 Serial pH Increments (~20 to 40 Milliseconds) in Water During Exposures to Weak, Physiologically Patterned Magnetic Fields: Implications for Consciousness
Serial pH Increments (~20 to 40 Milliseconds) in Water During Exposures to Weak, Physiologically Patterned Magnetic Fields: Implications for Consciousness
Murugan NJ1,2,3, Karbowski LM2,3, Persinger MA1,2,3*
1Department of Biology, Laurentian University, Sudbury, Ontario, Canada P3E 2C6
2Behavioural Neuroscience Program, Laurentian University, Sudbury, Ontario, Canada
3Biomolecular Sciences Program, Laurentian University, Sudbury, Ontario, Canada
*Correspondence E-mail: mpersinger@laurentian.ca
Key Words: Water, Complex magnetic fields, pH shifts, MicropH jumps, Intrinsic ~40 Hz electromagnetic fields
Received Aug 17th, 2013; Revised Oct 18th, 2013; Accepted Feb 26th, 2014; Published March 25th, 2014; Available online April 15th, 2014
Abstract
The pH values for volumes (50 ml) of spring water were measured for 12 hours while being exposed to a weak (8±4 µT) decelerating frequency-modulated magnetic field that has been shown to diminish the growth of cancer cells and inhibit the movement of planarian. Compared to sham field-exposed water, the magnetic field-exposed water displayed a greater increase in pH (towards alkalinity) that involved an increase between 0.5 and 1 pH units after about 7 to 8 hr. This shift occurred slowly as successive 0.02 pH transient peaks (about 7 per s) that were between 20 to 40 ms in duration. This pattern was not observed in water exposed to background conditions (0.11 µT). These results are consistent with the hypothesis that properties of water exposed to specific patterns of magnetic fields produced by a patterned series of 3 ms voltage durations generated from computer software can produce transient temporal properties in water that converge with those associated with the cerebral cortical activity coupled to consciousness. Several quantitative solutions for small and very large volumes of water support this possibility.
Article Outline
- Introduction
- Materials and Methods
- Results
- Discussion
- Acknowledgments
- References
- Discussion with Reviewers
Introduction
The chemical and electromagnetic properties of water (House, 1974) have been considered the fundamental substrate that defines the boundaries of living systems (Pollack, 2003; Pollack et al., 2009). The continuous flux of H+ ions mediated through transient hydronium ions (H3O+) within a maintained structure (a matrix of H2O molecules) indicates that the spatial pattern of the aggregate is maintained indefinitely while specific microstructures are ephemeral (Decoursey, 2003). This physical combination of statics and dynamics sets the conditions for a substance that can interact with its environment and represent these interactions yet maintain a structural momentum that defines the individuality of the set (DeMeo, 2011).
The universality of the water molecule and its connection to fundamental forces is reflected by its diffusivity (0.88∙10-7 m2∙s-1) obtained by dividing the magnetic moment of a proton (1.41∙10-26 A∙m2) by the unit charge (1.6∙10-19 A∙s). When the diffusivity is multiplied by the typical viscosity of water (1.0∙10-3 kg∙m-1s-1) at 20°C the resulting force of 8.80∙10-11 kg∙m∙s-2 across the width of two O-H bonds (1.92∙10-10 m) is 1.7∙10-20 J. This value is within the range of measurement error for the energy of the second shell hydrogen bond which is ~1.8∙10-20 J and reflects proton mobility (Decoursey, 2003). The quantum of 10-20 J (Persinger, 2010) may be a universal value that reflects the intrinsic average forces within the fine structure of space distributed across the neutral hydrogen wavelength (Persinger et al., 2008).
The differentiation and measurement of water as bulk and interfacial phases indicated that when this compound was near any surface electrical properties arose that simulated the conditions hereto attributed to plasma membranes and the separation of ions (Del Giudice and Preparata, 1994). Several authors (Pollack, 2003; Del Giudice et al., 2010; 2011) have shown that water adjacent to a surface displays ten times the viscosity than bulk water and exhibits a narrow shell of enhanced proton charges at the interface between the bulk-interfacial zones. The range of potential differences is comparable to the resting plasma membrane potential (~100 mV). The role of proton flux, per se, as the source for all membrane phenomena (traditionally attributed to disparities of charges from ions across the membrane) is suggested by the copious nature of proton channels within membranes and the fact that an incredible range of currents within these channels is pH dependent (Decoursey, 2003). In addition clusters of molecules within a coherent phase have the capacity to maintain or represent electromagnetic fields applied exogenously or originating within the interactions between water molecules (Del Giudice and Preparata, 1994).
