 Spontaneous Particle Separation and Salt Rejection by Hydrophilic Membranes
Spontaneous Particle Separation and Salt Rejection by Hydrophilic Membranes
Zhang Y, Takizawa S*, and Lohwacharin J
Department of Urban Engineering, Graduate School of Engineering, the University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
*Correspondence E-mail: takizawa@env.t.u-tokyo.ac.jp
Key Words: Exclusion-zone (EZ); Hydrophilic surface; Nafion®; Particle separation; Salt rejection
Received March 29th 2015; Revised May 21st; Accepted June 15th; Published July 1st; Available online July 7th, 2015
Abstract
The interactions between a hydrophilic surface and water can produce a particle-free zone of several hundred micrometers referred to as the exclusion zone (EZ). We examined the effects of particle concentration and surface hydrophilicity on the EZ using a vertical surface–suspension interface. According to the literature, the EZ develops within a period of several minutes and then stably persists at low particle concentrations. However, we observed that, at high particle concentrations, the flow of water from the vertical EZ region formed a distinctive supernatant from which both particles and chloride ions were rejected.
This spontaneous phase separation was affected by the hydrophilicity and area of the surface. Among the various EZ formation mechanisms proposed in the literature, our results supported the long-range water ordering hypothesis, which states that EZ water has an ordered structure that can reject both particles and ions and can move upward due to the density-driven buoyancy force. The spontaneous phase separation observed in this study has never been previously reported and may lead to breakthroughs in the applications of hydrophilic-surface-induced solid–liquid separation processes.
Outline
- Introduction
- Materials and Methods
- Results and Discussion
- Salt Rejection and the Structure of the EZ Water
- Conclusions
- Acknowledgements
- References
- Discussion with Reviewers
Introduction
The interactions between water and hydrophilic surfaces are critically important in biological, chemical, and environmental studies (Israelachvili and Wennerström, 1996; Granick and Bae, 2008). Interfacial water, generally a few layers of water molecules, has been well established through both experimental and theoretical studies to substantially differ from bulk water (Nimtz and Weiss, 1987; Ruan et al., 2004; Goertz et al., 2007; Köfinger et al., 2008; Stanley and Rau, 2011). Water molecules at a hydrophilic surface are in an ordered structure composed of a network of hydrogen bonds. This structure is responsible for the specific physical and chemical properties of interfacial water (Speedy, 1997; Smith et al., 2004; Head-Gordon and Johnson, 2006; Stiopkin et al., 2011).
The long-range effect of a hydrophilic surface on a colloidal suspension has not been well recognized until recently. Zheng and Pollack (2003) reported the exclusion of colloidal particles near hydrophilic surfaces, which left a clear water region several hundred micrometers from the surface. Different terms are used to refer to this clear water region, including “unstirred layer” (Barry and Diamond, 1984), “long-range surface induced water” (Totland et al., 2013), and “Nafionated water” (Elia et al., 2013); however, it is usually referred to as the exclusion zone (EZ) (Zheng et al., 2006). Water in the EZ was observed to have physicochemical characteristics (e.g., light absorption, fluorescence, higher viscosity, and birefringence) that distinctly differed from those of the bulk water (Zheng et al., 2006; Chai et al., 2008; Yoo et al., 2011; Bunkin et al., 2014). Hence, long-range-ordered water at the hydrophilic surface is considered to be a possible cause of EZ formation (Chai et al., 2008, 2009; Segarra-Martí et al., 2013, 2014; Totland et al., 2013). Besides, Del Giudice et al. (2014) proposed that the coherent oscillations caused by quantum electrodynamics contiguous to hydrophilic surfaces is the cause of an EZ and suggested the similarities, such as the domain size, strong negative charge and higher viscosity than that of the bulk water between a coherent domain and an EZ as evidences to support their proposed model. More recently, other researchers have proposed an alternative model for EZ formation; this model involves concentration gradients of ions, e.g., chemotaxis (Schurr, 2013; Schurr et al., 2013) and diffusiophoresis (Florea et al., 2014).
EZ formation is affected by various factors, including surface hydrophilicity (Zheng et al., 2006), solution chemistry (Zheng and Pollack, 2003; Zheng et al., 2006), and incident radiant energy (Chai et al., 2009). The EZ is formed near many hydrophilic surfaces that contain functional groups that can interact with the water molecules via hydrogen bonding, whereas no EZ forms adjacent to hydrophobic surfaces or hydrophilic surfaces without functional groups, e.g., gold wire (Zheng et al., 2006). The particle concentration of the suspension had no effect on the EZ in the range reported by Zheng and Pollack (2003), whereas the addition of salt made the EZ smaller, and no EZ formed at a high salt concentration (Zheng and Pollack, 2003; Zheng et al., 2006). In contrast, the input of incident radiant energy, especially mid-infrared radiation, distinctly increased the EZ size (Chai et al., 2009). Although the exclusion of microspheres and dyes with a small molecular weight has been previously reported (Zheng et al., 2006), the rejection of salt by the EZ has never been reported.
Because studies of the EZ formation in the literature have been conducted at low particle concentrations with horizontal surfaces, this study was designed to evaluate the long-term stability of an EZ formed by vertical immersion of different types of hydrophilic membranes into suspensions containing a wide range of particle concentrations and surface functional groups. This design made it possible for us to discover that the flow of EZ water leads to the formation of a supernatant on top of the bulk water, which we termed “spontaneous phase separation.” On the basis of this finding, this study also aimed to provide further insight into EZ formation mechanisms by evaluating the desalting ability of the EZ. To the best of our knowledge, this paper is the first report of spontaneous phase separation and salt rejection by EZ water, which could lead to a better understanding of EZ formation mechanisms and enable the application of this phase separation mechanism in particle–liquid separation processes.
Materials and Methods
Membranes
We used Nafion-117 (187 µm thick, Sigma-Aldrich, St. Louis, MO, USA), cellulose acetate (Toyo Roshi Kaisha, Tokyo, Japan), regenerated cellulose, mixed cellulose ester membranes, and hydrophobic polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Billerca, MA, USA) in these particle separation experiments. Because Nafion generates relatively large EZs (Zheng et al., 2006), we used it in most of our experiments. Nafion is hydrophobic when dry, but becomes hydrophilic after full hydration, with an advancing water contact angle of 22.3 ± 0.5° (Goswami et al., 2008; Bass et al., 2010).
Particle Suspensions
Six suspensions were used in our experiments: polystyrene microspheres, amino microspheres, carboxylate microspheres, sulfate microspheres, silica microspheres, and carbon black. The microsphere suspensions were purchased from Polysciences, Inc., Warrington, PA, USA, and the hydrophilic carbon black was obtained from Tokai Carbon Co., Ltd., Tokyo, Japan. The volumetric mean diameters of the particles (Table 1) were measured with a Nanotrac 150 particle size analyzer (Nikkiso Co., Ltd., Tokyo, Japan) using dynamic light scattering.
Experimental Procedure
The authors of previous studies (Zheng and Pollack, 2003; Zheng et al., 2006) of the EZ phenomenon reported in the literature usually used a horizontal setup, where EZ formation was observed from a top view. In this study, we conducted experiments using a vertical setup with a side view (Figure 1). A 9 mm × 10 mm piece of a membrane sheet was placed onto a side wall of a plastic cuvette (either 10 mm × 10 mm × 40 mm or 4 mm × 10 mm × 18 mm). Membranes were soaked in Milli-Q water (resistivity ≥ 18.2 mΩ·cm) for 10 min before use. Then, 1.0 or 0.4 mL of the colloidal suspension (all the six particle types formed colloidal suspensions) was slowly added into the cuvette. The suspensions were prepared by diluting the stock suspensions with Milli-Q water immediately before they were added into the cuvette. The cuvette was then covered with a plastic cap to prevent evaporation under ambient laboratory temperature and illumination. Phase separation—the emergence of a supernatant above the bulk colloidal suspension—was observed for 3 h using a digital microscope (VHX-1000, Keyence Corp., Osaka, Japan) equipped with a 20× objective lens and a halogen lamp (Figure 1). Microscope images were captured by a digital camera (18 megapixels; high-resolution CCD) with a minimum detection limit of 1 µm.
