 The Manipulation of Water with Non-Ablation Radiofrequency Energy: A Repetitive Molecular Energy Conversion Loop under Non-Ionizing Electromagnetic Forces
The Manipulation of Water with Non-Ablation Radiofrequency Energy: A Repetitive Molecular Energy Conversion Loop under Non-Ionizing Electromagnetic Forces
McRury ID1, Morgan RE1, Augé II WK1,*
1Department of Research and Development, NuOrtho Surgical, Inc. at the Advanced Technology & Manufacturing Center, University of Massachusetts Dartmouth, Fall River, MA 02723
*Correspondence: Tel: 617-848-8999; Email: wauge@nuorthosurgical.com;
Key Words: radiofrequency, non-ablation, water splitting, arthroscopy, fuel cell
Received 14 June 2010; accepted 10 November; Published 27 November 2010; available online 27 November 2010
doi:10.14294/WATER.2010.8
Summary
The effects produced by surgical devices that deploy an electrical circuit between electrodes are dependent on the nature of electrical work performed upon the conductive media in and around biologic tissues. Because this conductive media is water-based, this study characterizes the effects that non-ablation radiofrequency energy exerts upon saline interfacing media typically encountered during surgical applications. Non-ablation radiofrequency surgical devices were deployed in a bulk 0.9% sodium chloride solution at 300 mOsm/L at 20oC. During energy delivery, temperature and pH changes; gaseous species production, gas condensation behavior, and gas generation dynamics; and ionized charged particle generation were measured in the region of a constrained primary reaction zone surrounding an active electrode. Saline temperature change demonstrated three functional domains commensurate with a decrease in pH at steady-state at the constrained primary reaction zone without changes to the bulk fluid. Gas chromatography, thermal conductivity detector, and flame ionization detection evaluations measured a uniform 2:1 ratio of hydrogen and oxygen comingled non-condensable gas production indicative of split water without heat transfer or gas generation dynamics of water vapor. The presence of ionized charged particles was not detected. These results allowed formulation of a stoichiometric model depicting a repetitive molecular energy conversion loop from water under non-ionizing electromagnetic forces. Non-ablation radiofrequency applications utilize the energy from the molecular bonds of interfacing media water to perform surgical work without delivering ionizing electromagnetic radiation.
Article Outline
Introduction
Methods and Materials
Near-Field Characterization
Far-Field Characterization
Treatment Site Characterization
Discussion
Conclusions and Clinical Relevance
References
Discussion with Reviewers
Introduction
Surgical devices that deploy an electrical circuit between electrodes do so in an electrically conductive medium, which may be either in vivo biologic tissues or delivered media such as electrolyte solutions (Edwards et al, 2008; Jossinet, 2008). The tissue effects produced by these devices are dependent upon the events occurring at or around the electrodes as electrical energy is converted to therapeutically useful forms. Converted energy forms can be either near-field at the electrode surface or far-field projected away from the electrodes. Near-field effects are produced by electrical current and include physiochemical events like electrothermal and electrochemical conversions; far-field effects are produced by electromagnetic radiation forces like magnetic flux densities, voltage potentials, or displacement currents generated around the electrodes. Gross electrical conduction in biological tissues is principally due to the conductivity of in situ interstitial fluids which are electrolyte water-based and thus predominantly ionic (Jossinet, 2008). Since the electrical charge carriers in metal electrodes are primarily electrons, the transition between electronic and ionic conduction is governed by physiochemical processes at the electrode-to-water interface within the conductive media (Edwards et al, 2008; Matyushov, 2009; Mayer et al, 1992; Soderberg et al, 2006), even though this process can be altered by electrode contact with macromolecular biologic material (Zheng et al, 2000). Electrically conductive solutions have been used for many decades to complete surgical device circuits and no longer alone serve as a proprietary method of circuit completion (Elässer and Roos, 1976). Water is the common operational media for both direct current and alternating current formulations that have been deployed in surgical device designs.
Surgical use of direct current induces tissue necrosis as a means to destroy unwanted tissue through near-field electrical current effects delivered into biologic structures (Baxter et al, 1998; Gravante et al, 2009). Electrolytic ablation, or tissue electrolysis, is a technique which consists of placing an anode electrode and cathode electrode at various points within or adjacent to tissue and driving direct current (40-100 mA) between them and through the biologic mass to induce tissue electrolysis. The products of tissue electrolysis kill cells by creating, in a spherical area surrounding the each electrode, local changes within tissue pH too large for cells to survive. These pH changes are created by toxic products such as chlorine, oxygen, and hydrogen ions at the anode electrode and hydrogen gas and sodium hydroxide at the cathode electrode. The region surrounding the anode becomes very acidic (~ pH 2) and surrounding the cathode becomes strongly alkaline (~ pH 12) with the amount of necrosis dependent upon the total electrolysis dose measured in coulombs as a product of tissue current delivery and time. A pH less than 6.0 at the anode and greater than 9.0 at the cathode reflects total cellular necrosis. Direct current applications deliver static electromagnetic fields that have inconsequential energy quanta in the region of non-necrotic tissue (Okafur et al, 2009; van Rongen et al, 2007; Yamashita et al, 2003). Electrolytic ablation does not rely upon a thermal effect as tissue temperatures rise minimally during these procedures to levels not associated with cell death (Baxter et al, 1998; Wemyss-Holden et al, 2004).
Surgical use of alternating current has been designed to induce therapeutic necrosis for volumetric tissue removal, coagulation, or dissection through near-field electrical current effects within biologic tissues. Radiofrequency wavelengths and frequencies do not directly stimulate nerve or muscle tissue; and, so are prevalent in medical applications (Paniagua et al, 2009). Radiofrequency surgical devices utilize tissue as the primary medium like in direct current applications; however, these surgical devices produce resistive tissue heating (ohmic or Joule heating) by an alternating current induced increase in molecular kinetic or vibrational energy to create thermal necrosis (Foster and Glaser, 2007; Haines, 2004; Nath et al, 1994). In order to obtain the desired levels of thermal necrosis through resistive heating in a media with the exceptionally large specific heat capacity of water found in and around biologic tissues, high-levels of alternating current deposition are required to maintain heat production and conduction to remote tissue in the presence of treatment site thermal convection (Schramm et al, 2006). In certain settings, high-level energy radiofrequency devices can be configured to produce water vapor preferentially through very rapid and intense resistive heating, overcoming the high heat of vaporization at the treatment site (Floume et al, 2010; Thompson et al, 2009; Wood et al, 2005). Coincident with this method, the far-field time-varying electromagnetic forces of these devices deliver energy quanta able to generate charged plasma particles within the water vapor cloud (Priglinger et al, 2007; Stadler et al, 2001; Graham and Stadler, 2007). This ionizing electromagnetic radiation can induce an electron cascade, which operates over very short distances (Debye sphere) and with electron temperatures of several thousand degrees Celsius, to produce therapeutic molecular disintegration of biologic tissues as its action decays into heat. Radiofrequency thermal ablation and plasma-based techniques display use limitations associated with their design. Thermal and plasma lesions spread according to induced gradients; but, because of the variable energy transfer coefficients in the treatment settings of biologic tissues, iatrogenic tissue charring, necrosis, and collateral damage from imprecise heating or excess energy deposition can occur (Palanker et al, 2008; Shrivastava and Vaughn, 2009).
Electrolytic ablation, radiofrequency thermal ablation, and radiofrequency plasma-based surgical devices are designed for a direct electrode-to-tissue interface, concentrating near-field electrical energy to perform surgical work centered upon therapeutic necrosis. Collateral damage is a normal procedural consequence since the application locales to which these devices are deployed can often accommodate an excess or imprecise application of energy to ensure expedient procedural efficacy within varying treatment site conditions (Palanker et al, 2008). From a surgical work energy procurement standpoint, these procedures are defined by an inefficient use of electrical energy due to the excess energy deposition that occurs within biologic tissue producing iatrogenic collateral damage. Far-field electromagnetic forces, although present, are confounded by tissue current deposition or, in the case of plasma-based radiofrequency devices, are of such a high intensity constituting local ionizing electromagnetic radiation. Electrolytic ablation, radiofrequency thermal ablation, and radiofrequency plasma devices all struggle in balancing volumetric tissue removal with healthy tissue loss because of excess collateral energy deposition into tissue.
Newer surgical uses of alternating current include non-ablation radiofrequency systems which deliver low-level energy to tissues through a protective tip architecture that prevents active electrode-to-tissue contact and therefore do not rely upon a direct electrode-to-tissue interface. The devices are deployed in a saline immersion setting with the protected electrode creating a more controlled and directed energy delivery to modify or precondition tissue allowing tissue preservation even during resection or débridement applications. Because the electrodes do not contact tissue during activation, electrical current deposition is concentrated into an interfacing media within the protective housing rather than directly into and through biologic tissue as in ablation-based devices. The protective housing provides the ability to move, manipulate, and segregate the near-field effects both tangentially and perpendicularly to the tissue surface during modification or preconditioning; and, it can serve as a mechanical implement and selective throttling vent/plenum during use. For example, the near-field effects are often configured to match current density dispersion with biologic tissue surfaces in a procedure-specific manner. This design allows more consistent electrical current near-field effects at the electrode surface because the circuit is not required to accommodate widely fluctuating impedance changes that tissue contacting electrodes create (Avitall et al, 1997; Floume et al, 2010; Jossinet and Desseux, 2004; Nath et al, 1994). Accordingly, tissue electrolysis and resistive (ohmic or Joule) tissue heating can be prevented. These devices allow a more efficient surgical work energy procurement as iatrogenic collateral tissue damage is minimized without compromising procedural efficacy. Non-ablation devices can deliver useable far-field electromagnetic forces to surface and subsurface tissues designed to create quantitatively and qualitatively larger strengths in tissue not damaged by excessive current deposition or ionizing electromagnetic radiation. These devices are used to permit normal tissue healing responses during modification and preconditioning through segregated near-field effects, while creating far-field electromagnetic intensities designed to induce tissue healing responses within the preserved tissue not subjected to collateral damage.