The spatial segregation of H+, which defines pH, indicates that this phenomenon could be employed to assess the effects of appropriately-patterned magnetic fields upon water. Minute changes in pH are associated with brain activity (Kaila and Ransom, 1968). Alkaline pH shifts exhibit a rapid onset and can be maintained as long as the stimulus is applied. Increasing extracellular pH and lowering intracellular pH often precede neuronal activity within the cerebral cortices and hippocampus and are associated with the opening of H+ channels (Elder and Decoursey, 2001). Shifts of pH from 7.9 to 7.2 produce currents in outside membrane patches which occur within temporal intervals of 10 and 40 ms (Bevan, 1998). For comparison the three major enzyme complexes of the respiratory chain donate and receive an electron once every 5 to 20 ms (Alberts et al., 2002).
The water molecule has been hypothesized to be the central thread through which even more complex processes, such as consciousness, are mediated if not created (Amoroso, 1999). The fundamental dynamic unit of the neuron, the single ion channel, is associated with a discrete change of 0.2 µV (Kandel et al., 2000). The energy exerted on a charge (1.6∙10-19 A∙s) is 0.5∙10-25 J (Persinger and Lavallee, 2012). When divided by the mass of a proton (1.67∙10-27 kg) the average velocity is ~4.5 m∙s-1. This is the bulk velocity of cortical fields moving through the cerebral cortices (Nunez, 1995) and would require ~20 to 30 ms to traverse its linear distance. The time required for the coherent electromagnetic fields (McFadden, 2002; 2007) associated with different cognitive states (particularly waking and dreaming) to move along the rostral-caudal axis of the cerebral cortices is about 20 to 25 ms with phase shifts in the order of 10 to 12 ms (Llinas and Pare, 1991; Llinas and Ribary, 1993). Emoto (2011) has suggested that properties of consciousness may be an intrinsic feature of water or that its kinetics can be influenced by cerebral activity. Radin et al. (2006) recently provided experimental evidence to support the latter possibility.
The most conspicuous physical feature of consciousness is its multiple electromagnetic properties (Persinger and Lavallee, 2012; 2010). Our recent research indicates that application of weak (µT range) physiologically-patterned magnetic fields with the appropriate point voltage durations produced marked and predictable changes in activity within the cerebrum (Saroka and Persinger, 2013) as measured by quantitative electroencephalography and sLORETA (low resolution electromagnetic tomography). Specific changes are associated with subjective alterations in consciousness and phenomena consistent with altered states. The mechanism is more related to the representation of the energy from the applied field within the cerebral volume rather than a Faradic-related mechanism (Persinger et al., 2010) although the latter could be significant at quantum energy levels as secondarily induced magnetic fields from the electric fields associated with the applied magnetic fields. It has been known for some time that global shifts in brain pH towards alkalinity are proconvulsant while shifts towards acidity are anticonvulsant (Kaila and Chesler, 1989).
Given these considerations we examined the effects of the same complex time-varying magnetic field (that produced altered states of consciousness in human subjects) upon changes in pH in spring water. It was selected because of its similarity to cellular and extracellular fluids. When 50 ml of spring water were placed in open beakers and not disturbed for 12 hr there was a gradual drift towards alkalinity (e.g., pH 7.75 to 8.21). Such slow dynamics have been considered optimal to allow interaction with applied magnetic fields. When this volume was exposed to the frequency-modulated magnetic field that is known to induce the conditions necessary to produce altered states in human subjects (Saroka and Persinger, 2013) and electrical lability in epileptic rats (Persinger and Belanger-Chellew, 1999), we observed multiple discrete bursts or shifts, with durations primarily between 20 and 40 ms, in pH. The effect was so conspicuous we designed a series of experiments to investigate this phenomenon.
Materials and Methods
“Beakers” (105 ml flint glass jars) containing 50 ml of spring water (4 mM of HCO3; 1.77 mM Ca; 76 µM of Cl; 1.3 mM; of Mg, 41.9; µM of NO3; 61 µM SO4 ; 17.9 µM K; 43.5 µM Na) were exposed continuously for 12 hours to either a physiologically patterned magnetic field (4.4 to 11.5 μT, RMS) or to background conditions. The intensity within the background (8 m from the coil), from 60 Hz power frequencies, was about 0.11 µT and was typical for the general area. By “physiologically patterned” we mean magnetic fields whose complex and asymmetrical time-variations are similar to those generated by aggregates of neurons. There were a total of 8 replicates for the field and 8 replicates for the reference conditions. The shape of the applied magnetic field was frequency modulated (Figure 1), that is, it displayed an irregular decrease in phase modulation. The pattern was generated by converting 859 numbers, each with a value between 0 and 256, to increments between -5 to + 5 V (127=0 V); they were transformed by a digital-to-analogue converter to the appropriate current within a coil. It was created by wrapping 305 m of 30 AWG (Belden 9978) wire in a single layer (18 cm wide) around plastic milk crates which were 38 cm x 33 cm x 27 cm.