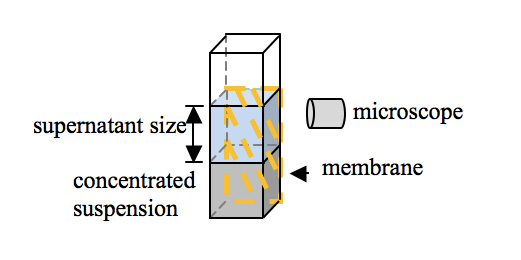
Figure 1: Schematic diagram of the microscope observation setup.
The size of the supernatant was defined as the vertical distance between the air–water interface and the water–suspension interface. The terminal size was the size at the end of microscopic observation, which was usually at 3 h. Chloride concentration in the supernatant was analyzed by ion chromatography (861 Advanced Compact IC, Metrohm Ltd., Herisau, Switzerland).
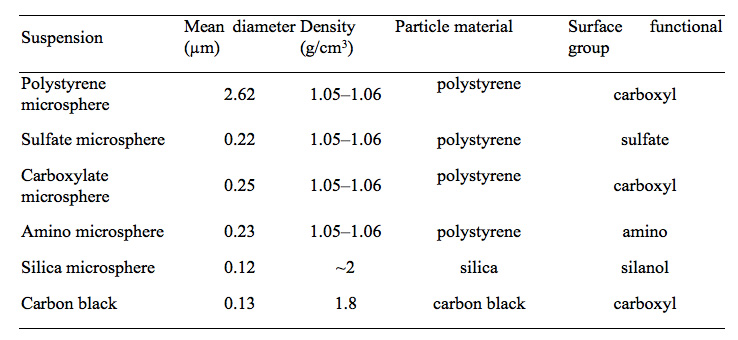
Table 1: Properties of the tested particles used in colloidal suspensions.
Results and Discussion
Observation of the Phase Separation
As reported by Zheng et al. (2006), Nafion generates a particle-free interfacial water zone of several hundred micrometers within a few minutes after being introduced into an aqueous suspension. Although we used a vertical setup that differed from most of the setups reported in the literature, an EZ was observed along the strip of Nafion membrane in the suspensions at concentrations higher than those reported in the literature (Zheng and Pollack, 2003; Zheng et al., 2006).
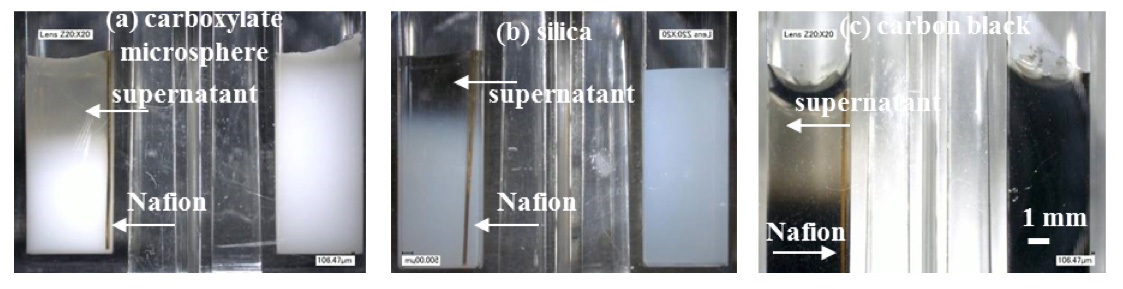
Figure 2: Phase separation phenomenon (left cuvette, with Nafion; right cuvette, without Nafion) in (a) 0.25 μm carboxylate microspheres (5.68 × 1011 /mL, 3 h), (b) 0.12 μm silica microspheres (4.8 × 1012 /mL, 1 h), and (c) 0.13 μm carbon black (4.8 × 1012 /mL, 3 h).
In addition, as shown in Figure 2, a notable supernatant devoid of particles formed on the top of the suspension during the long-term observations (e.g., 1 or 3 h), which has not been reported in the literature. In the present study, we refer to this phenomenon as spontaneous phase separation. The supernatant appeared in suspensions of carboxylate microspheres, silica microspheres, and carbon black in the presence of Nafion (left cuvettes in Figure 2); however, it did not appear in the absence of Nafion (right cuvettes in Figure 2). We observed that, after the supernatant appeared, it persisted for at least 48 h, the longest experimental observation period in this study. Even nanoparticles, e.g., carbon black, could not re-disperse into the supernatant. This persistence of the supernatant is one of its most significant characteristics. These results indicated that the exclusion of particles from the supernatant is maintained long after it is formed, which is similar to the EZ (Zheng et al., 2006).
Furthermore, this phase separation phenomenon occurred spontaneously in all the six tested colloidal suspensions of particles with different surface functional groups and sizes, as shown in Table 1. This result indicates that the phase separation occurs regardless of the particles’ properties. Generally, the size of the supernatant increased rapidly within the first hour and reached a plateau between < 1 mm and 5 mm after 2 h. Because colloidal particles are usually considered to be non-settling as a consequence of Brownian motion (Piazza et al., 2012), the observed sizes of the supernatants were significantly greater than the settling distance of each particle type after 3 h, as estimated on the basis of Stokes’ law. Therefore, gravity settling is not the cause of the phase separation. Under certain conditions, the exclusion of particle occurs at the air-water interface without Nafion in microspheres suspensions (Ovchinnikova and Pollack, 2009), but such phenomenon was not found in the cuvettes without Nafion in this study (Figure 2). Moreover, the supernatant formed even when the Nafion strip was underneath the water surface. Therefore, the effect of the Nafion membrane on the colloidal suspension may be a determining factor because, only in the presence of Nafion, the supernatant excluded particles in the same manner as the EZ.
Effect of the Hydrophilic Surface
The phase separation phenomenon is not specific to the Nafion membrane; it has been observed with the other types of hydrophilic membranes, including membranes composed of cellulose acetate, regenerated cellulose, and mixed cellulose ester. However, the phase separation did not occur in the presence of the hydrophobic PVDF membrane within 3 h when the membrane was immersed in a polystyrene microsphere suspension. Hence, the presence of a hydrophilic membrane is critical to the phase separation. To further elucidate the role of the hydrophilic surface, we measured the supernatant size as a function of surface area by increasing the number of 90 mm2 Nafion strips from zero to four. In the carbon black suspension, the supernatant size increased faster and more distinctly with increasing number of Nafion strips (Figure 3). A linear relationship between the supernatant size and the numbers of Nafion strips is shown in Figure 3a. These results indicate that a larger hydrophilic surface area can produce a greater volume of supernatant water. A larger hydrophilic surface area (e.g., Nafion) also created more EZ water (Figueroa and Pollack, 2011), suggesting that the supernatant might stem from the EZ.