Although non-ablation systems have been shown to be very useful in tissue preservation settings, their mechanism of action is less well known. The purpose of this study is to characterize the effects of non-ablative radiofrequency energy deposition upon saline interfacing media typically encountered during surgical applications. Because non-ablation radiofrequency devices do not rely upon a direct electrode-to-tissue interface, this study evaluates both the near-field effects on the saline interfacing medium and the far-field effects which can be delivered into biologic tissue. By characterizing these water-based events, the beneficial surgical outcomes observed with non-ablation radiofrequency energy can be further clarified.
Methods and Materials
Figure 1 depicts a representative non-ablation radiofrequency surgical device exhibiting a protective housing that prevents active electrode-to-tissue contact, ensuring direct energy delivery to the saline interfacing media at the electrode surface. The electrode was comprised of stainless steel (Call et al, 2009; Mayer et al, 1992) containing a small amount of titanium (0.5%) used to stabilize its structure at higher temperatures, to prevent carbide precipitation from the grain boundaries, and to protect the metal from corrosion. The protective housing was comprised of an electrical and thermal insulating ceramic designed to prevent electrode-to-tissue contact and create a constrained primary reaction zone around the surface of the active electrode. The devices were configured in a bipolar fashion by connecting to an electrosurgical generator delivering radiofrequency energy at varying power outputs (0-350W), voltage potentials (0.1-4.5 kV), and frequencies (100kHz-1MHz). A general distinguishing characteristic of non-ablation, when compared to ablation, radiofrequency energy is a low current density bias combined with a high voltage potential bias.
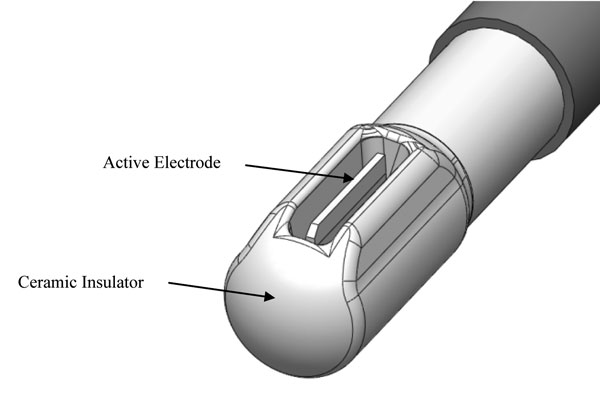
Figure 1: Representative radiofrequency device tip with a protected active electrode designed for non-ablation surgical treatments in a saline immersion setting. The area within the ceramic insulator and around the active electrode is the primary reaction zone wherein the saline interfacing media is worked upon by the radiofrequency energy. Electrical current is delivered to the interfacing media at the electrode surface and the precipitant reaction products can be directionalized by the configuration of the ceramic insulator openings to the treatment site. Note that the active electrode does not protrude from the edge of the ceramic housing.
The devices were tested in the apparatuses depicted in Figures 2-4 with the device tips fully immersed in bulk 0.9% sodium chloride at 300 mOsm/L at 20oC typically used during surgical applications. During testing, the devices were driven to steady-state conditions unless otherwise noted.
Figure 2 apparatus was used to evaluate the near-field effects of non-ablation radiofrequency energy that occur at the active electrode surface within the primary reaction zone of the protective tip housing. Temperature (TrueRMS Supermeter, Newport Electronics, Inc.; Santa Ana, California) and pH (VWR Scientific Products; West Chester, Pennsylvania) changes of the interfacing media were measured in both the primary reaction zone at the protective housing opening and the bulk solution away from the device during probe activation. Produced gas was collected and analyzed by ASTM D-1946 gas chromatography, thermal conductivity detector, and flame ionization detection evaluations (GC/TCD/FID) for constituent species (Air Toxics, LTD; Folsom, CA). A separate glass container of collected gas was allowed to stand at ambient conditions to determine condensation behavior as an additional determinant as to whether water vapor was present.
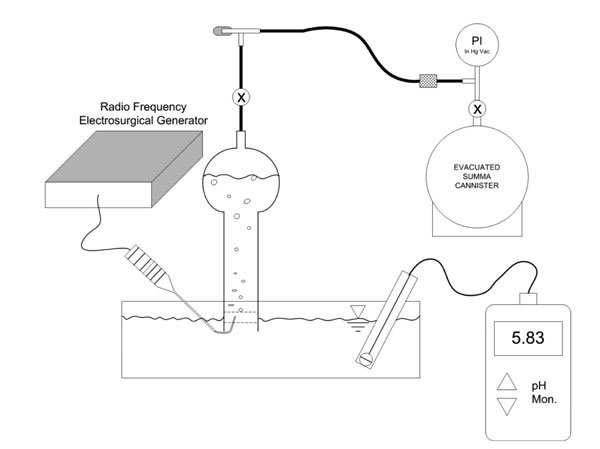
Figure 2: Experimental laboratory set-up designed to evaluate the near-field effects of non-ablation radiofrequency manipulation of saline interfacing media. The pH detector is shown away from the probe’s primary reaction zone for purposes of illustration. The temperature probe is not shown. The temperature and pH of both the primary reaction zone and the bulk solution was measured independently. The gas collection process included an inverted glass collection tube fully filled with the same interfacing media as in the reaction reservoir to create a manometer fluid column that could be displaced by collected gas. Generated gas bubbles were allowed to naturally float into the capture section of collecting tube via buoyancy forces to displace approximately 95% of its total volume. Thereafter, the gas was evacuated from the collection tube by partially opening the stop-cock valve to form a restriction and then sequentially opening the needle valve allowing the gas to fill the summa canister. The combined flow restrictions allowed inlet gas rate metering to avoid unwanted water uptake into the summa canister. The summa canister was allowed to maintain an intact partial vacuum with an attached pressure gauge so that the receiving laboratory could verify whether inadvertent uptake of contaminating atmosphere had occurred during transport.
Gas generation dynamics at the electrode surface were characterized by video assessment and digitized (1188HD 3-Chip camera with SDC digital capture; Stryker Corporation; Kalamazoo, MI) to allow comparison to a control of water vapor bubble production typical of ablation-based radiofrequency devices. Bubble time to release state from the electrode, diameter and volume, shape and conformational fluctuation, coalescent tendencies, directional mass transfer fluid delivery properties, and relative terminal velocity were assessed qualitatively.
Figure 3 apparatus was used to evaluate the far-field effects of non-ablation radiofrequency energy that might occur within the electromagnetic fields generated by the surgical device as a result of the near-field energy conversions. The production of ionizing electromagnetic radiation was monitored using a radiation particle detector in the treatment field sensitive to 200 disintegrations per minute at 1 mm distance from the air-water interface, a distance over which a 0.5 keV particle would be transmitted as the removal of shell electrons emits characteristic energies from a few keV to over 100 keV. This sequential phase interface design allowed particles to be detected if produced in any appreciable quantity above normal background radiation. The device was activated for a continuous 30 minutes.
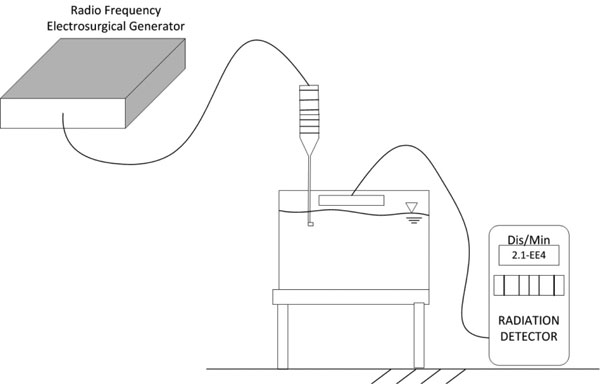
Figure 3: Experimental laboratory set-up designed to determine whether generation of charged particles occurs with non-ablation radiofrequency manipulation of saline interfacing media. The distances between the electrode and the water surface are exaggerated for purposes of illustration.
Figure 4 apparatus was used to time integrate roentgenographic film exposure by ionizing electromagnetic particle generation. The surgical device was fully immersed and placed with the active electrode within 1 mm of the roentgenographic cassette wall of the reservoir and activated for a continuous 30 minutes allowing any ionized reaction zone species to integrate over time and expose the film. A control emitter source of alpha (α) particles and low energy gamma rays of 60 keV, americium-241, was adhesively affixed to the roentgenographic wall with the same spacing of 1mm to demonstrate time dependent control exposure.