The point duration is the time each point with a specific number between 0 and 256 (and the corresponding voltage) was generated; it was 3 ms. Consequently, the time required for the completion of the “physiological pattern” was 3 ms per point∙859 points or 2.58 s. This cycle was continuously repeated. More details regarding the hardware and software employed to generate the magnetic field patterns have been reported elsewhere (Murugan et al., 2013a). The 3 ms duration was selected because it diminishes the division of cancer cells but not normal cells in culture (Buckner, 2011), reduces the growth of melanoma tumors in mice (Hu et al., 2010) and enhances analgesia (equivalent to 4 mg/kg of morphine) to thermal nociceptive stimuli for rats (Martin et al, 2004). Point durations less than 3 ms or greater than 3 ms are less effective or not effective. This was predicted by the neurophysics model of Persinger and Koren (2007) and recently supported experimentally (Persinger, 2013). The pH of the volume of water was continuously measured at intervals of either 1 s, 100 ms, 10 ms or 1 ms depending on the experiment. The pH values for both beakers in each experiment were recorded separately once per second by Dr. Daq systems (Pico Technology, United Kingdom) which are sensitive to the .01 pH unit. The data were recorded continuously on a laptop operating the appropriate software. The pH measurements, collected every mi-nute for 12 hrs for the magnetic field-exposed and control water, were partitioned into hourly averages for statistical analyses. For the discernment of the optimal increment of time to measure the water (sampling intervals of 1 ms, 10 ms, 100 ms or 1000 ms) the numbers of positive displacements (increased pH), which were almost always about .02 to .03 pH, were calculated for triplicates of experiments completed in each condition. To more precisely isolate the typical duration of the increased pH shift 1000 successive sequences of 10 ms sampling rates were obtained from three different records (replications) from different trials for the magnetic field and control treated conditions. The numbers of positive shifts towards increased pH that displayed 0 to 10, 11 to 20, 21 to 30, 31 to 40, 41 to 50, and 51 to 60 ms increments were counted. All statistical analyses involved SPSS 16 for PC.
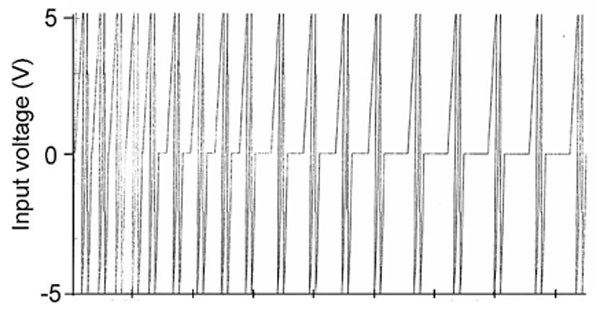
Figure 1: The shape of the repeated magnetic field pattern to which the water samples were exposed. The graph reflects the integrated picture of 859 points (horizontal axis), each generated for 3 ms; the total duration to complete the sequence was 2.58 s.
Results
The typical shift towards higher pH (0.5 to 1 unit) of spring water over time (12 hrs) contained within open beakers is shown in Figure 2. Although the water volumes exposed to both the magnetic field and no field conditions display an increased pH over the 12 hr periods of measurement, the volumes exposed to the magnetic field pattern displayed a greater pH shift. The numbers of discrete pH shifts during hour 1, 3, 4, 6, 7, 9, 10 and 12 for the volumes of water exposed to the no field or magnetic field condition are shown in Figure 3. Two way analysis of variance as a function of condition (no field, field) and time (increments between 1 and 12 hrs) showed statistically significant differences between field conditions [F(1,32)=497.70, p <.001] and time [F(7,32)=55.69, p <.001]. The two-way interaction was statistically significant as well [F(7,32)=14.86, p <.001]. Polynomial analysis for the control (non-exposed) water indicated a significant linear trend for the increase in pH [F(1,16)=80.83, p <.001] over time. For the water exposed to the magnetic fields there were both a significant linear trend [F(1,16)=300.00, p <.001] and a deviation from the linear term [F=5.18, p <.001]. Although there was a weak but significant quadratic term [F=6.64], there was also significant deviations from this as well as the cubic, 4th-order and 5th order terms (all Fs>4.89, p <.01].