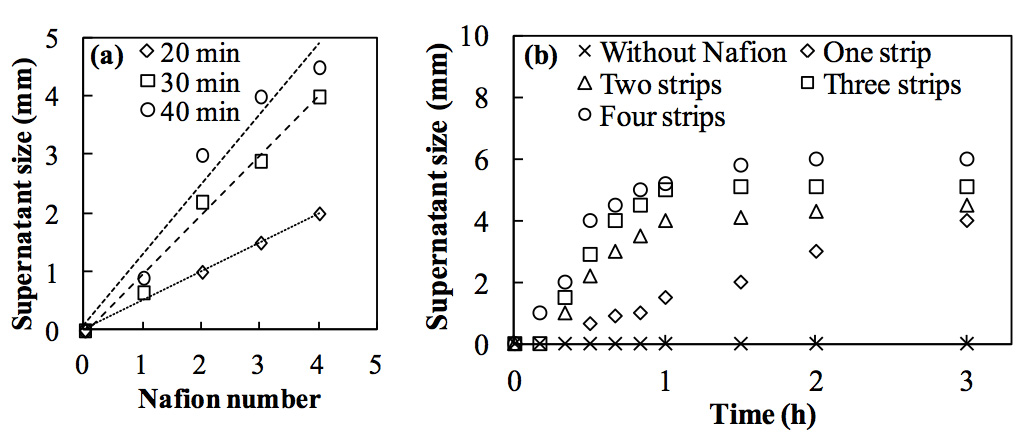
Figure 3: Effect of Nafion surface area on the supernatant size in a carbon black suspension (2.4 × 1011 /mL). (a) Relationship between the number of Nafion strips and the supernatant size at 20, 30, and 40 min. (b) Time course of supernatant sizes with 0 to 4 Nafion strips.
However, the effects of a larger surface area were less obvious after 3 h, particularly with three and four strips (Figure 3b). The terminal sizes of the supernatant were ca. 4 mm with one strip, 4.5 mm with two strips, 5 mm with three strips, and 6 mm with four strips. The differences in these terminal sizes are not as large as the initial differences; specifically, the surface area of the hydrophilic membrane slightly affected the terminal supernatant size under the experimental conditions employed in this study. These results may be due to (1) the reduction of EZ water volume caused by the significant decrease in the effective Nafion area in contact with the bulk suspension, (2) increase in the particle concentration in the bulk solution, and (3) pH decrease in the bulk solution (Zheng et al., 2006; Chai et al., 2009). The Nafion surface in contact with the bulk solution is effective in excluding particles and generating the EZ; however, the surface in contact with the supernatant is no longer effective. Because EZ formation and phase separation depend on the effective surface area of the Nafion, a decrease in the effective area after the first hour slowed the supernatant formation process. At the same time, as the supernatant gradually developed, the bulk suspension became concentrated. Hence, an increase in the particle concentration in the suspension during the course of the phase separation might also interfere with EZ formation, which is described further in the following section. Furthermore, the solution pH may affect the structure of interfacial water as well (Ye et al., 2001). We confirmed that no EZ forms at a bulk solution pH of < 3 and that Nafion is a strong-acid ion-exchange membrane that releases protons and lowers the pH. Thus, in the experiments with three or four Nafion strips, the supernatant sizes did not increase significantly after 1 h as a result of the loss of the effective Nafion surface, the relatively slow rate of the EZ formation and the rapid decrease in the bulk solution pH.
Effect of the Particle Concentration
In addition to the effects of the vertical experimental setup, we studied the role of the particle concentration of the suspension. We observed both an EZ and phase separation at a relatively high particle concentration (e.g., 5.68 × 1011 /mL in Figure 2a), whereas, in the literature, the EZ phenomenon was observed only in dilute suspensions at low particle concentrations (e.g., 8.5 × 106 /mL). Hence, the particle concentration, which has drawn less attention in the literature, was considered to be an important factor both in the long-term process of EZ formation and in the ability to achieve phase separation. To investigate the effect of the particle concentration, we performed phase separation experiments in carbon black suspensions with a volumetric mean diameter of 0.13 μm over a wide range of particle concentrations. No distinct supernatant was observed at 3 h in the case of the low concentration (4.8 × 109 /mL), consistent with the results reported in the literature; however, the supernatant reached the terminal sizes of 3, 4, 4.5, and 5 mm at particle concentrations of 2.4 × 1010, 4.8 × 1010, 4.8 × 1011, and 4.8 × 1012 /mL, respectively (Figures 4a, b). These results imply that the minimum particle concentration required for supernatant formation in the carbon black suspension is between 4.8 × 109 /mL and 2.4 × 1010 /mL. Furthermore, the supernatant size increased with increasing particle concentration. As shown in Figure 4b, when the particle concentration was increased from 2.4 × 1010 to 4.8 × 1011 /mL, the supernatant size increased dramatically. However, the size increased only slightly more when the concentration was increased further to 4.8 × 1012 /mL. Because the specific density of the carbon black solution increased with increasing particle concentration, the formation of the supernatant was apparently accelerated by the density difference between the EZ water and the bulk suspension.
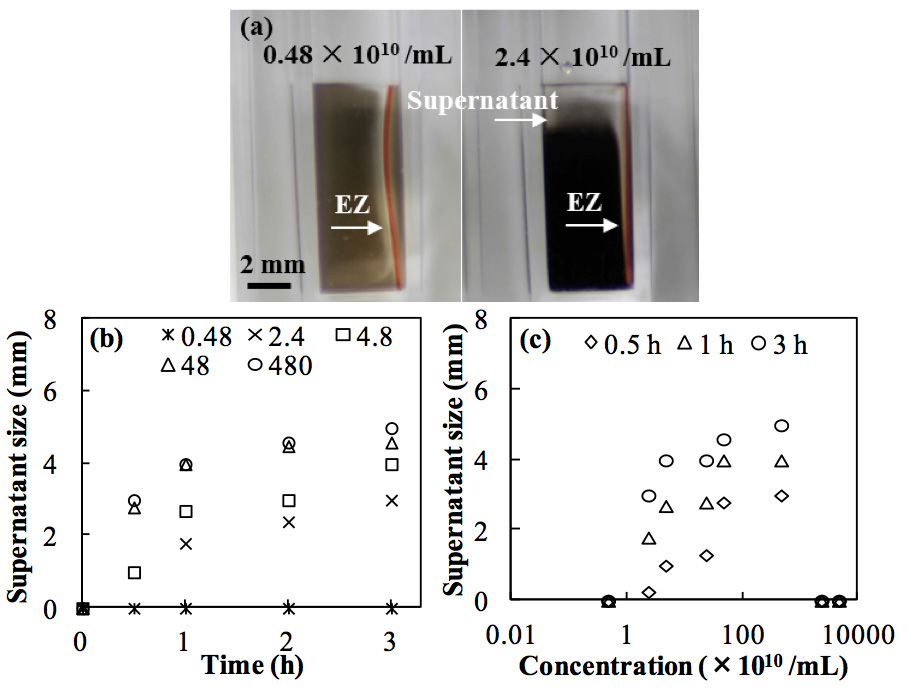
Figure 4: Effect of carbon black concentrations on the development of phase separation (measured as supernatant size) caused by Nafion. (a) Supernatant sizes at low (left) and high (right) concentrations at 1 h. (b) Growth of supernatant size at various concentrations (×1010 /mL) up to 3 h. (c) Supernatant sizes at various times at concentrations of 4.8 × 109 to 4.8 × 1013 /mL.
In contrast, at extremely high particle concentrations of 2.4 × 1013 and 4.8 × 1013 /mL (Figure 4c), an unclear supernatant appeared after the first 10 min and then diminished, even though the EZ formed and persisted for more than 10 min. This result suggests that extremely high particle concentrations perturb supernatant formation and that a critical concentration range exists to achieve phase separation. The Stokes–Einstein equation gives a diffusion coefficient of 3.28 × 10-12 m2/s for carbon black; thus, the average displacement distance by Brownian motion is approximated as 20 µm in 1 min, 154 µm in 1 h and 266 µm in 3 h. However, the average EZ was approximately 120 µm in 1 min, which is much greater than the displacement distance of carbon black. As a result, the initial formation of EZ water was dominant compared to the diffusion of carbon black, and thus the EZ could emerge and develop. When the EZ became sufficiently large, the EZ water moved upward and formed the supernatant because of the density difference between the EZ water and the bulk suspension. Because the supernatant and EZ water were particle-free, carbon black could diffuse back into them, following the concentration gradients between them and the bulk suspension. As the concentration of the bulk suspension increased during the phase separation, the formation of the EZ and the phase separation were perturbed by back-diffusion of the carbon black particles driven by their concentration gradient between the clear water and the concentrated suspension. Although the EZ water has been estimated to have a more ordered structure than bulk water (Yoo et al., 2011; Segarra-Martí et al., 2013), it could still be disrupted by back-diffusion of the colloidal particles at extremely high particle concentrations. Thus, the occurrence of phase separation was limited to a certain range of particle concentrations in our vertical setup, whereas the EZ could be observed within 10 min at all of the concentrations tested in this study.