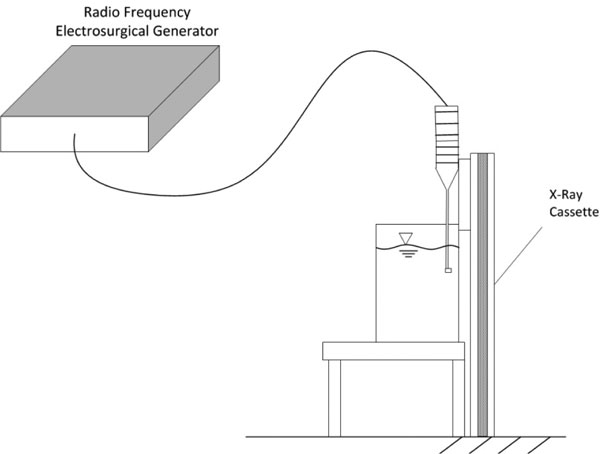
Figure 4: Time integrated experimental laboratory set-up designed to determine whether generation of charged particles occurs with non-ablation radiofrequency manipulation of saline interfacing media. The distances between the electrode and the roentgenographic wall are exaggerated for purposes of illustration. The americium-21 control source is not shown.
General Observations
Two non-ablation radiofrequency energy conversion modes were evident based upon visual cues that can be used to define surgical work on water: one during which the device deploys energy levels that do not produce non-soluble gas; the other during which non-soluble gas is produced. As demonstrated in Figure 5, these modes were part of an observable continuum that was dependent upon power level applied to the interfacing media. In all instances, a steady state was achieved with probe activation by 3 seconds. The threshold for non-soluble gas production detectable by gross visualization was a power delivery of 35W. Voltage and frequency influences on steady state for a given power delivery level did not significantly alter the threshold for gas production within the ranges tested.
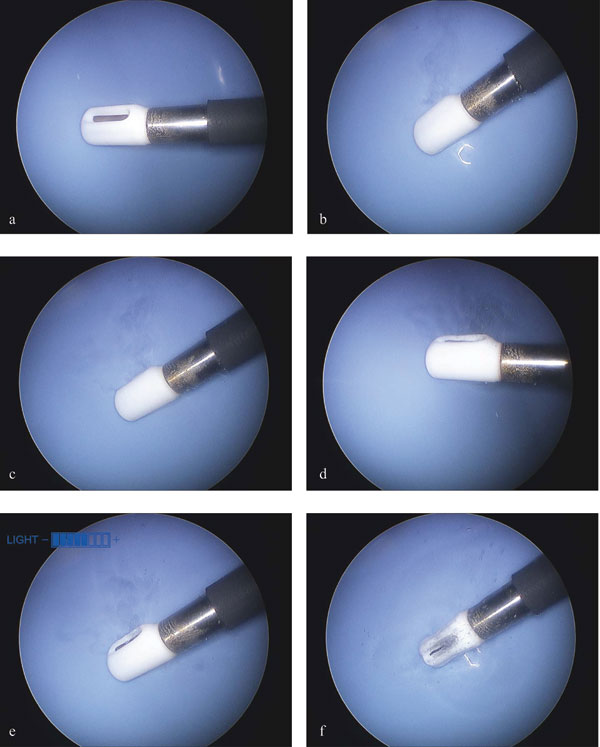
Figure 5: General observations of non-ablation radiofrequency energy manipulation of saline interfacing media. Electrothermal, electrochemical, and gas generation dynamics. Static images taken during videography and digitization. Power delivery: a, 0 Watts; b, 25W; c, 50W; d, 75W; e, 100W; f, 120W. Note that early non-soluble gas (bubble) production does not begin until 35W, after which the non-soluble gas production level remained consistent without overwhelming the dynamics of the primary reaction zone until 75 W when the turbulence and mass effect of the increased gas production facilitated the removal of the reactants/products from the primary reaction zone more dramatically.
Electrothermal Effects
Electrothermal effects of the primary reaction zone are depicted in Figure 6. Temperature at steady-state was generated well below the level at which water vapor could be produced. The thermal gradients migrated from the electrode based upon typical thermodynamic behavior but could be altered by the configuration of the protective housing. The bulk saline bath did not change temperature significantly during the testing with probe activation.
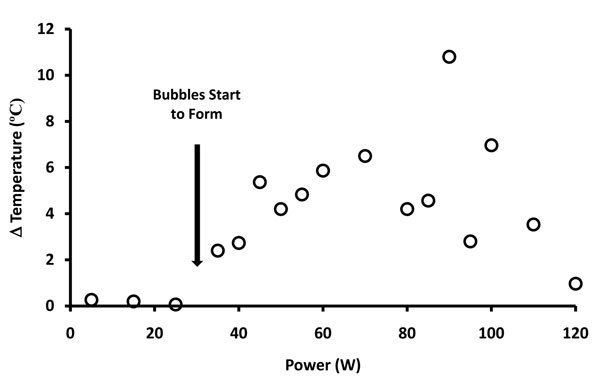
Figure 6: Graphic representation of temperature changes versus power delivery at the primary reaction zone when non-ablation radiofrequency energy is delivered to saline interfacing media. The temperature distribution demonstrated three distinguishable functional domains: the first domain (0-35W) revealed no temperature change associated with the lack of non-soluble gas formation; the second domain (35-75W) revealed a linear relationship of temperature increase during low-level non-soluble gas formation; the third domain (75-120W) revealed a decrease in temperature associated with more pronounced non-soluble gas formation despite the increased power delivery to the primary reaction zone.
Electrochemical Effects
Electrochemical effects of the primary reaction zone are depicted in Figure 7. These effects were evident visually as a pH fluid wave with the acid-base shift migrating based upon typical solution densities, but could be directionalized based upon configuration of the protective housing (see Figure 5 noting the varying probe positions). The pH of the primary reaction zone demonstrated a linear relationship between power delivery and unit pH drop until energy delivery was terminated at which time rapid normalization occurred. The bulk saline bath did not change pH significantly during the testing with probe activation.
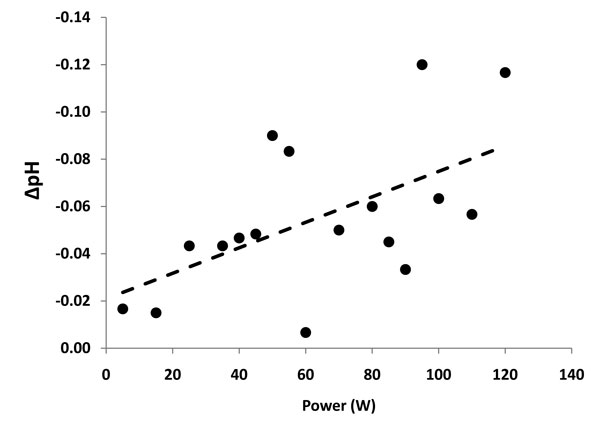
Figure 7: Graphic representation of pH changes versus power delivery at the primary reaction zone when non-ablation radiofrequency energy is delivered to saline interfacing media. R2 = 0.311; p<0.02. Note that the goodness-of-fit linear regression is better for the segment during which low level non-soluble gas formation occurs (35-75W) with increasing scatter as the primary reaction zone turbulence increased.
Collectable Gas Production
Non-soluble gas production correlated with temperature and pH observations. From 0-35W of energy delivery (Phase 1), non-soluble gas was not produced, temperature did not increase, but pH decreased. From 35-75W (Phase 2), non-soluble gas was produced at levels that did not overwhelm the dynamics of the primary reaction zone commensurate with a linear temperature increase and linear pH decrease. From 75-120W (Phase 3), non-soluble gas production increased to a level that overwhelmed the primary reaction zone dynamics and was associated with a decrease in temperature despite the increased energy delivery and a more scattered but linearly decreasing pH.
ASTM D-1946 GC/TCD/FID analysis yielded uniform species results in all instances with a 2:1 ratio of hydrogen and oxygen comingled gas without significant atmospheric contamination or evidence of water vapor. The collected gas was not condensable within the separate glass collection container confirming the ASTM D-1946 GC/TCD/FID analysis lacking water vapor.
Consistent with the constituent make-up of the collected gas, the gas bubble dynamics were different from that of water vapor bubble production used as a control as noted in Figure 8. When compared to water vapor bubble generation, the comingled oxygen and hydrogen gas bubbles reached release state from the electrode very rapidly, were small in size on the order of a 125x smaller volume, remained spherical without confirmation fluctuations typical of the much larger water vapor bubbles, did not coalesce with other bubbles, demonstrated directional mass transfer fluid delivery properties, and displayed a slower terminal velocity. Gas bubble flow dynamics were easily modulated with the protective housing throttling vent/plenum (see also Figure 5).