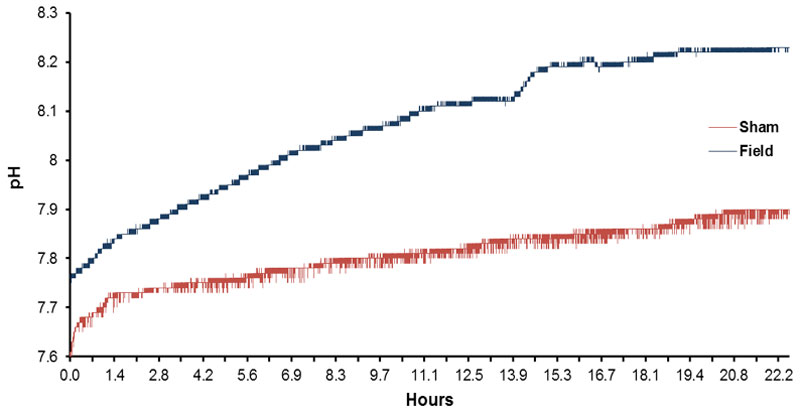
Figure 2: Typical record of successive, slow shift in pH as a function of time (12 hr) for spring water exposed to either the sham field condition or to the temporally patterned magnetic field.
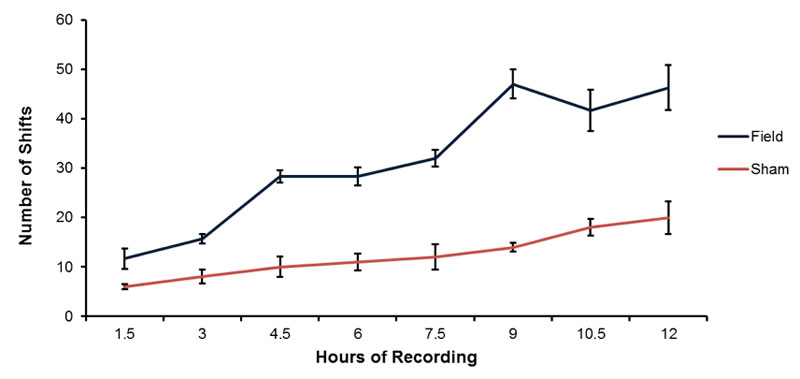
Figure 3: Means and standard errors of the mean for the numbers of discrete shifts during hourly intervals for the field and sham field conditions. Vertical bars indicated standard deviations.
To discern the optimal Δt or increment of analysis by which to discern the pH shifts, the numbers of shifts obtained when the data were collected with intervals of 1 ms, 10 ms, 100 ms, or 1000 ms (1 s) were analyzed as a function of field or no field conditions. As seen in Figure 4, the optimal duration for revealing the numbers of the pH shifts involved 10 ms increments of sampling. Two way ANOVA as a function of duration of sampling and field vs no field condition was dominated by a strong interaction that explained 67% of the variance. Post hoc analyses indicated that the major source of the interaction was due to the more frequent pH shifts for the water exposed to the magnetic field when the durations of observation were 10 ms compared to the no field condition and in conjunction with the field and no field conditions for the other three sampling durations that did not differ significantly from each other.
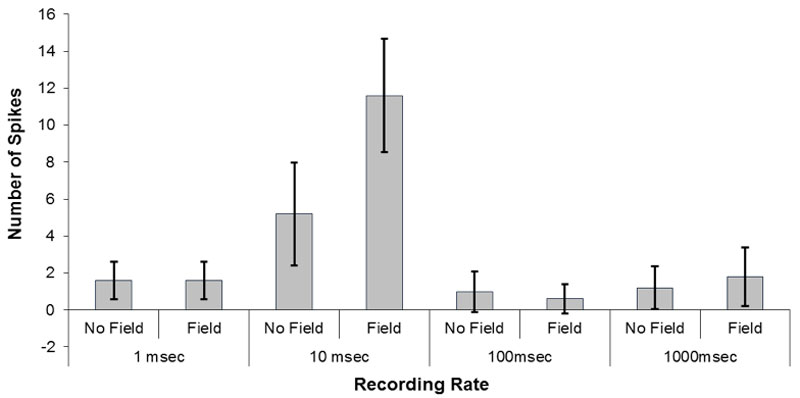
Figure 4: The numbers of pH shifts (>0.02 pH) recorded during increments of sampling of either 1 ms, 10 ms, 100 ms or 1000 ms. Vertical bars indicate standard deviations.