Generally, the phase separation proceeded for 2–3 h and the supernatant sizes reached their maxima even at moderately high particle concentrations (Figure 4b). This behavior can be explained by the changes in the particle concentrations during the course of the EZ formation and the phase separation due to the limited volume of the bulk water in our experiments; i.e., concentration-driven particle back-diffusion could interrupt EZ formation and phase separation after 2–3 h. This explanation is supported by the aforementioned results, which show that no phase separation occurred at carbon black concentrations greater than 2.4 × 1013 /mL. Furthermore, EZ formation was slow at a high particle concentration as we observed in the polystyrene microsphere suspensions, and a decrease in the pH also interrupted both EZ formation and phase separation, as explained previously.
Therefore, a high particle concentration promotes phase separation because of the great density difference between the clear water and the concentrated suspension. However, when the particle concentration becomes excessively high in the suspension, both the EZ and supernatant formation processes are interrupted.
Mechanism of Phase Separation
Based on the above evidence, we postulated that the phase separation was driven by the density difference between the EZ water and the bulk suspension, which caused a buoyant movement of the EZ water to form the supernatant. Furthermore, the supernatant was believed to have an ordered structure that repels particles in a manner similar to the EZ water. To verify our hypothesis, we monitored both the phase separation and EZ formation phenomena using Nafion in microsphere suspensions at particle concentrations of 8.4 × 106 and 8.4 × 107 /mL for 2 or 3 h. The EZ size was defined as the distance between the Nafion surface and the boundary of the bulk suspension in the vertical setup observed from the top, whereas the supernatant size was measured in the vertical setup observed from the side in the same way as in the preceding sections. Because the minimum observation size of the microscope was ca. 1 µm, we used 2.62-µm polystyrene microspheres to better define the EZ size. These microspheres settle by gravity; therefore, we estimated the net supernatant size as the difference between the sizes with Nafion (i.e. the supernatant generated by both phase separation and gravity settling) and without Nafion (i.e. the supernatant generated by only gravity settling).
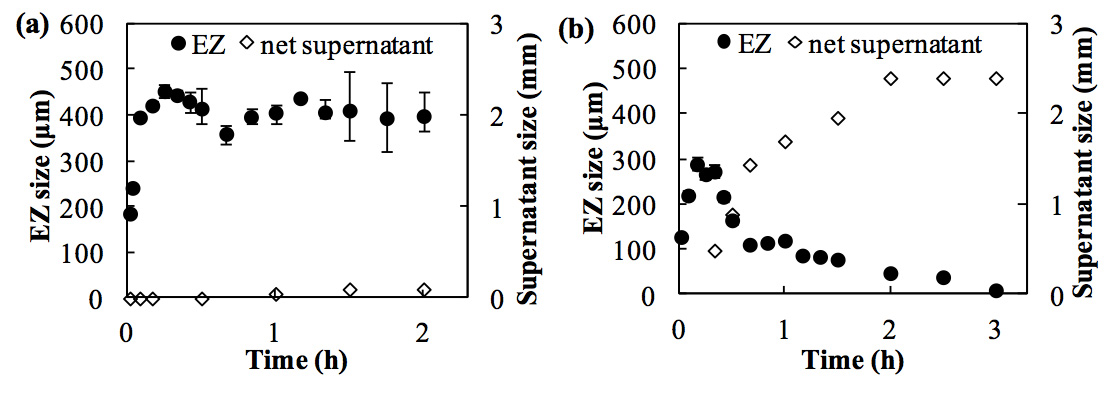
Figure 5: Long-term observation of the EZ size and the net supernatant size in the presence of Nafion in a 2.62-µm polystyrene microsphere suspension at (a) low (8.4 × 106 /mL) and (b) high (8.4 × 107 /mL) particle concentrations. Error bars show the maximum and minimum EZ size (n = 3).
At the lower particle concentration (8.4 × 106 /mL, Figure 5a), the EZ developed quickly within the first 10 min, as reported by Zheng et al. (2006) and Figueroa and Pollack (2011). Afterwards, the EZ size stabilized at approximately 400 ± 50 µm. No phase separation occurred within 2 h, which was in agreement with the report by Figueroa and Pollack (2011), wherein the EZ size was approximately 350–400 µm in a 99-min observation. The maximum size of the EZ was determined by the interfacial effect of the hydrophilic membranes on water, which can reach only a limited distance. This was verified by measuring the EZ force field with an infrared laser-tweezers system (Chen et al., 2012).
However, we observed that, at higher particle concentrations, the EZ size increased initially and then decreased until it became too small to be observed by the digital microscope. Conversely, the supernatant appeared after 20 min and its size grew progressively over a period of 3 h (Figure 5b). These results show a clear distinction between the low and high concentrations of particles for the EZ and phase separation behaviors, and the existence of a minimum particle concentration necessary for the phase separation to occur was confirmed. Because the EZ size shrank during the process of phase separation in all of the tested colloidal suspensions at high concentrations, the EZ water is thought to contribute to the growth of the supernatant.
Figure 6 shows the dynamic formation process of the phase separation. Evidently, the supernatant water is brought in by upward flow of the EZ water. The supernatant first appeared near the Nafion side of the cuvette (Figure 6a) and then gradually covered the surface of the suspension within 20 min (Figures 6b–f). To measure this flow, we plotted the volumes of the EZ water, the net supernatant, and the sum of these two volumes generated at a particle concentration of 8.4 × 107 /mL (Figure 7). The volume of EZ water was calculated by multiplying the Nafion surface area and the EZ size. The total clear water volume, which is the sum of the EZ water and the supernatant, grew progressively over a period of a few hours and then reached a plateau. This behavior clearly differed from the EZ formation behavior at a low particle concentration (8.4 × 106 /mL), as shown in Figure 5a, wherein EZ formation stabilized in less than 10 min. Conversely, Figure 7 shows that, at a high particle concentration (8.4 × 107 /mL), the EZ was produced with a relatively slower formation rate during the phase separation. Although the regeneration of the EZ is not well understood in the literature, we observed that the EZ existed for nearly 3 h even when the phase separation occurred (Figures 5b, 6f). We postulated that the water molecules are attracted from the bulk solution to form a new EZ and that the EZ is continually recreated under ideal conditions. Nevertheless, as explained previously, the regeneration of EZ could be interrupted by changes in the effective surface area, particle concentration, and solution pH. Hence, the formation of the EZ and supernatant terminated after 3 h.
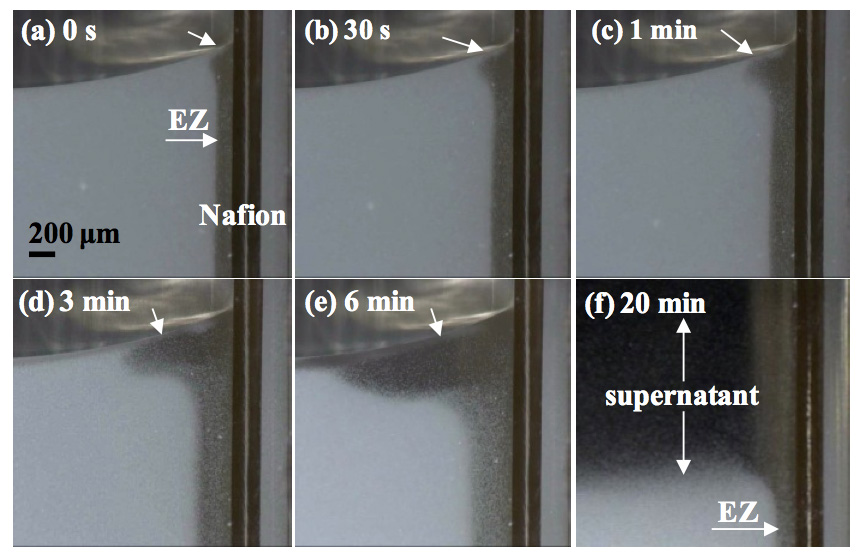
Figure 6: Time course of EZ formation and phase separation in the presence of Nafion in a 2.62-µm polystyrene microsphere suspension (8.4 × 107 /mL).