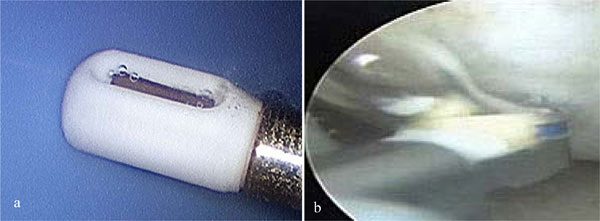
Figure 8: Gas general dynamics of non-ablation (a) versus ablation (b) radiofrequency energy deposition upon saline interfacing media. The ablation electrode is shown at tissue contact during use; whereas the non-ablation electrode is shown without tissue present as it cannot touch tissue during use. The larger bubble in (a) has a diameter of 0.3 mm; the singular bubble in (b) has a diameter of 3.9 mm. Water vapor bubbles (b) typically were larger, with a surface tension, adhesion dependant stalk connecting it to the electrode prior to release.
Far-Field Characterization
Electromagnetic Field Characterization
During operation, particles were not sensed by the radiation particle detector above standard background which averaged approximately 2.5 mSv/yr at the testing locale. After 30 minutes of exposure to both non-ablation radiofrequency energy deposition and americium-241 source, only the americium-241 source area was exposed. The area immediately adjacent to the electrode remained unexposed and clear of any image. Non-ablation radiofrequency energy produced only non-ionizing electromagnetic forces.
Treatment Site Characterization
Stoichiometry
The defined reactants (0.9% sodium chloride aqueous solution, radiofrequency energy) and resultant products (2:1 ratio of H2 and O2 gas, pH drop, heat) present in this study, along with the generation dynamics and lack of ionizing electromagnetic radiation observed, allow formulation of a uniform stoichiometric thermochemical description of non-ablation radiofrequency deposition upon saline interfacing media. This formulation is depicted in Figure 9a-d.
The overall process utilizes alternating current to rapidly split and reconstitute water in a repetitive molecular energy conversion loop. The general, electrothermal, electrochemical, and gas production observations are governed by the relative availability of the reactants and products within the primary reaction zone. The initial splitting of water is slightly endothermic driven by the low current and high activation overpotential of non-ablation radiofrequency energy. In this setting, gas emanation is inefficient as bubble threshold fluencies and bubble lifetime dictate aqueous nano-sized bubble production that are immediately converted back to water. As gas emanation is produced, bubble size remained very small with high release rates; therefore, the electrode-to-water interface surface area was not significantly altered by gas production at any setting thereby limiting significant electrode current density or impedance fluctuations. This phenomenon was further supported by the high voltage potentials delivered which diminish any minimal effect of bubble induced conduction area reduction. As gas emanation occurred and gas was liberated from the primary reaction zone by buoyancy forces, complementary liberation of additional acid-base pairs necessarily occurs, both of which may be modulated by the protective housing throttling vent/plenum.
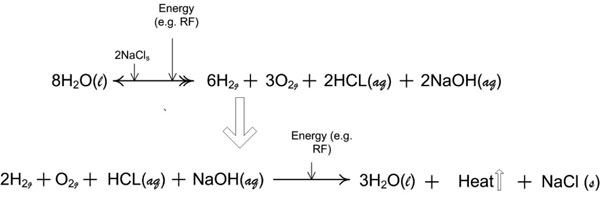
Figure 9a: Reaction stoichiometry of the near-field effects of non-ablation radiofrequency manipulation of saline interfacing media. Two half-reactions of the thermochemical cycle that describe the quantitative relationships between the reactants and products for the repetitive molecular energy conversion loop. [(aq) = aqueous; (g) = gas; (l) = liquid; (s) = solute].
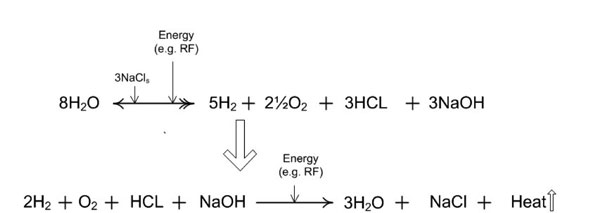
Figure 9b: With loss of reactants or products from the primary reaction zone, such as gas emanation modulated by the protective housing throttling vent/plenum, the electrochemical effects can become more visible. These electrochemical effects are termed an acid-base shift.

Figure 9c: A more general case in which the ionic salt is represented by variable X, where X is any appropriate group 1, period 1-7 element of the periodic table. The salt-bridge catalytic efficiency is dependent upon the salt’s elemental properties. [(aq) = aqueous; (g) = gas; (l) = liquid; (s) = solute].
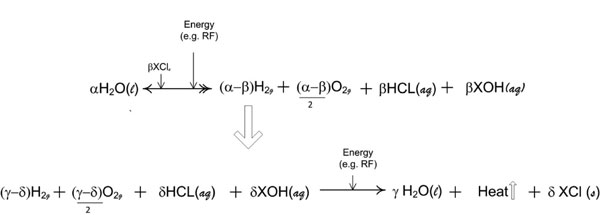
Figure 9d: The repetitive molecular energy conversion loop is demonstrated by variables consisting of α, β, γ and δ wherein, the molar quantities required are any value that appropriately satisfies the oxidation reduction valence requirements for the overall reaction. [(aq) = aqueous; (g) = gas; (l) = liquid; (s) = solute].
Representational Model
Figure 10 illustrates a representational model summarizing non-ablation radiofrequency energy manipulation of saline interfacing media with overlaid equations on the depicted physical flow-field of surgical application. The electrode provides conducted electrical energy to the electrode-water interface through the a salt ion solution whereby water splitting causes the accumulation of oxygen and hydrogen gases immediately about the electrode which rapidly reduce to water and heat. As the reaction takes place, buoyancy forces allow non-soluble gas to escape the primary reaction zone; while acid-base pairs of greater density descend away from the electrode with artifacts visible as density streak-lines. As the acid-base pairs move away from the electrode, cooling takes place which results in a normal precipitation. This reactant-product escape, although modulated by the protective housing, is facilitated by normal fluid flow in the surgical environment that, in addition, simultaneously induces considerable reaction zone quenching while preventing reaction zone water-starvation. Therefore, the repetitive molecular energy conversion loop does not result in any volumetric loading of the primary reaction zone. This reaction is not possible to deploy without the protective housing around the active electrode due to the large fluid flow fields present during surgical application.
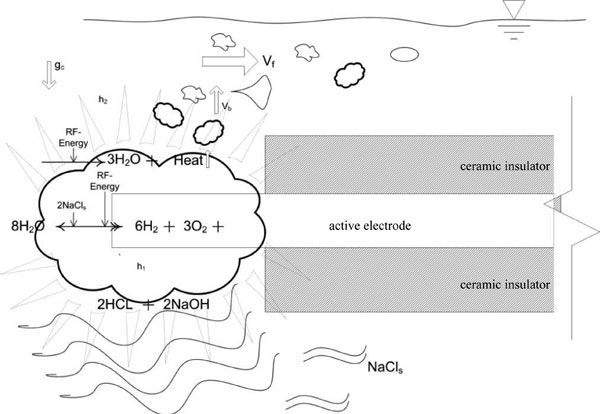
Figure 10: Diagrammatic representation of the manipulation of saline interfacing media by non-ablation radiofrequency energy. Note that the protective housing is not shown for the purposes of illustration. Ablation devices have exposed electrodes making any attempt at low energy physiochemical conversions inconsequential due to the large physical fluid flow and convective forces present during surgical application; hence their design necessitates a large amount of energy delivery. Faded triangles represent electrothermal effects; wavy lines represent electrochemical effects. Vf represents the convective force velocity of the fluid flow outside of the protective housing; Vb represents bubble buoyancy force velocity of non-soluble gas production; gc represents gravitational forces exerted upon the denser acid-base precipitants; h1 represents electrothermal heat within the protective housing; and h2 represents the electrothermal heat that may leave the primary reaction zone.
Phase 1 observations (0-35W, inefficient water splitting, limited water reconstitution)
At this energy input level, alternating current is very inefficient at splitting water and producing non-soluble gas, an endothermic reaction. Non-soluble gas is not produced indicating the reaction zone has yet to reach gas saturation characteristics to generate non-soluble gas production. Therefore, the reconstitution of water, an exothermic reaction, does not occur to a level that would demonstrate a significant increase in temperature at the unconstrained edge of the protective housing. The noted decrease in pH is indicative of water splitting.
Phase 2 observations (35-75W, efficient water splitting and reconstitution)
Increasing alternating current delivery becomes more efficient at splitting water as non-soluble gas is produced consistent with gas saturation characteristics of the primary reaction zone. Therefore, more split water is available for reconstitution, producing an increase in temperature as power increases consistent with the increased frequency of water reconstitution, an exothermic reaction. pH continues to drop consistent with the process of splitting water and reactant/product migration from the primary reaction zone.
Phase 3 observations (75-120W, more efficient water splitting and less efficient water reconstitution)
Further increasing alternating current induces even larger amounts of non-soluble gas production facilitating increased primary reaction zone turbulence and mass transport effect removing reaction reactants/products from the primary reaction zone more rapidly. This non-soluble gas removal and increased acid-base shift decreases the efficiency of water reconstitution which in turn decreases the frequency of exothermic water reconstitution resulting in the noted temperature decrease. pH continues to drop consistent with the process of splitting water and reactant/product migration from the primary reaction zone, although more scattered based upon the altered primary reaction zone dynamics.