The more precise measurement of samples of the 1 ms measurements is shown in Figure 5 for the mean numbers of occurrences of pH shifts for 10 different random samples for 1000 points each with increments of 10 ms (i.e., 10 s). The peak in the numbers of occurrences of pH shifts occurred for spike durations between 20 to 30 ms and 30.1 to 40 ms for the samples exposed to the patterned magnetic field. Figure 5 also shows the peaks in the numbers of occurrences in pH units for comparable periods within the water exposed to reference conditions. There was no obvious non-linear effect with a peak between 20 and 40 ms.
A simple summation of the means of the numbers of positive shifts towards basic for 10 ms increments between 1 and 60 ms indicated an average occurrence of 68 per 10 s or ~6.8 shifts per s. However these shifts were not maintained but returned to antecedent levels when the transient was completed. This would indicate that in an 8 hour period, when the effect began to inflect towards asymptote, there would have been ~2.9 ∙104 of these shifts even though the total shift was between 0.5 and 1 pH unit. The shape of the pH shift indicated that over time they did not return to the antecedent value but drifted upward. Consequently, on average, the discrepancy between the antecedent pH and the return pH after the shift would have been about 1.7∙10-5 of a pH unit, which was below the range of the sensitivity of our sensors. However the integrative effect over the ~8 hr resulted in a cumulative increase of between 0.5 to 1 pH units.
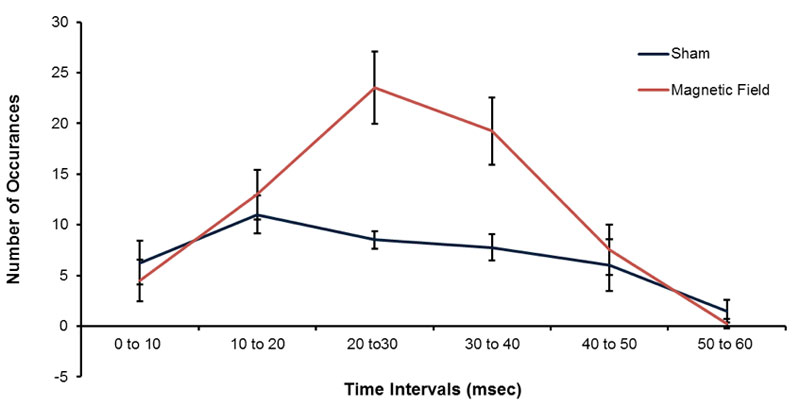
Figure 5: Means and standard errors of the mean (SEM) for the numbers of pH occurrences (increased pH transients) with intervals between 0-10 ms to 50 to 60 ms that were exposed to the sham (blue) or temporally patterned magnetic field (red).
Discussion
The results of these experiments indicate that when spring water was exposed to a physiologically-patterned magnetic field which has been shown to induce conspicuous diminishment of movement in planarian (Murugan et al., 2013b), inhibition of division of different lines of cancer cells but not normal cells (Buckner, 2011), marked analgesia in rodents (Martin et al., 2004) and invertebrates (Thomas et al., 1997), and altered states of consciousness in human participants (Saroka and Persinger, 2013), reliable changes in pH occurred. The changes were primarily toward alkalinity and involved between 0.5 to 1 pH units, compared to the water exposed to background conditions. Although the latter also displayed a drift towards higher pH, the change was slower, more linear, and of less magnitude.
The increase in pH of the water exposed to the magnetic field pattern displayed an inflection around 7 to 8 hrs. The mechanisms for this latency must still be examined. However it is interesting that Gang et al. (2012) found that the change in viscosity of water as inferred by diffusion of a solute for 50 ml volumes of water exposed to 0.16 T static magnetic fields was also within this range. Because the water was contained within open beakers the potential interaction of gas exchanges from the surrounding air and the volume of water must still be considered. Even small additions of O2, N2 or CO2 from the air could have been significant. In the mammalian brain, interstitial tissue oxygen levels are non-uniform between 1 to 5% relative to the atmosphere concentration of 21% (Sharp and Bernaudin, 2004). Serotonerigc neurons may help maintain pH homeostasis by functioning as CO2 sensors.
The measurement of the incremental shifts in pH of ~0.02 units as a possible “quantum” might be considered analogous to the 0.5 mV increments that were initially measured in postsynaptic space as incremental units. Only later were they associated with the energy from the “spontaneous” release of fixed quantities of neurotransmitter from a presynaptic vesicle. Although the shifts were evident in the same volumes of water exposed to background (“60 Hz”) intensities there were quantitatively more in the water exposed to the magnetic field pattern. That they were not artifacts of simple measurement or a variant of Faradic current by induced electric fields was shown by their maximum detection when the sampling interval was 10 ms rather than 1, 100, or 1000 ms increments.