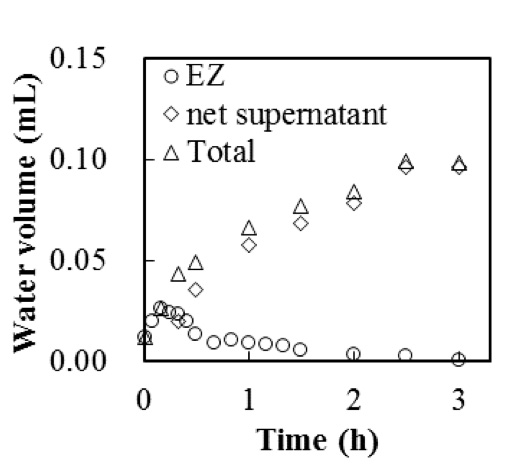
Figure 7: Volumes of EZ, supernatant, and total clear water generated in the presence of Nafion in a 2.62-µm polystyrene microsphere suspension (8.4 × 107 /mL) (data from Figure 5b).
Density differences between the clear liquid and a concentrated suspension have been reported to cause a buoyant movement of the clear liquid, even in a system of two miscible fluids (Boycott, 1920; Séon et al., 2004; Acrivos and Herbolzheimer, 2006). On the basis of our experimental results and the literature, we conclude that the formation of the supernatant is caused by the buoyant upward movement of EZ water generated by the hydrophilic surface. When an EZ forms, three regions with different densities exist: near the hydrophilic surface, the density is similar to the density of pure water because of particle exclusion in the EZ; in the vicinity of the EZ, the density of the suspension is higher than the initial bulk suspension because particles have accumulated in this region; far away from the hydrophilic surface, the density of the bulk suspension is equal to that of the initial bulk suspension. Conventional Rayleigh–Taylor instability dictates the behavior at the liquid interface between two fluids (Read, 1984; Sharp, 1984), and a lower density layer is placed on top of a denser layer.
In our system, the lighter layer of the EZ water is lifted to the top by buoyancy to form the supernatant. This process requires sufficient buoyancy to overcome the viscosity of water; hence, it occurs only when the EZ is sufficiently large and/or when the particle concentration is sufficiently high, because interfacial water usually has viscosity greater than that of the bulk water (Nimtz and Weiss, 1987; Smith et al., 2004; Goertz et al., 2007; Totland et al., 2013). In this regard, critical values of EZ size and particle concentration beyond which the EZ water starts moving upward may exist. For instance, because the average EZ size (236µm) in the carbon black suspensions was approximately half of the EZ size in the microsphere suspensions (Figure 5a), a higher density difference between the bulk carbon black suspension and the EZ water would be necessary for supernatant formation.
Meanwhile, we found the supernatant sizes produced by Nafion in amino, carboxylate, and sulfate microspheres suspensions varied significantly in 3 h, although the EZ sizes of these three microsphere suspensions were similar in 5-min observation. These three microspheres have similar particle sizes and the same density. The supernatant size in the amino microsphere suspension was even greater than that in the carbon black or silica suspension, which has a higher particle density than the amino microspheres. These results indicate that the growth of supernatant is dependent on the long-term development of EZ formation. In addition, the buoyant movement indicates that the EZ, which is estimated to have an ice-like, ordered structure, can flow like a liquid, as is the case with liquid crystals (McMillan, 1971; Blinov, 2011).
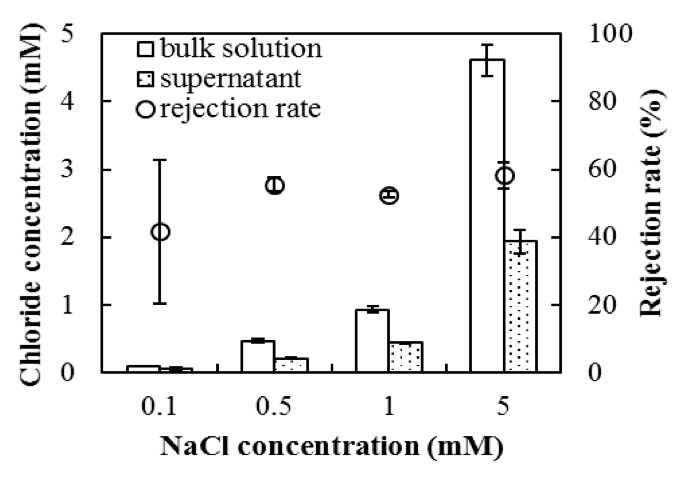
Figure 8: Rejection of chloride ion in the supernatant after 1 h of phase separation (n = 5). The error bars show the SD.
Therefore, the regeneration of the EZ by the hydrophilic surface and the circulation of EZ water by a buoyancy force leads to phase separation. The formation of a supernatant is expected to proceed for longer than 3 h when the effective surface area, particle concentration, and solution pH remain the same during the phase separation process. The exclusion of particles in the supernatant implies that the EZ water structure is maintained for a long period, even when the EZ is separated from the surface–suspension interface to form the supernatant.
Salt Rejection and the Structure of the EZ Water
As the long-range ordered water hypothesis for EZ formation has suggested (Zheng et al., 2006; Chai et al., 2009), the EZ water has an ice-like structure; thus, the EZ repels various substances, e.g., low-molecular-weight dyes and colloidal particles (Zheng et al., 2006), just as ice does (Schulson, 1999). However, the rejection of salt in the EZ was still unknown because of the difficulty in monitoring the EZ at the microscale level. Because we demonstrated that the origin of the supernatant is the EZ water, we can study the EZ water by analyzing the supernatant. Thus, to further clarify the structure of the EZ, sodium chloride was added into the carbon black suspension and the concentration of chloride ions in the supernatant was analyzed. Because Nafion is a cation-exchange membrane with sulfonic acid functional groups, the concentration of the sodium ions could change due to ion exchange (Mauritz and Moore, 2004). However, the chloride ions cannot be exchanged by Nafion. In addition, because carbon black is coated with carboxylate functional groups, the chloride ions cannot interact with carbon black.
As shown in Figure 8, the concentration of chloride ions in the supernatant was lower than their initial concentration in the bulk suspension and the average rejection rate was in the range of 40–60%. These results appear to be independent of the initial salt concentration in the range of 0.1–5 mM.
Thus, not only were particles excluded, but a solute, i.e., chloride ion, was also excluded from the EZ by Nafion. To our knowledge, such a salt rejection in an EZ has not been previously reported. However, water has long been desalinated by the freezing–melting of seawater, even as far back as the 1600s (Nebbia and Menozzi, 1968). According to historical techniques, fresh water is produced by freezing a saline solution and melting the ice crystals (Rahman and Al-Khusaibi, 2014), which are ideally free of salts. Hence, we posit that the EZ, which is the origin of the supernatant, can repel dissolved ions by forming an ice-like structure with liquid-like structure boundaries, which is analogous to the freezing process (Rubinsky, 1983; Schulson, 1999; Vrbka and Jungwirth, 2005). In addition, the electrostatic repulsion between the EZ and chloride ions might also contribute to the exclusion of chloride ions because the EZ created by Nafion is negatively charged (Zheng et al., 2006). The initial EZ layer forms near the hydrophilic surface, and the layers of the EZ then stack onto each other through hydrogen bonding (Tiezzi, 2003; Yoo et al., 2011; Segarra-Martí et al., 2013, 2014). Finally, the EZ stops extending into the bulk solution because of the limitation of the effective range of hydrophilic surfaces. Particles and ions cannot pass through the planar EZ layer because of the hexagonal ice-like structure (Segarra-Martí et al., 2014), and they are therefore excluded from the EZ lattice in the long-range water ordering process.