Discussion
The results of this study demonstrate that non-ablation radiofrequency energy produces distinct near-field and far-field effects as electrical energy is converted to a therapeutically useful form. Near-field effects to perform surgical work are created by a thermochemical cycle originating directly from the molecular bond energy of water. This electrosurgical refinement creates an energy efficient procurement system that is a sister technology to other methods designed to capture released molecular energy from water like fuel cells, photolysis, and photosynthetic machinery (Lee et al, 2010; McKinlay and Harwood, 2010). Non-ablation surgical devices utilize alternating current to rapidly split and reconstitute water in a repetitive molecular energy conversion loop as a means to modify or precondition biologic tissues. Active electrode current density dispersion is manipulated by the protective housing to limit current delivery into tissues as current can be detrimental through tissue electrolysis and/or resistive (ohmic or Joule) heating. The near-field effects of current are delivered to the tissue surface rather than relying upon an electrode-to-tissue interface as in ablation-based devices designed to eliminate, coagulate, or dissect tissues. Because the near-field effects of current are geographically constrained within the protective housing, these effects can be manipulated based upon procedure-specific needs with the protective housing serving as a mechanical adjunct to and selective throttling vent/plenum for energy and reactant/product delivery. The devices allow far-field electromagnetic forces to manifest within tissue unencumbered by current deposition and which are of intensities that do not create ionizing forces. A differential between current density dispersion and electromagnetic field strength is exploited to allow a normal healing response of tissues in reaction to the near-field treatment effects of tissue modification and preconditioning, while permitting far-field effects designed to induce therapeutic responses in the treated tissues that have been protected from the collateral damage of electrode-to-tissue interfaces.
The application of radiofrequency energy upon an electrically conductive media can follow distinct pathways based upon the nature of electrical work desired (Chelli et al, 2005; England et al, 2008; Graziano, 2006; Iuchi et al, 2007; Tan and Luo, 2007; Vaitheeswaran et al, 2005). These pathways are determined by structural rearrangements of water molecules that are subjected to the radiofrequency energy effects upon the interfacing media molecular dynamics. Whether the interfacing media is in or around biologic tissues, it is governed by hydrogen bond behavior and proton transport (Chen et al, 2010; Eaves et al, 2005; Elsaesser, 2009; Fayer et al, 2009; Livesay et al, 2008; Teixeira, 2009; Wernet et al, 2004) that allow for widely malleable structural fluctuations of liquid water molecules. These fluctuations are due to water’s very dynamic hydrogen bond network which displays the inherent ability to both exhibit simultaneous behavioral states and to rapidly reconfigure to accommodate physiochemical perturbations (Matharoo et al, 2009; Pártay and Jedlovszky, 2005; Poole et al, 1994). With ablation- and plasma-based radiofrequency systems, resistive heating is produced predominantly by molecular kinetic and vibrational motions occurring within and amongst the hydrogen bond network. Rapid and intense resistive heating can produce a phase transition from liquid water to water vapor as vibrational motions further exert a predominate role in the ultrafast loss of liquid water’s structural configuration leading toward phase transition (Fayer et al, 2009; Park et al, 2009; Wernet et al, 2004; Zahn, 2004). This process is energy intensive due the high specific heat capacity and heat of vaporization of water (Raabe and Sadus, 2007). In the presence of charged species like salts, this temperature driven phase transition process from rapid resistive heating at the electrode is slowed by 3-4 times, which further increases the amount of energy required to reach phase transition (Fayer et al, 2009; Nucci and Vanderkooi, 2008). Once phase transition occurs, water vapor and other elements can be ionized by the electromagnetic forces associated with this radiofrequency energy level required to drive the heating process to phase transition (Priglinger et al, 2007; Stadler et al, 2001; Graham and Stadler, 2007).
In contrast, non-ablation radiofrequency energy requirements are low because the requisite energy input is limited to splitting water which then creates a repetitive molecular energy conversion loop that self-fuels due to the exothermic reaction of water reconstitution. Charged species like salts, in distinction to their effect during resistive heating, decrease the system energy requirements because they serve as an energy salt-bridge catalyst facilitating water splitting by forming, breaking, and nucleating hydrogen bonds between acid-base pairs and water molecules (Nahtigal and Svishchev, 2009). As this study demonstrates, water splitting is a low energy initiation process associated with non-ionizing electromagnetic forces. Without the protective housing around the active electrode, this physiochemical process would be rendered inconsequential due to the large fluid flow and convective forces present during surgical application. It is for this reason that ablation-based systems have been designed with ever increasing energy levels and associated ionizing electromagnetic radiation while non-ablation systems have focused upon limiting energy requirements by refining the energy procurement and delivery process to preserve tissue.
The near-field electrothermal effects of non-ablation radiofrequency energy are governed by the nature of electrical work performed upon the intermolecular hydrogen bonds of water-based interfacing media. Energy generation is created by a repetitive molecular energy conversion loop rather than by high energy resistive heating of water. Splitting water is a mildly endothermic reaction that is driven by the low-energy near-field effects of non-ablation current; whereas, reconstitution back to water is exothermic providing assistive energy for further repetitive molecular energy conversion loops ultimately deployed for surgical work. The alternating current allows each electrode to perform each redox half-reaction, but the effects can vary between electrodes because of architectural nuances. The initial reaction activation barrier is the four electron oxidation of water to oxygen during the anode phase of water splitting. This barrier is overcome by increased voltage potentials between the electrodes rather than by increased current so that architectural nuances of the electrodes are primarily due to the magnitude of voltage potential difference rather than current density disparities (Lewis et al, 2010; Rahimi and Mikkelsen, 2010). At the frequencies employed, this process is very inefficient at producing non-soluble gas (Kikuchi et al, 2006; Kikuchi et al, 2009; Yang et al, 2009; Zhang et al, 2006). When non-soluble gas is produced, it is limited to molecular hydrogen and oxygen which is effectively managed by the protective housing throttling vent/plenum. Water vapor is not produced demonstrating the low-level energy deployment well below water’s heat of vaporization. As a corollary, excessive water vapor production during resistive heating has been shown to significantly impair visualization of the ablation treatment site (Varghese et al, 2004).
The near-field electrochemical events of non-ablation radiofrequency energy are also governed by the nature of electrical work performed upon the water-based interfacing media (Hammes-Schiffer, 2009; Saulis et al, 2005). During the repetitive molecular energy conversion loop, alternating current can also facilitate an otherwise inefficient and more complex chemical reaction within the interfacing media rather than simple phase transition to water vapor as in ablation-based devices. The intermediary products and reactants of the repetitive molecular energy conversion loop may combine to create an acid-base shift desirable for therapeutic interventions through techniques such as capacitive deionization and concentration enrichment (Biesheuvel, 2009; Perdue et al, 2009). Because of the protective housing throttling vent/plenum, these products can be delivered in a controlled and localized fashion through precipitation, sedimentation, thermal, or chemical gradient forces into the treatment site through redox magnetohydrodynamic fluid flow (Anderson et al, 2010; Brown et al, 2001; Ramos et al, 2003; Ramos et al, 2007). Much like the electrothermal gradients, these electrochemical modification gradients can be driven toward tissue surfaces. For example, sodium hypochlorite can be precipitated preferentially based upon device design configuration to react with a wide variety of biomolecules including nucleic acids, fatty acid groups, cholesterol, and proteins at tissue surfaces (Schiller et al, 1994). Additionally, pH shifts have been shown to produce tissue surface alterations effecting transport properties and extracellular composition (Loret and Simões, 2010; Wachtel and Maroudas, 1998). Water vapor itself is not a therapeutic product or event, limiting ablation-based devices to thermal interventions.
The far-field effects of non-ablation radiofrequency devices can manifest due to a minimal current density at or within biologic tissues, and hence magnetic field flux densities within the protective housing, and an high voltage potential force resulting in non-ionizing electromagnetic intensities designed for therapeutic use (Weaver, 2002). Not only do these high voltage potentials increase the ability to perform redox reactions in conductive media by facilitating the repetitive molecular energy conversion loop, voltage potentials not coincidentally have been shown to be a principle driver of non-ionizing electromagnetic effects upon biologic tissue (Andocs et al, 2009; Szasz et al, 2009). Because these electromagnetic forces carry energy that can be imparted to biologic tissue with which it interacts, higher voltage potentials enable oxidization or reduction of energetically more demanding tissue constituent macromolecular compounds other than water. These forces are deployed at the protective housing-to-tissue interface, unencumbered by current deposition, typically scaled at 0.1- 1.5 mm distances from the electrode, rather than processes at the electrode-to-tissue interface as in, for example, plasma-based systems where the ionizing electromagnetic radiation generates high energy thermal particles that interact with biologic tissue.