In addition, careful measurement of the positive spikes (increased pH) of these discrete shifts indicated there was a non-linear effect as a function of duration for only the water exposed to the patterned magnetic field. There was a conspicuous increase in numbers of increased pH shifts with durations between 20 and 40 ms, that is the “40 Hz” band. This range of duration is within time required for the bulk velocity of the transcerebral electromagnetic fields associated with consciousness (the waking and dream states) to move along the rostral-caudal axis of the cerebrum. It may be relevant that the strength of a magnetic field in Tesla (kg∙A-1∙s-2) divided by the mass-to-charge ratio of a H3O+ ion [(3.15∙10-26 kg) ∙(1.6∙10-19 A∙s)-1] results in frequency. Assuming an average strength of 8∙10-6 T for the field strength in the present study and ~2∙10-7 kg∙A-1∙s-1 for the ratio, the emerging frequency is ~40 Hz.
That the phenomenon measured in our experiments is directly relevant to neuronal and brain activity is indicated by the discrete changes in pH that occur during dynamic processes. Increasing extracellular pH and lower intracellular pH often precede neuronal activity within the cerebral cortices and are associated with the opening of H+ channels (Elder and DeCoursey, 2001). Discrete shifts in pH occur within temporal increments of 10 and 40 ms (Bevan, 1998), which is what we observed in spring water within which a physiologically patterned magnetic field was applied. We suggest that the intrinsic, physiologically patterned magnetic fields generated from neuronal activity through out the aqueous matrix of the cerebrum volume might produce a comparable process coupled to complex cognition. In vivo shifts, as little as 0.2 pH (well within the range we observed), within the medulla of rats evoked an immediate increase of Ca++ across the syncytium of astroglial processes.
Implications for the Role of Water, Electromagnetic Fields and Consciousness
The results of the present experiment as well as previous calculations (Persinger and Lavallee, 2010) strongly indicate there are properties within water that might mediate if not create the cerebral cortical conditions that are experienced as consciousness. That the same physiologically-patterned magnetic field that produced the pH shifts in the study with durations of 20 to 40 ms (the same duration for the “refresh rate” of consciousness) also alters states of consciousness when experimentally applied across the cerebrum (Saroka and Persinger, 2013) indicates that extracellular and intracellular fluids may be the major source of these phenomena. There is now empirical evidence that these externally applied, physiologically-patterned “weak” magnetic fields are not attenuated significantly through cerebral space (Persinger and Saroka, 2013a). The classical arguments against the influence of weak magnetic fields because of the “kT boundary” apply only in certain conditions (Cifra et al., 2011).
A shift of .02 units of pH from 7.75 to 7.77, for example, would be equivalent to a shift from 1.7∙10-8 M to 1.6∙10-8 M of H+ or a net change of 10-9 M. From Avagadro’s Number (6.023∙1023 molecules per Mole), which would be 18 ml of water volume), a nanoMole, would be a change of 6.0∙1014 H+. When multiplied by the unit charge (1.6∙10-19 A∙s) the change would be associated with 9.6∙10-5 A∙s. Because each increment had a mean of 30 ms (3∙10-2 s) the current equivalent would be ~3.2∙10-3 A. This is the range (mA) required to elicit powerful subjective experiences and images, when brain tissue is directly stimulated by intracerebral electrodes (see Persinger and Saroka, 2013b for a review). With a typical shift of voltage associated with the rostral-caudal domain in the order of 50 to 100 µV (5 to 10∙10-5 V), the average steady-state resistance (V∙A-1) would be between .15 to 0.3 Ω-12 cm (length of cerebrum) or about 1 to 2.5 Ω ∙m. This range of values includes the resistivity of extracellular fluid, 2 Ω m, according to Barnes (1986).
The mean rate of occurrence of shifts in 0.02 pH units was equivalent to about 6.8 per second. Considering the larger numbers of events during the first few hours, the value may have been proximal to 7 to 8 Hz. With a bulk velocity of about 4.5 m∙s-1 as measured by Nunez (1995) and the recurrent phase-shifting transcerebral electromagnetic fields measured by Llinas and his colleagues (1991, 1993) , this would indicate that the standing wave of the cerebrum with a functional circumference of about 60 cm would be between 7 and 8 Hz. These solutions are consistent with hypothesis that discrete transient shifts in pH in the order of the duration involved with the recurrent rostral-caudal fields associated with consciousness (particularly dreams and the waking state) could mediate or even be a major contributor to these processes. A shift towards alkalization in the extracellular fluid adjacent to neuronal membranes precedes their activation. As measured by Bevan (1998), shifts in pH comparable to the ones noted over a longer interval in our study, occurred as 20 to 40 ms bursts.