However, unlike the exclusion of particles, some chloride ions remained in the supernatant, demonstrating that the EZ water interacts with dissolved ions in a different manner than it interacts with particles. Because ions are typically considered as “structure breakers” because of their hydration effects (Chen et al., 2007; Yang et al., 2009; Ninham et al., 2011), the low rejection rate of ions in the EZ is attributed to the interplay between dissolved ions and the molecular structure of the EZ water. Using a spectroscopic technique, the hydrogen-bonding network of the interfacial water was revealed to consist of ordered (ice-like) and disordered (liquid-like) structures (Ostroverkhov et al., 2005). Because ions cannot be repelled by a disordered structure, the rejection rate of the ion cannot reach 100%. Even in the case of ice, ions can be trapped inside the ice crystals at high salt concentrations (Rubinsky, 1983; Vrbka and Jungwirth, 2005). Therefore, the EZ contains a mixture of ordered and disordered structured water and some chloride ions, but at concentrations less than their concentration in the bulk solution. During the phase separation, the EZ moves to the surface and forms a supernatant. During the upward movement of the EZ water, sodium chloride from the bulk solution might be entrained into the EZ water; thus, the chloride rejection in the supernatant of 40–60% might be less than that in the EZ water.
In summary, the unique characteristics of the supernatant—specifically, the exclusion of particles and ions—originate from the EZ water with an ice-like structure. Our experimental results of salt rejection favor the long-range-ordered water hypothesis for EZ formation over other models (Schurr, 2013; Schurr et al., 2013; Florea et al., 2014) that suggest that the exclusion of particles in the EZ is driven by ionic concentration gradients.
Conclusions
Spontaneous particle separation and salt rejection by a hydrophilic surface were observed in the supernatant formed above a colloidal suspension with a high particle concentration. The supernatants (with sizes from < 1 mm to 5 mm) were free of colloidal particles and had chloride concentrations 40–60% lower than those in the initial bulk suspensions. The supernatants are derived from the upward flow of EZ water induced by the hydrophilic surface. The density difference between the EZ water and the concentrated particle suspension drives the flotation of the EZ water, which consequently forms the supernatant. The phase separation phenomenon can be observed only in a certain range of particle concentrations and only in the presence of a hydrophilic surface. Increasing the particle concentration promotes flotation of the EZ water by a buoyancy force, but extremely high particle concentration disrupts EZ formation; thus, the phase separation only occurs in a certain range of particle concentrations. A larger area of hydrophilic surfaces generates larger volumes of the EZ water and the supernatant. However, the benefit of the increased surface area is limited by the decrease of the effective surface area over the course of the phase separation.
The particle-exclusion and desalting properties of the supernatant demonstrate an ordered structure of the EZ water. This evidence implies that the EZ is reproducible under ideal conditions and that the EZ water remains organized to some extent after it is removed from the hydrophilic surface. Because the EZ water could be harvested during the phase separation, its properties could be probed further. Because of the intrinsic simplicity of this phase separation, this process may lead to the development of new methods for solid–liquid separations and desalination.
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research #26303013 provided by the Japan Society for the Promotion of Science (JSPS).
References
Acrivos A and Herbolzheimer E (2006) Enhanced sedimentation in settling tanks with inclined walls. J. Fluid Mech. 92, 435.
Barry PH and Diamond JM (1984) Effects of unstirred layers on membrane phenomena. Physiol. Rev. 64, 763–872.
Bass M, Berman A, Singh A, Konovalov O, Freger V (2010) Surface Structure of Nafion in Vapor and Liquid. J. Phys. Chem. B 114, 3784–3790.
Blinov LM (2011) Magnetic, Electric and Transport Properties, in: Structure and Properties of Liquid Crystals. Springer Netherlands, Dordrecht, pp. 151–187.
Boycott AE (1920) Sedimentation of Blood Corpuscles. Nature 104, 532–532.
Bunkin NF, Gorelik VS, Kozlov VA, Shkirin AV, Suyazov NV (2014) Colloidal Crystal Formation at the “Nafion–Water” Interface. J. Phys. Chem. B 118, 3372–3377.
Chai BH, Yoo H, Pollack GH (2009) Effect of Radiant Energy on Near-Surface Water. J. Phys. Chem. B 113, 13953–13958.
Chai BH, Zheng JM, Zhao Q, Pollack GH (2008) Spectroscopic Studies of Solutes in Aqueous Solution. J. Phys. Chem. A 112, 2242–2247.
Chen CS, Chung WJ, Hsu IC, Wu CM, Chin WC (2012) Force Field Measurements within the Exclusion Zone of Water. J. Biol. Phys. 38, 113–120.
Chen X, Yang T, Kataoka S, Cremer PS (2007) Specific Ion Effects on Interfacial Water Structure near Macromolecules. J. Am. Chem. Soc. 129, 12272–12279.
Del Giudice E, Tedeschi A, Vitiello G, Voeikov V (2014) The origin and the special role of coherent water in living systems, Fields of the Cell: 91-107 Editors: Daniel Fels and Michal Cifra
Elia V, Napoli E, Niccoli M (2013) Physical–Chemical Study of Water in Contact with a Hydrophilic Polymer: Nafion. J. Therm. Anal. Calorim. 112, 937–944.
Figueroa XA and Pollack GH (2011) Exclusion-Zone Formation from Discontinuous Nafion Surfaces. Int. J. Des. Nat. Ecodynamics 6, 286–296.
Florea D, Musa S, Huyghe JMR, Wyss HM (2014) Long-Range Repulsion of Colloids Driven by Ion Exchange and Diffusiophoresis. Proc. Natl. Acad. Sci. U.S.A. 111, 6554–6559.
Goertz MP, Houston JE, Zhu XY (2007) Hydrophilicity and the Viscosity of Interfacial Water. Langmuir 23, 5491–5497.
Goswami S, Klaus S, Benziger J (2008) Wetting and Absorption of Water Drops on Nafion Films. Langmuir 24, 8627–8633.
Granick S and Bae SC (2008) A Curious Antipathy for Water. Science 322, 1477–1478.
Head-Gordon T and Johnson ME (2006) Tetrahedral Structure or Chains for Liquid Water. Proc. Natl. Acad. Sci. U.S.A. 103, 7973–7977.
Israelachvili J and Wennerström H (1996) Role of Hydration and Water Structure in Biological and Colloidal Interactions. Nature 379, 219–225.
Köfinger J, Hummer G, Dellago C (2008) Macroscopically Ordered Water in Nanopores. Proc. Natl. Acad. Sci. U.S.A. 105, 13218–13222.
Mauritz KA and Moore RB (2004) State of Understanding of Nafion. Chem. Rev. 104, 4535–4586.
McMillan WL (1971) Simple Molecular Model for the Smectic A Phase of Liquid Crystals. Phys. Rev. A 4, 1238–1246.
Nebbia G and Menozzi GN (1968) Early experiments on water desalination by freezing. Desalination 5, 49–54.
Nimtz G and Weiss W (1987) Relaxation time and viscosity of water near hydrophilic surfaces. Z. Für Phys. B Condens. Matter 67, 483–487.
Ninham BW, Duignan TT, Parsons DF (2011) Approaches to Hydration, Old and New: Insights through Hofmeister Effects. Curr. Opin. Colloid Interface Sci. 16, 612–617.