Once non-ionizing electromagnetic fields have been produced from a given charge distribution, other charged objects within the field, such as biologic tissue, will experience a force, creating a dynamic entity that causes other tissue charges and currents to move as their strengths are typically lower (Hart, 2010; Lai et al, 2000; Prezhdo and Pereverzev, 2009). When non-ionizing electromagnetic radiation is incident on biologic tissue, it may produce mild thermal and/or weaker non-thermal field effects. The complex biological consequences of these fields, exerted through such mechanisms as tissue voltage sensor domains (Börjesson and Elinder, 2008; Okamura, 2007), stress response gene expression (Blank and Goodman, 2009; Goodman and Blank, 2002), and direct voltage-to-force energy conversion molecular motors (Bai et al, 2009; Haila et al, 2001; Junge and Nelson, 2005; Kere, 2006), and their therapeutic potential for tissue healing (Blank and Goodman, 2009; Challis, 2005; Funk et al, 2009; Goodman and Blank, 2002; Sheppard et al, 2008) are becoming more fully understood.
Conclusions and Clinical Relevance
Non-ablation radiofrequency surgical devices create a repetitive molecular energy conversion loop for surgical work as determined by reconciling the molecular species present; and, non-ionizing electromagnetic forces are deployed at strength levels that can produce thermal and non-thermal biologic tissue effects as determined by the absence of ionizing species detection by typical measuring means. Non-ablation radiofrequency surgical devices are deployed in an immersion setting utilizing a protective housing that prevents electrode-to-tissue contact facilitating electrodes to be fully wetted by the interfacing media. A differential between current density dispersion and electromagnetic field strength is exploited to allow normal tissue healing responses to the near-field effects of tissue modification and preconditioning while permitting far-field effects, which are useful for inducing therapeutic biologic responses, to manifest in treated tissues that have been protected from electrical current generated collateral damage. The devices provide, based upon procedure-specific needs, the ability to move, manipulate, and segregate near-field effects both tangentially and perpendicularly to the tissue surface; to deliver far-field electromagnetic effects to tissue unencumbered by current deposition; and to serve as a mechanical adjunct to and a selective throttling vent/plenum for energy delivery.
References
Anderson EC, Weston MC, Fritsch I (2010). Investigations of redox magnetohydrodynamic fluid flow at microelectrode arrays using microbeads. Anal Chem 82:2643-2651.
Andocs G, Vincze GY, Szasz O, Szendro P, Szasz A (2009). Effect of curl-free potentials on water. Electromagn Biol Med 28:166-181.
Avitall B, Mughal K, Hare J, Helms R, Krum D (1997). The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol 20:2899-2910.
Bai JP, Surguchev A, Montoya S, Aronson PS, Santos-Sacchi J, Navaratnam D (2009). Prestin’s anion transport and voltage-sensing capabilities are independent. Biophys J 96:3179-3186.
Baxter PS, Wemyss-Holden SA, Dennison AR, Maddern GJ (1998). Electrochemically induced hepatic necrosis: the next step forward in patients with unresectable liver tumours? Aust N Z J Surg 68:637-640.
Biesheuvel PM (2009). Thermodynamic cycle analysis for capacitive deionization. J Colloid Interface Sci 332:258-264.
Blank M, Goodman R (2009). Electromagnetic fields stress living cells. Pathophysiology 16:71-78.
Börjesson SI, Elinder F (2008). Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell Biochem Biophys 52:149-174.
Brown AB, Smith CG, Rennie AR (2001). Pumping of water with ac electric fields applied to asymmetric pairs of microelectrodes. Phys Rev E Stat Nonlin Soft Matter Phys 63:016305.
Call DF, Merrill MD, Logan BE (2009). High surface area stainless steel brushes as cathodes in microbial electrolysis cells. Environ Sci Technol 43:2179-2183.
Challis LJ (2005). Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics 7:S98-S106.
Chelli R, Barducci A, Bellucci L, Schettino V, Procacci P (2005). Behavior of polarizable models in presence of strong electric fields. I. Origin of nonlinear effects in water point-charge systems. J Chem Phys 123:194109.
Chen H, Voth GA, Agmon N (2010). Kinetics of proton migration in liquid water. J Phys Chem B 114:333-339.
Eaves JD, Loparo JJ, Fecko CJ, Roberts ST, Tokmakoff A, Geissler PL (2005). Hydrogen bonds in liquid water are broken only fleetingly. Proc Natl Acad Sci USA 102:13019-13022.
Edwards PP, Gray HB, Lodge MT, Williams RJ (2008). Electron transfer and electronic conduction through an intervening medium. Angew Chem Int Ed Engl 47:6758-6765.
Elässer E, Roos E (1976). Uber ein instrument zur leckstromfreien transurethralen resektion [An instrument for transurethral resection without leakage of currents]. Acta Medico Technica 24:129- 134.
Elsaesser T (2009). Two-dimensional infrared spectroscopy of intermolecular hydrogen bonds in thecondensed phase. Acc Chem Res 42:1220-1228.
England JL, Park S, Pande VS (2008). Theory for an order-driven disruption of the liquid state in water. J Chem Phys 128:044503.
Fayer MD, Moilanen DE, Wong D, Rosenfeld DE, Fenn EE, Park S (2009). Water dynamics in salt solutions studied with ultrafast two-dimensional infrared (2D IR) vibrational echo spectroscopy. Acc Chem Res 42:1210-1219.
Floume T, Syms RR, Darzi AW, Hanna GB (2010). Optical, thermal, and electrical monitoring of radio-frequency tissue modification. J Biomed Opt 15:018003.
Foster KR, Glaser R (2007). Thermal mechanisms of interaction of radiofrequency energy with biological systems with relevance to exposure guidelines. Health Phys 92:609-620.
Funk RH, Monsees T, Ozkucur N (2009). Electromagnetic effects – From cell biology to medicine. Prog Histochem Cytochem 43:177-264.
Goodman R, Blank M (2002). Insights into electromagnetic interaction mechanisms. J Cell Physiol 192:16-22.
Graham WG, Stadler KR (2007). Plasmas in saline solutions. J Physics: Conference Series 71:012013.
Gravante G, Ong SL, Metcalfe MS, Bhardwaj N, Maddern GJ, Lloyd DM, Dennison AR (2009). Experimental application of electrolysis in the treatment of liver and pancreatic tumours: Principles, preclinical and clinical observations and future perspectives. Surg Oncol Dec 31.
Graziano G (2006). Scaled particle theory study of the length scale dependence of cavity thermodynamics in different liquids. J Phys Chem B 110:11421-11426.
Haila S, Hästbacka J, Böhling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U (2001). SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem 49:973-982.
Haines D (2004). Biophysics of ablation: application to technology. J Cardiovasc Electrophysiol 15:S2-S11.
Hammes-Schiffer S (2009). Theory of proton-coupled electron transfer in energy conversion processes. Acc Chem Res 42:1881-1889.
Hart FX (2010). Cytoskeletal forces produced by extremely low-frequency electric fields acting on extracellular glycoproteins. Bioelectromagnetics 31:77-84.
Iuchi S, Izvekov S, Voth GA (2007). Are many-body electronic polarization effects important in liquid water? J Chem Phys 126:124505.
Jossinet J (2008). Elementary electrodynamics. Technol Health Care 16:465-474.
Jossinet J, Desseux A (2004). Electrical impedance endotomography: sensitivity distribution against bipolar current patterns. Physiol Meas 25:355-364.
Junge W, Nelson N (2005). Structural biology. Nature’s rotary electromotors. Science 308:642-644.
Kere J (2006). Overview of the SLC26 family and associated diseases. Novartis Found Symp 273:2-11; discussion 11-18, 261-264.
Kikuchi K, Tanaka Y, Saihara Y, Maeda M, Kawamura M, Ogumi Z (2006). Concentration of hydrogen nanobubbles in electrolyzed water. J Colloid Interface Sci 298:914-919.
Kikuchi K, Ioka A, Oku T, Tanaka Y, Saihara Y, Ogumi Z (2009). Concentration determination of oxygen nanobubbles in electrolyzed water. J Colloid Interface Sci 329:306-309.
Lai WM, Mow VC, Sun DD, Ateshian GA (2000). On the electric potentials inside a charged soft hydrated biological tissue: streaming potential versus diffusion potential. J Biomech Eng 122:336-346.
Lee HS, Vermaas WF, Rittmann BE (2010). Biological hydrogen production: prospectives and challenges. Trends Biotechnol Feb 26.
Lewis PM, Sheridan LB, Gawley RE, Fritsch I (2010). Signal amplification in a microchannel from redox cycling with varied electroactive configurations of an individually addressable microband electrode array. Anal Chem 82:1659-1668.
Livesay DR, Huynh DH, Dallakyan S, Jacobs DJ (2008). Hydrogen bond networks determine emergent mechanical and thermodynamic properties across a protein family. Chem Cent J 2:17.
Loret B, Simões FM (2010). Effects of pH on transport properties of articular cartilages. Biomech Model Mechanobiol 9:45-63.
McKinlay JB, Harwood CS (2010). Photobiological production of hydrogen gas as a biofuel. Curr Opin Biotechnol Mar 18.
Matharoo GS, Razul MS, Poole PH (2009). Spectral statistics of the quenched normal modes of a network-forming molecular liquid. J Chem Phys 130:124512.
Matyushov DV (2009). Standard electrode potential, Tafel equation, and the solvation thermodynamics. J Chem Phys 130:234704.
Mayer S, Geddes LA, Bourland JD, Ogborn L (1992). Faradic resistance of the electrode/electrolyte interface. Med Biol Eng Comput 30:538-542.