According to Martinez-Banaclocha (2007) the potential change from resting membrane potential to activation (action potential) corresponds to approximately 0.2 µT (2∙10-7 T) at 0.5 mm around a neuronal surface. Alfven (magnetohydrodynamic) waves propagate in the medium with a velocity of
(1) v=B∙(µod)-1/2
where B is the magnetic field strength, µo is magnetic permeability, and d is the mass density of the charge carriers (Alvager and Moga, 1997). A change of 0.02 pH units (from 7.77 to 7.75 for example) would be associated with a mass density of 5.6∙10-8 kg [(10-9 M ∙ 6.023∙1023 H+ per M) ∙ (5.55∙104 L per m3) ∙(1.67∙10-27 kg per H+)]. The square root of the product of this value with uo (1.26∙10-6 N∙A-2) is 2.6∙10-7. The quotient is in the order of 1 m∙s-1. This suggests that small changes in pH within this range would be associated with functional charge densities of H+ that could mediate the bulk velocity that defines the ~4.5 m∙s-1 of the recursive waves associated with consciousness.
Diffusivity (µoσ)-1, where σ~ 2.1 S∙m-1 is for physiological saline, can reveal the temporal component of the dispersion within water. The value is 0.38∙106 m2∙s-1. The diffusion time scale, employing Ryskin’s (2009) logic, would be estimated by the radius and the thickness of the shell of the volume. Assuming a radius of 6 cm for the cerebrum and an average thickness or 4 mm, the magnetic diffusivity of water within the human cerebrum would be in the order of 10-9 s. For an action potential with durations of 10-3 s this is the same order of magnitude as the time required for each ion of the ~106 ions (Kandel et al., 2000) to traverse a channel that is temporally integrated into the total change in voltage. Thus even at the dynamic level the characteristics of water appear to define the boundary of the likely bases of consciousness (Persinger and Lavallee, 2012).
Global Implications
The conspicuous number of pH shifts per sec are within the range of the fundamental standing resonance of 7 to 8 Hz for the human cerebrum, as inferred by the frequency generated from the bulk velocity (4.5 m∙s-1) divided by the typical circumference of the skull (60 cm), and the Schumann resonances [(3∙108 m∙s-1) ∙ (4∙107 m)-1] generated around the Earth’s circumference between the surface and the ionosphere (Cherry, 2002). Although the amplitudes are small, in the order of a pT for the magnetic field component and tens of mV∙m-1 for the electric field component, they are persistent. They were also present during the origins of life (Cole and Graf, 1974). These electric and magnetic intensities are within the same order of magnitude as those generated during global cerebral activity in the human being. Cosic et al. (2006) have shown, as have others, that experimental application of the fundamental frequency and its harmonics affects cerebral systems.
Although water containing various solutes constitutes approximately 70% of the earth’s surface, its significance has been considered primarily for its contribution to climate. The intrinsic biological significance may be even greater. If we assume the (median value) volume of water on the surface of the planet is ~1.3·1018 m3, then the energy (E) contained within it from the geomagnetic steady state field strength would be:
(2) E=T2 ·(2·4π10-7 NA-2)-1 m3
or (25·10-10 T2) divided by 25·10-6 N∙A-2 multiplied by the volume. The intrinsic energy is ~1.3·1015 J contained within the oceans. The product of the volume of the oceans (1.3·1018 m3), 103 L M-1, 5.56·101 M L-1, and 6.023·1023 molecules per M, indicates there would be 43.5·1045 molecules. The quotient of 1.3·1015 J and 43.5·1045 molecules indicates about 3·10-32 J per water molecule. When this value is divided by the quantum indicator (Planck’s constant, 6.626∙10-34 J∙s) the intrinsic frequency is ~45 Hz. This could mean that intrinsic variations would be occurring about once every 20 to 25 ms, which is within the range of responsiveness of water observed in the present study.
Within the human brain volume a more direct potential connection to consciousness, which is assumed to be correlated with transcerebral ~40 Hz cortical waves, is suggested. Assuming an average volume of 1350 ml for a cerebrum which is ~945 ml (70%) water, there would be 55.5 M of water (18 ml per Mole). This would involve 3.16∙1025 water molecules. The magnetic energy from the static magnetic field from the earth potentially stored within this volume of water would be 0.945∙10-6 J or 2.9∙10-32 J per water molecule.
