 19: 397-402.
Kumar YP, King P, Prasad VSRK (2006). Equilibrium and kinetic studies for the biosorption system of copper(II) ion from aqueous solution using Tectona grandis L.f. leaves powder. J. Hazard. Mater. B137: 1211–1217.
Lagergren S (1898). Zur theorie der sogenannten adsorption geloester stöffe, Kungliga Svenska Vetenskapsakad, Handl. 24: 1–39.
Lombard K et al (2005). Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Comp. Anal. , 18: 571–581.
McKay G, Ho YS (1999). Pseudo-second order model for sorption processes. Proc. Biochem. 34: 451–465.
Oliveira WE, Franca AS, Oliveira LS, Rocha SD (2008). Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 152: 1073–1081.
Özer A, Özer D, Özer A (2004). The adsorption of copper(II) ions on to dehydrated wheat bran (DWB):determination of the equilibrium and thermodynamic parameters. Proc. Biochem. 39: 2183–2191.
Rahman MS, Islam MR (2009). Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. J. Chem. Eng. 149: 273–280.
Saeed A, Akhter MW, Iqbal M (2005). Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep. Purif. Technol. 45: 25–31.
Sen TK, Mohammod M, Maitra S, Dutta BK (2010). Removal of Cadmium from Aqueous Solution Using Castor Seed Hull: A Kinetic and Equilibrium Study. Clean 38: 850–858.
Sun GG, Shi W (1998). Sunflower stalks as adsorbents for the removal of metal ions from wastewater. Ind. Eng. Chem. Res. 37: 1324–1328.
Valdés H, Sánchez-Polo M, Rivera-Utrilla J, and Zaror CA (2002). Effect of ozone treatment on surface properties of activated carbon. Langmuir 18: 2111–2116.
Varshney R, Bhadauria S, Gaur MS (2011). Biosorption of Copper (II) from electroplating wastewaters by Aspergillus terreus and its kinetics studies. Water 2: 142-151.
Wahab MA, Jellali S, Jedidi N (2010). Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 101: 5070–5075.
Wang X, Li ZZ, Sun C (2009). A comparative study of removal of Cu(II) from aqueous solutions by locally low-cost materials: marine macroalgae and agricultural by product. Desalination 25: 146–159.
Wasewar KL, Atif M, Prasad B, Mishra IM (2008). Adsorption of Zinc using Tea Factory Waste: Kinetics, Equilibrium and Thermodynamics. Clean 36: 320 – 329.
Yahaya YA, Don MM, Bhatia S (2009). Biosorption of copper(II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: Equilibrium and kinetic studies. J. Hazard. Mater. 161: 189–195.
Zhu C-S, Wang Li-P, Chen W-B (2009). Removal of Cu(II) from aqueous solution by agricultural by-product: Peanut hull. J. Hazard. Mater. 168: 739–746.
Discussion with Reviewers
Anonymous Reviewer: Fig. 2 shows the number of adsorption sites (carboxylic and phenolic groups) in mmole/g. Can the authors give an estimate of the spatial separation between the sites in the wet adsorbant from these measurements?
A. Chowdhury, A. Bhowal and S. Datta: The equilibrium uptake results indicated that all the adsorption sites were not occupied. This is one of the reasons which would limit the estimation of spatial separation between the sites.
Reviewer: Four thermodynamic adsorp-tion models are numerically analyzed, but what do they show qualitatively about the molecular adsorption process, for example, if the Freundlich model is a better fit than others, can we rule some putative adsorption steps in or out?
Chowdhury, Bhowal and Datta: In the present study we have found that Freundlich isotherm model yielded best fit with the highest R2 values at majority of the temperatures compared to other models. This concludes:
• Heterogenous surface of the adsorbents (onion and garlic skin)
• Non-uniform distribution of heat of adsorption
The Temkin model did not give a good fit concluding that the present adsorption process is not characterized by uniform distribution of binding energy.
Reviewer: The article is appearing in Water Journal, so it is relevant to mention the role of water in the investigations. How many water molecules are accepted to be in the hydration shell of Cu, and does the heat of desorption from the ion fall into the same range (10-20 kJ/mol) as the adsorption values calculated from Fig 9?
Chowdhury, Bhowal and Datta: Cu2+ ion in aqueous solution exists as Cu (H20)42+. (Chemical Principles in the Laboratory by Emil J. Slowinski, Wayne C. Wolsey, William L. Masterton, Brookes/Cole, 2005). The hydration enthalpy of Cu2+ is -2100 kJmol-1 (Electronic Structure and Solvation of Copper and Silver Ions: A Theoretical Picture of a Model Aqueous Redox Reaction, J. Blumberger, L. Bernasconi, R. Vuilleumier, I. Tavernelli, M. Sprik, J. Am. Chem. Soc. 126 (2004) 3928-3938). Therefore, the heat of desorption of water from the Cu2+ is far higher than the heat of adsorption of the metal ion to the biosorbent (which is also endothermic).