Ostroverkhov V, Waychunas GA, Shen YR (2005) New Information on Water Interfacial Structure Revealed by Phase-Sensitive Surface Spectroscopy. Phys. Rev. Lett. 94, 046102.
Ovchinnikova K and Pollack GH (2009) Cylindrical phase separation in colloidal suspensions. Phys. Rev. E 79, 036117.
Piazza R, Buzzaccaro S, Secchi E (2012) The unbearable heaviness of colloids: facts, surprises, and puzzles in sedimentation. J. Phys. Condens. Matter 24, 284109.
Rahman MS and Al-Khusaibi M (2014) Freezing-Melting Desalination Process, in Desalination: Kucera, J. (Ed.), John Wiley & Sons, Inc.: Hoboken, U.S.A., pp. 473–501.
Read KI (1984) Experimental Investigation of Turbulent Mixing by Rayleigh-Taylor Instability. Phys. Nonlinear Phenom. 12, 45–58.
Ruan CY, Lobastov VA, Vigliotti F, Chen S, Zewail AH (2004) Ultrafast Electron Crystallography of Interfacial Water. Science 304, 80–84.
Rubinsky B (1983) Solidification Processes in Saline Solutions. J. Cryst. Growth 62, 513–522.
Schulson EM (1999) The Structure and Mechanical Behavior of Ice. JOM 51, 21–27.
Schurr JM (2013) Phenomena Associated with Gel–Water Interfaces. Analyses and Alternatives to the Long-Range Ordered Water Hypothesis. J. Phys. Chem. B 117, 7653–7674.
Schurr JM, Fujimoto BS, Huynh L, Chiu DT (2013) A Theory of Macromolecular Chemotaxis. J. Phys. Chem. B 117, 7626–7652.
Segarra-Martí J, Coto PB, Rubio M, Roca-Sanjuán D, Merchán M (2013) Towards the Understanding at the Molecular Level of the Structured-Water Absorption and Fluorescence Spectra: A Fingerprint of π-stacked Water. Mol. Phys. 111, 1308–1315.
Segarra-Martí J, Roca-Sanjuán D, Merchán M (2014) On the Hexagonal Ice-Like Model of Structured Water: Theoretical Analysis of the Low-Lying Excited States. Comput. Theor. Chem. 1040–1041, 266–273.
Séon T, Hulin JP, Salin D, Perrin B, Hinch EJ (2004) Buoyant Mixing of Miscible Fluids in Tilted Tubes. Phys. Fluids 16, L103–L106.
Sharp DH (1984) An Overview of Rayleigh-Taylor Instability. Phys. Nonlinear Phenom. 12, 3–18.
Smith JD, Cappa CD, Wilson KR, Messer BM, Cohen RC, Saykally RJ (2004) Energetics of Hydrogen Bond Network Rearrangements in Liquid Water. Science 306, 851–853.
Speedy RJ (1997) Waterlike Anomalies from Repulsive Interactions. J. Chem. Phys. 107, 3222–3229.
Stanley C and Rau DC (2011) Evidence for Water Structuring Forces between Surfaces. Curr. Opin. Colloid Interface Sci. 16, 551–556.
Stiopkin IV, Weeraman C, Pieniazek PA, Shalhout FY, Skinner JL, Benderskii AV (2011) Hydrogen Bonding at the Water Surface Revealed by Isotopic Dilution Spectroscopy. Nature 474, 192–195.
Tiezzi E (2003) NMR Evidence of a Supramolecular Structure of Water. Ann. Chim. 93, 471–476. PMID: 12817648
Totland C, Lewis RT, Nerdal W (2013) Long-Range Surface-Induced Water Structures and the Effect of 1-Butanol Studied by 1H Nuclear Magnetic Resonance. Langmuir 29, 11055–11061.
Vrbka L and Jungwirth P (2005) Brine Rejection from Freezing Salt Solutions: A Molecular Dynamics Study. Phys. Rev. Lett. 95, 148501.
Yang Z, Li Q, Chou KC (2009) Structures of Water Molecules at the Interfaces of Aqueous Salt Solutions and Silica: Cation Effects. J. Phys. Chem. C 113, 8201–8205.
Ye S, Nihonyanagi S, Uosaki K (2001) Sum Frequency Generation (SFG) Study of the pH-Dependent Water Structure on a Fused Quartz Surface Modified by an Octadecyltrichlorosilane (OTS) Monolayer. Phys. Chem. Chem. Phys. 3, 3463–3469.
Yoo H, Paranji R, Pollack GH (2011) Impact of Hydrophilic Surfaces on Interfacial Water Dynamics Probed with NMR Spectroscopy. J. Phys. Chem. Lett. 2, 532–536.
Zheng JM, Chin WC, Khijniak E, Khijniak Jr E, Pollack GH (2006) Surfaces and Interfacial Water: Evidence that Hydrophilic Surfaces Have Long-Range Impact. Adv. Colloid Interface Sci. 127, 19–27.
Zheng JM and Pollack GH (2003) Long-Range Forces Extending from Polymer-Gel Surfaces. Phys. Rev. E 68, 031408.
Discussion with Reviewers
Editor: According to your data, a supernatant with EZ-water-like properties forms on top of the aqueous system containing a high concentration of particles and kept in a vessel with highly hydrophilic vertical wall. Supernatant is located at the water-air boundary at which according to Pollack et al. data EZ-water is formed (Pollack’s book “The Fourth Phase…”, pp. 283-foll.). According to him EZ-water there was formed at “low” microsphere concentration and without Nafion. How can you explain the discrepancy between his and your results? Can the formation of the EZ-zone on water-air surface depend upon the geometry of the vessel, surface area or something else?
Zhang Y, Takizawa S, and Lohwacharin J: In the studies of Pollack’s group (Ovchinnikova and Pollack, 2009; Mork and Pollack, 2009), the particle exclusion underneath the water-air interface without Nafion occurred in beakers under certain condition with the success rate of less than 50%. However, in our study, as shown in Figure 2, the supernatant appeared in all the experiments with Nafion, whereas no supernatant formation was observed in all the cuvettes without Nafion or with hydrophobic membranes. These results suggested that hydrophilic surfaces such as Nafion is necessary for the supernatant formation. In addition, the ratios of height to width in our study were 1, 2, and 4, which were beyond the experimental range of Ovchinnikova and Pollack’s study; this implies that the geometry of the container is irrelevant to the supernatant formation. Based on these results, we think our finding is different from the phenomenon reported by Ovchinnikova and Pollack.
Editor: Can supernatant in your case appear if you use a cuvette completely filled with particle suspension covered with a cap isolating water from the air?
Zhang, Takizawa, and Lohwacharin: Yes, we found the supernatant even when water was isolated from the air. This result also indicates that our findings are different from the results of Ovchinnikova and Pollack (2009).
Editor: Pollack suggests that in the oceans an EZ-water like phase may extend down from the surface by tens of meters. Do you think that if you take water with a high concentration of salts present in ocean water (e.g. Na-Mg chloride) you’ll see the formation of supernatant in your experimental system?
Zhang, Takizawa, and Lohwacharin: We did not find the formation of EZ and supernatant with Nafion when the concentration of NaCl in carbon black suspensions was greater than 0.5 M. We do not think supernatant can be generated in suspension with high concentration of salt, because no EZ forms under such condition.
Anonymous Reviewer 1: Is there a difference between the tested types of microspheres and their functional groups (Table 1) in their ability to reveal / interact with / EZ water formation?
Zhang, Takizawa, and Lohwacharin: The formation of EZ was found in all the tested suspensions, while the sizes of EZs varied. Similar to the result of Zheng and Pollack (2003), we found that EZ size decreased with the diameter of particles. As for the effects of particle functional groups, the EZ sizes for the microspheres with different functional groups were almost the same in the 5-min observation in our study.
Reviewer 1: Are colloidal suspensions only indicators of Exclusion Zones, or do they contribute to their formation, beyond just modifying local densities?