Nahtigal IG, Svishchev IM (2009). Generation and integration of NaOH into NaCl clusters in supercritical water: a molecular dynamics study on hydrolysis product partitioning. J Phys Chem B 113:14681-14688.
Nath S, DiMarco JP, Haines DE (1994). Basic aspects of radiofrequency catheter ablation. J Cardiovasc Electrophysiol 5:863-876.
Nucci NV, Vanderkooi JM (2008). Effects of salts of the Hofmeister series on the hydrogen bond network of water. J Mol Liq 143:160-170.
Okafor ENC, Okon PE, Okoro CC (2009). Magnetic field mapping of a direct current electrical machine using finite element method. J Appl Sci Res 5:1889-1898.
Okamura Y (2007). Biodiversity of voltage sensor domain proteins. Pflugers Arch 454:361-371.
Palanker DV, Vankov A, Huie P (2008). Electrosurgery with cellular precision. IEEE Trans Biomed Eng 55:838-841.
Paniagua JM, Rufo M, Jiménez A, Antolín A, Sánchez M (2009). Electrical stimulation vs thermal effects in a complex electromagnetic environment. Sci Total Environ 407:4717-4722.
Park S, Odelius M, Gaffney KJ (2009). Ultrafast dynamics of hydrogen bond exchange in aqueous ionic solutions. J Phys Chem B 113:7825-7835.
Pártay L, Jedlovszky P (2005). Line of percolation in supercritical water. J Chem Phys 123:24502.
Perdue RK, Laws DR, Hlushkou D, Tallarek U, Crooks RM (2009). Bipolar electrode focusing: the effect of current and electric field on concentration enrichment. Anal Chem 81:10149-10155.
Poole PH, Sciortino F, Grande T, Stanley HE, Angell CA (1994). Effect of hydrogen bonds on the thermodynamic behavior of liquid water. Phys Rev Lett 73:1632-1635.
Prezhdo OV, Pereverzev YV (2009). Theoretical aspects of the biological catch bond. Acc Chem Res 42:693-703.
Priglinger SG, Palanker D, Alge CS, Kreutzer TC, Haritoglou C, Grueterich M, Kampik A (2007). Pulsed electron avalanche knife: new technology for cataract surgery. Br J Ophthalmol 91:949-954.
Raabe G, Sadus RJ (2007). Influence of bond flexibility on the vapor-liquid phase equilibria of water. J Chem Phys 126:044701.
Rahimi M, Mikkelsen SR (2010). Cyclic biamperometry. Anal Chem 82:1779-1785.
Ramos A, González A, Castellanos A, Green NG, Morgan H (2003). Pumping of liquids with ac voltages applied to asymmetric pairs of microelectrodes. Phys Rev E Stat Nonlin Soft Matter Phys 67:056302.
Ramos A, González A, García-Sánchez P, Castellanos A (2007). A linear analysis of the effect of Faradaic currents on traveling-wave electroosmosis. J Colloid Interface Sci 309:323-331.
Saulis G, Lape R, Praneviciũte, Mickevicius D (2005). Changes of the solution pH due to exposure by high-voltage electric pulses. Bioelectrochemistry 67:101-108.
Schiller J, Arnhold J, Gründer W, Arnold K (1994). The action of hypochlorous acid on polymeric components of cartilage. Biol Chem Hoppe Seyler 375:167-172.
Schramm W, Yang D, Haemmerich D (2006). Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Conf Proc IEEE Eng Med Biol Soc 1:5013-5016.
Sheppard AR, Swicord ML, Balzano Q (2008). Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys 95:365-396.
Shrivastava D, Vaughan JT (2009). A generic bioheat transfer thermal model for a perfused tissue. J Biomech Eng 131:074506.
Soderberg JN, Co AC, Sirk AH, Birss VI (2006). Impact of porous electrode properties on the electrochemical transfer coefficient. J Phys Chem B 110:10401-10410.
Stadler KR, Woloszko J, Brown I, Smith CD (2001). Repetitive plasma discharges in saline solutions. Applied Physics Letters 79: 4503-4505.
Szasz A, Vincze G, Andocs G, Szasz O (2009). Do field-free electromagnetic potentials play a role in biology? Electromagn Biol Med 28:135-147.
Tan YH, Luo R (2007). Continuum treatment of electronic polarization effect. J Chem Phys 126:094103.
Teixeira J (2009). Dynamics of hydration water in proteins. Gen Physiol Biophys 28:168-173.
Thompson N, Lustgarten D, Mason B, Mueller E, Calame J, Bell S, Spector P (2009). The relationship between surface temperature, tissue temperature, microbubble formation, and steam pops. Pacing Clin Electrophysiol 32:833-841.
Vaitheeswaran S, Yin H, Rasaiah JC (2005). Water between plates in the presence of an electric field in an open system. J Phys Chem B 109:6629-6635.
van Rongen E, Saunders RD, van Deventer ET, Repacholi MH (2007). Static fields: biological effects and mechanisms relevant to exposure limits. Health Phys 92:584-590.
Varghese T, Techavipoo U, Zagzebski JA, Lee FT Jr (2004). Impact of gas bubbles generated during interstitial ablation on elastographic depiction of in vitro thermal lesions. J Ultrasound Med 23:535-544.
Wachtel E, Maroudas A (1998). The effects of pH and ionic strength on intrafibrillar hydration in articular cartilage. Biochim Biophys Acta 1381:37-48.
Weaver JC (2002). Understanding conditions for which biological effects of nonionizing electromagnetic fields can be expected. Bioelectrochemistry 56:207-209.
Wemyss-Holden SA, Dennison AR, Berry DP, Maddern GJ (2004). Local ablation for unresectable liver tumors: is thermal best? J Hepatobiliary Pancreat Surg 11:97-106.
Wernet P, Nordlund D, Bergmann U, Cavalleri M, Odelius M, Ogasawara H, Näslund LA, Hirsch TK, Ojamäe L, Glatzel P, Pettersson LG, Nilsson A (2004). The structure of the first coordination shell in liquid water. Science 304:995-999.
Wood MA, Shaffer KM, Ellenbogen AL, Ownby ED (2005). Microbubbles during radiofrequency catheter ablation: composition and formation. Heart Rhythm 2:397-403.
Yamashita M, Duffield C, Tiller W (2003). Direct current magnetic field and electromagnetic field effects on the pH and oxidation-reduction potential equilibration rates of water. 1. Purified water. Langmuir 19:6851–6856.
Yang S, Tsai P, Kooij ES, Prosperetti A, Zandvliet HJ, Lohse D (2009). Electrolytically generated nanobubbles on highly orientated pyrolytic graphite surfaces. Langmuir 25:1466-1474.
Zahn D (2004). How does water boil? Phys Rev Lett 93:227801.
Zhang L, Zhang Y, Zhang X, Li Z, Shen G, Ye M, Fan C, Fang H, Hu J (2006). Electrochemically controlled formation and growth of hydrogen nanobubbles. Langmuir 22:8109-8113.
Zheng X, Walcott GP, Hall JA, Rollins DL, Smith WM, Kay GN, Ideker RE (2000). Electrode impedance: an indicator of electrode-tissue contact and lesion dimensions during linear ablation. J Interv Card Electrophysiol 4:645-654.
Discussion with Reviewers
Reviewer: How would this technology work on specific tissue types like hyaline cartilage and what are the advantages of non-ablation over ablation techniques?
McRury ID, Morgan RE, Augé II WK: Historically, technology development based upon biophysical tissue characteristics proves most efficacious. In the instance of hyaline cartilage commonly encountered during arthroscopy for which this technology can be applied, early articular cartilage damage manifests as surface matrix changes such as that observed with the initial stages of osteoarthritis. Despite the heterogeneity of this damage, safe lesion stabilization (i.e. damaged tissue removal) is required to permit intrinsic homeostatic and repair responses since damaged tissue serves as a biologic and mechanical irritant impeding such responses and leading to symptoms and disease progression. Lesion stabilization for early articular cartilage disease constitutes a tissue rescue, allowing biologic tissue response properties to more fully manifest unencumbered rather than allowing the tissue to progressively convert to a mechanical adaptation construct characterized by further matrix failure. Because early intervention presupposes that tissue surrounding the lesion retains effective differentiated function, chondrocyte viability and a healing phenotype are important attributes to retain within subadjacent tissue. Thermal and plasma ablation technologies which deliver electrical current directly into tissue have been deemed inappropriate for articular cartilage tissue preservation procedures as a result of significant induced iatrogenic damage to subadjacent tissue associated with the high energy deployment necessitated by device design. Hyaline cartilage is a tissue type retaining a high water content ensuring that ablation technology will effectively pool electrothermal energies within cartilage tissue to a detrimental level. Ablation technologies cannot distinguish between normal and abnormal tissues because device design is not based upon tissue specific biology and consequently induce necrosis. This necrosis is caused for a variety of reasons, including the formation of subsurface tissue heat capacitance due to water permeability constraints at normal surfaces adjacent to lesions, the overwhelming metabolic disturbances of internal tissue electrolysis, and the surface entry wounds typical of electrical injury; all of which further impair tissue integrity and local biologic responses by expanding the size of the original lesion and further progressing disease. Non-ablation technology allows for the targeted removal of diseased tissue without expanding lesion size or compromising subsurface tissue with electrical current deposition. Device architecture ensures that the near-field reaction products are delivered only to tissue surfaces, not within tissues, and can selectively target damaged tissue, preparing it for mechanical débridement through inherent cleavage planes. Diseased articular cartilage is characterized by deteriorating surface-layered shear properties of collagen fibril disruption and orientation changes, weak collagen-to-proteoglycan bonds, proteoglycan depletion, aberrant water content, and decreased fixed charge density; this compromised tissue is further altered by the physiochemical loading delivered by non-ablation technology to a state amenable to gentle shear débridement during lesion stabilization. Shear stabilization in this instance illustrates treatment design relative to a tissue’s perturbation failure specificity; understandably, safe lesion stabilization remains an advance inextricably necessary for disease burden mitigation.