Zhang, Takizawa, and Lohwacharin: We used colloidal suspensions to visualize the EZ formation process. However, as we mentioned in our answer to the previous question, the properties of particle influence the formation of EZ; namely, the EZ size is dependent on the colloidal particles used in our study. In addition, as reported previously (Schurr, 2013; Schurr et al., 2013; Florea et al., 2014), the migration of particles in suspensions can be driven by other mechanisms, e.g. diffusiophoresis, which makes it difficult to define and accurately measure the EZ formation.
Reviewer 1: In tree and wood research, lunar phase – related variations have been observed in the binding forces between wood and water. The wood cell walls are hydrophilic by nature, have additionally dielectric properties and can be considered as anisotropic gels. So, could you imagine the possibility of lunar variations in the formation of EZ-water and of supernatant layers? What would be the simplest device for testing this hypothesis?
Zhang, Takizawa, and Lohwacharin: Radiant energy is found to be the energy source for the EZ formation (Chai et al. 2009), but it is possible that there are other energy sources for the EZ formation because Earth receives all kind of energies from the cosmos, e.g., gravitation or electromagnetic fields. Lunar rhythm may cause the variation of external cosmic energy input and thus lead to the change of coherent oscillations between liquid water and surrounding surfaces. As a result, the formation rate of EZ or EZ-like water near the wood cell walls fluctuates and the variation of the ratio of bound water and free water in the cells influences the growth of tree and wood. On the other hand, since our study pointed out that EZ water could flow under some condition, the intertubular flow of water might also be generated inside xylem vessels. This water flow can be increased with more external energy input and somehow plays a positive role on the growth of tree and wood. This hypothesis may be tested by conducting the nuclear magnetic resonance (NMR) analysis of the water in xylem vessels.
Anonymous Reviewer 2: I would like to request the authors to discuss the basic substance comprising the microspheres listed in Table 1. This is important to note, because the basic material comprising the microspheres will affect the density of the microspheres, and the density, of course, plays a role in gravitational effects including buoyancy or sinking. For example, carboxylated polystyrene microspheres have a density about the same as water and will not tend to settle out, but silica microspheres have a density about twice that of water and will sink faster and tend to settle out. Could they also discuss the influence of colloid particle density on the supernatant formation observed?
Zhang, Takizawa, and Lohwacharin: The formation of supernatant is dependent on the EZ formation process and the EZ formation is affected by various factors, e.g., the properties of particles and solution chemistry. In some cases, because these factors influence the EZ formation in a confounding manner, it is hard to isolate the effect of each factor, especially in the long-term observation. For example, the amino, carboxylate, and sulfate microspheres have the same particle size and density as shown in Table 1, and produced nearly the same EZ size in 5-min observation. However, we found the supernatant sizes varied significantly in three hours in these three microsphere suspensions. The supernatant size in the amino microsphere suspension was even greater than those in carbon black or silica suspension. Thus, we think it is hard to draw conclusion in this manuscript, but it is worth clarifying the effect of particle density on the supernatnat formation in our future investigation.
Reviewer 2: Please read the paper by Jabs & Rubik (Self-Organization at Aqueous Colloid-Membrane Interfaces and an Optical Method to Measure the Kinetics of Exclusion Zone Formation. 2014. Entropy 16(11), 5954-5975.), which describes the visible movement of microscopic vortices in the fluid near the Nafion membrane interface. Do you think such vortices may be involved in the formation of the “supernatants” that you observe in your experimental setup?
Zhang, Takizawa, and Lohwacharin: The vortices observed by Jabs and Rubik (2014) may be related to our finding, as the EZ was generated by the edge of Nafion strip in their study. They immersed a Nafion strip (0.187 cm thick) in the microsphere suspension that was sandwiched between two glass slides. In other words, the experimental setup was vertical. We observed similar phenomena in suspensions with relatively low particle concentrations. We believe the density difference between the EZ water and the bulk suspension is not large enough to float the EZ water to form the supernatant at the relatively low particle concentrations. However, this density difference may be great enough to cause EZ water flow or circulate in the suspension and thus lead to the buildup of vortices in suspensions. Besides, the curvature of the Nafion strip in contact with suspension may also be relevant because the distribution of EZ along with the Nafion strip cannot be uniform if the strip is not flat. One more factor is that a complex flow patterns of the suspension can be generated when the gravitational force is perpendicular to the direction of particle-exclusion (Florea et al., 2014).
Reviewer 2: Why did the phase separation take place only within a certain particle concentration range?
Zhang, Takizawa, and Lohwacharin: At low particle concentration, the density difference between the EZ water and the bulk water is too small to cause buoyancy force, whereas at high particle concentration, the EZ formation is disrupted.
Reviewer 2: Ambient light has been shown to be an energy source for the formation of exclusion zones. What is the role of ambient light on the kinetics of formation and size of the “supernatants” that you observe? How might light or infrared energy impact this self-organization and non-equilibrium process of supernatant formation?
Zhang, Takizawa, and Lohwacharin: We did not investigate the effect of light on the supernatant formation systematically, but we’ve conducted the phase separation experiment both in the dark and under the ambient laboratory illumination. We found the formation of supernatant in both cases and the supernatant sizes in 3 h was almost the same. This result suggests that the ambient laboratory illumination has little effect on the supernatant formation. As Chai et al. (2009) pointed out, the input of infrared energy effectively increased EZs. Since it is impossible to shield the input of infrared energy even we turn off all the light in the laboratory, infrared energy may be more important for the supernatant formation. Moreover, it is reasonable to believe that the formation rate of supernatant increases with the input of infrared energy, because the supernatant originates from the EZ water.
Reviewer 2: If we make reference to Gerald Pollack’s book, at p. 35 – 37 (Figure 3.13b), and at many other places, EZ-water has a specifically high energy absorption in the 270 nm range. My problem is that this range is NOT the infrared range of the light spectrum, but the lower limit of the UV-B zone (UV-B: 280 – 315 nm) of the CIE-classification.
Zhang, Takizawa, and Lohwacharin: It was reported that the incident radiant energy in the UV-visible and IR ranges expanded the EZ size (Chai et al., 2009 and p. 87, the book authored by Pollack). However, this light-induced expansion was spectrally sensitive; the greatest expansion of the EZ size was observed in the mid-IR range (i.e. 3.1 μm), which corresponds to the O-H stretch vibration (2.9-3.25um). This resonance with O-H bonding and the consequent vibrational excitation may lead to the reorganization of water molecules. Hence, the IR energy is considered to be the major energy source for the EZ formation.
DWR References
Mork AJ, Pollack GH (2009). New Observation at the Air-Water Interface. J. Undergrad. Res. Bioeng. Univ. Wash. 105–113.
Chai B, Yoo H, and Pollack GH (2009). Effect of Radiant Energy on Near-Surface Water. J Phys Chem B 113, 13953–13958.
Florea D, Musa S, Huyghe JMR, and Wyss HM (2014). Long-range repulsion of colloids driven by ion exchange and diffusiophoresis. Proc. Natl. Acad. Sci. U.S.A. 111, 6554–6559.
Ovchinnikova K, Pollack GH (2009). Cylindrical phase separation in colloidal suspensions. Phys. Rev. E 79, 036117.
Pollack GH (2013). The Fourth Phase of Water. Ebner & Sons Publishers, Seattle WA, USA.
Schurr JM (2013). Phenomena Associated with Gel–Water Interfaces. Analyses and Alternatives to the Long-Range Ordered Water Hypothesis. J. Phys. Chem. B 117, 7653–7674.
Schurr JM, Fujimoto BS, Huynh L, and Chiu DT, (2013). A Theory of Macromolecular Chemotaxis. J. Phys. Chem. B 117, 7626–7652.
Zheng JM and Pollack GH (2003). Long-range forces extending from polymer-gel surfaces. Phys. Rev. E 68, 031408.