Reviewer: What is the relevance of tissue fluids or water in the development of orthopedic surgical technologies? How will it be affected in presence of edema?
McRury ID, Morgan RE, Augé II WK: When creating surgical technologies that preserve normal tissue at treatment sites, the role of water becomes an important factor to consider because of its ubiquitous presence in biologic assemblies. Tissue preserving surgical procedures can be difficult to create since they require balancing macroscopic treatment events with microscopic physiologic function. For example, many surgical treatment venues reside at tissue surfaces due to tissue integrity failures originating from surface forces or processes overloading tissue capacity to maintain integrity. Intact surfaces, whether articular cartilage, tendon, ligament or even other representative tissue types like gastric mucosa or lung pleura, are structured by water, often through variations in hydrophobic adhesion, to create a protective barrier designed to maintain tissue integrity against tissue-specific perturbations. Surface active phospholipid organization and absorption into lamellar superficial collagen layers constraining proteoglycan moieties is a common finding at the water-to-tissue interface that create the robust physiochemical charge barrier of tribiologic systems. These surface active phospholipid layers are often amorphous (without collagen), non-fibrous, or gel-like and can reconstitute via self-assembly after removal, even removal deep to collagen layers, through polymorphic aggregation forces like the hydrophobic effect governed by water. It is interesting to note that many anatomic tissue surface sites subjected to repetitive perturbation have similar tissue homeostatic and repair mechanisms which allow for collagen based layered or cleavage plane failure as a back-up mechanism to topographic loss of water-structured amorphous surface barrier regimes that can occur during physiologic loading. Such surface-based collagen cleavage plane failure is generally a reversible lesion under certain circumstances, most notably with damaged tissue removal while maintaining cell viability and differentiated phenotype around a lesion site stabilized relative to perturbation specificity. Non-ablation technology exploits this common tissue surface characteristic for tissue preserving lesion stabilization by augmenting those structural planes during selective preconditioning or modification of diseased tissue that has become accessible due to the loss of the surface regime barriers. It is further interesting to note that these normal tissue surface regimes are rather robust because of water’s structural interfacial organization, such that the reaction products originating from the electrosurgical plenum at tissue preservation settings cannot disrupt this barrier; hence, undamaged surface tissue is protected. Indeed, disruption requires prolonged perturbations like enzymatic incubation, strong detergents, large single or cumulative insults, or even ablation energies. Additionally important is that the healthy bed of lesions being stabilized is also a barrier to such treatment due to the integrity of those same tissue constituents which when diseased are susceptible to tissue specific non-ablation physiochemical loading regimens.
Tissue edema, or an increase in tissue water content distinct from tissue surface water, is often an early event associated with injury or disease occurring prior to observable morphological changes. The increased water content can be due to either an alteration in tissue constituent structure or the re-localization of additional tissue components. Surgical targeting of tissue with an increased water content but without observable macroscopic alterations remains difficult. It is for this reason that most surgical device development is based upon observable criteria that the surgeon can readily identify during the procedure. Surface-based morphologic changes are uniquely suited as a therapeutic target, particularly since early intervention in these settings is governed by the ability to pursue tissue rescue as a result of creating an environment amenable at least to homeostasis and at best to self-repair.
Reviewer: Surgical capture of water’s energy is a unique approach to match treatment with tissue concerns. Is there a built-in safety profile with this technology since the temperature goes down at higher energy of the ranges examined? Does ambient water serve a protective role at tissue surfaces?
McRury ID, Morgan RE, Augé II WK: The use of an electrosurgical plenum serves many functions, one being primary reaction zone manipulation within its interior. Configurational changes in its architecture can alter the formation and delivery of reaction products during targeted physiochemical loading of tissue surfaces. Two reaction products, pH and temperature, were evaluated in this study because they are especially relevant to the function of water at tissue surfaces during physiochemical loading in a sodium chloride milieu, even though many other associated physiochemical phenomena are simultaneously occurring and warrant description. For instance, the pH change, if desired, can be configured toward a more strict linear regression by further shielding the primary reaction zone from the fluid-flow and convective forces at the treatment site. With regard to temperature, heat can be delivered to tissue surfaces by creating localized temperature changes in the interfacing media rather than within the tissue itself as occurs with ablation technologies. Because water has a high specific heat capacity and heat of vaporization, it buffers heat delivery in a protective manner. This architectural modulation of reaction product escape from the primary reaction zone combined with surgical technique dependent positioning of the electrosurgical plenum is a simple mechanism to control treatment-specific reaction product character that is delivered to tissue surfaces during physiochemical loading. In the configuration studied, temperature change as a function of initial interfacing media temperature has been designed to protect tissue surfaces from inadvertent temperatures that may have an undesirable efficacy. Although tissue surfaces, like phospholipid layers, can be sensitive to temperature changes, the device studied was designed to induce only a small temperature change of the interfacing media with a protective triphasic behavior; further, tissue preserving settings generally function within Phase 1 during which no temperature change is deployed.
In addition to the protective role that ambient water serves during non-ablation technology use, it also serves a protective role at tissue surfaces because it is absorbed and held by tissue surface constituents. These surfaces are robust due to water’s influence on their constituents’ polar regions with positively charged ends anchored to the negative charge density of proteoglycan typical in collagen constrained extracellular matrix. For example, hydration shells around phospholipids bind water via hydrogen and electrostatic bonds and when combined with hydrated ions become effective lubricants between sliding charged surfaces. This composition creates a strong laterally bonded network that is protective against shear forces by exhibiting lipid mobility and viscous resistance. For physical load bearing tissue, the surface amorphous layer can support the majority of a load within its water phase thereby altering the liquid-solid phase load sharing of subsurface tissue by protecting the solid phases from elevated stresses. This water-to-tissue interfacial phenomenon is important in boundary lubrication regimes; and, it is the loss of this layer that facilitates further matrix failure leading to collagen based tissue damage. Should this collagen level damage progress without effective repair, it will serve as a lesion site irritant impeding natural reconstitution of the amorphous boundary lubrication layer and lead to further tissue overload matrix failure through additional loading of a damaged and poorly structured biomechanical site. Because this layer has been noted to reform after removal, its reconstitution, along with the favorable biomechanical environment of damaged tissue removal that stimulates more appropriate mechanotransductive biosynthetic gene expression, validates the approach of early intervention designed as a tissue rescue by removing an irritant and allowing cellular and matrix component repair to manifest relative to perturbation specificity.
Reviewer: Can tissue water be a therapeutic target for electromagnetic forces used in these technologies?
McRury ID, Morgan RE, Augé II WK: Non-ablation technology allows therapeutic regimens to be formulated at tissue surface and subsurface levels independently, but which are nonetheless interrelated. Physiochemical loading of tissue surfaces as a treatment platform is a complex discipline because it requires an understanding of tissue biology in both the native and diseased state. This study characterized a limited number of phenomena as part of the emerging therapeutic category defined by Engineered IrrigantsTM for the treatment of tissue surfaces. Various physiochemical loading regimens can be created based upon tissue-specific therapeutic goals by modification of the reactants and products available in the primary reaction zone. Because the physiochemical loading of tissue surfaces is geographically decoupled from subsurface tissue, non-ionizing electromagnetic forces at and below tissue surfaces are enabled that are particularly useful for an early intervention strategy since subsurface tissue in this setting demonstrates retained cellular viability and a differentiated functional phenotype. Although the influences that targeted in situ non-ionizing electromagnetic forces exert upon tissue are also complex, this additional discipline is fertile for further exploration in a effort to facilitate or recruit repair responses to assist in tissue recovery enabled by early intervention. These electromagnetic fields facilitate charge flow through accelerated transfer rates and changing valence configurations and have been associated with increased enzymatic reaction efficiency, DNA stimulated biosynthesis, superficial extracellular matrix volume contraction, cellular cytoprotection, and other domain specific gene expression modulation. In biologic tissue, water remains a substrate for non-ionizing electromagnetic forces as a facilitator of charge transfer because of its mobility around hydrogen bonds. However, the mechanisms by which electron transfer (often associated with redox chemistry) interacts with proton transfer (often associated with acid-base phenomena) in the presence of charged macromolecular tissue constituents that depend upon water to organize tertiary and quaternary structure and bond interactions are not fully defined. Therefore, non-ionizing electromagnetic field induced changes in biologic tissue requires in most instances further characterization of a tissue’s specific elements within the native and diseased state available for targeted manipulation.