The equivalent frequency associated with the quantum of energy, obtained by dividing by Planck’s constant (6.626∙10-34 J∙s), is ~40 Hz. This is within the classic band associated with consciousness and the direct inverse of the 20 to 25 ms intervals associated with both the recursive rostral-caudal waves of electromagnetic coherence produced over the cerebral cortices and the peak duration of pH shifts recorded in this study. We think it is not spurious, in this context, that calcium transients can cause internal pH transients (Schwiening and Thomas, 1998) and the Liboff resonance solution of Ca++ in the earth’s static magnetic field is ~40 Hz. Our experimental results and their congruence with quantifications from calculations reiterate the perspicacious inferences by G.H. Pollack that the intrinsic properties of water and its interaction with the environment may reflect an entire domain of undiscovered possibilities.
Acknowledgments
The support from Dr. W. E. Bosarge, Jr., CEO of Capitol Technologies, Inc. is appreciated. We thank Dr. Peter Ryser and our Colleagues in the Department of Biology for the use of their laboratories. Special thanks to Viger Persinger for technical assistance.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell. (4th ed). Garland Science, N.Y., pp. 788.
Alvager T, Moga MM (1997) Magnetohydrodynamic wave resonance and the evocation of epileptiform activity by milliTesla DC magnetic fields. Int. J. Neurosci. 90(1-2):99-104.
Amoroso RL (1999) An introduction to noetic field theory: the quantization of mind. Noetic J. 2(1): 28-37.
Barnes FS (1986) Interaction of DC fields with living matter. In Polk C, Postow E (eds) CRC Handbook of Biological Effects of Electromagnetic Fields. CRC Press: Boca Raton, pp. 99-119.
Bevan S (1998) Proton-gated ion channels in neurons. In Kaila K, Ransom BR (eds). Ph and brain function. Wiley-Liss, N.Y., pp. 447-475.
Buckner C (2011) Effects of electromagnetic fields on biological processes are spatial and temporal-dependent. Ph.D. Dissertation, Biomolecular Sciences Program, Laurentian University.
Cherry N (2002) Schumann resonances, a plausible biophysical mechanism for the human health effects of solar/geomagnetic activity. Nat. Haz. 26: 279-331.
Cifra M, Fields JZ, Farhadi A (2011) Electromagnetic cellular interactions. Prog. Biophys. Mol. Biol. 105: 223-246.
Cole FE, Graf ER (1974) Precambrian ELF and biogenesis. In Persinger MA (ed) ELF and VLF Electromagnetic Field Effects. Plenum, N.Y.
Cosic I, Cvetkovic D, Fang Q, Jovanov E, Lazoura H (2006) Human electrophysiological signal responses to ELF Schumann resonance and artificial electromagnetic fields. FME Trans. 34: 93-103.
DeCoursey TE (2003) Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83: 475-579.
Del Giudice E, Preparata G (1994) Coherent dynamics in water as a possible explanation of biological membranes formation. J. Biol. Phys. 20: 105-116.
Del Giudice E, Spinetti PR, Tedeschi A (2010) Water dynamics at the root of metamorphosis in living organisms. Water, 2: 566-586.
Del Giudice E, Stefanini P, Tedeschi A, Vitiello G (2011) The interplay of biomolecules and water at the origin of the active behavior of living organisms. J. Phys. Conf. Ser. 329, 012001.
DeMeo J (2011) Water as a resonant medium for unusual external environmental factors. WATER Journal 3: 1-47.
Eder C, DeCoursey TE (2001) Voltage-gated proton channels in microglia. Prog. Neurobio. 64: 277-305.
Emoto M (2011). Secret Life of Water. Atria Books. New York.
Gang N, St-Pierre LS, Persinger MA (2012) Water dynamics following treatment by one hour of 0.16 Tesla static magnetic fields depends on exposure volume. WATER Journal, 3: 122-131.
House CR (1974) Water transport in cells and tissues. Edward Arnold, London.
Hu JH, St-Pierre LS, Buckner CA, Lafrenie RM, Persinger MA (2010) Suppression of growth of injected melanoma cells by whole body exposure to specific spatial-temporal configurations of weak intensity magnetic fields. Int. J. Rad. Biol. 86: 79-88.
Kaila K, Chesler, M (1998) Extracellular pH transients, in Kaila K, Ransom BR (ed) pH and brain function. Wiley-Liss, N.Y. pp. 329-330.
Kaila K, Ransom BR (1998) pH and brain function. Wiley-Liss, N.Y.
Kandel ER, Schwartz JH, Jessell TM (2000) Principles of Neural Science. McGraw-Hill, New York.
Llinas RR, dePare D (1991) Of dreaming and wakefulness. Neurosci. 44(3): 521-535.
Llinas RR, Ribary U (1993). Coherent 40 Hz oscillations characterizes dream state in humans. Proc. Nat. Acad. Sci. 90(5): 2078-2081.
Martin LJ, Koren SA, Persinger MA (2004) Thermal analgesic effects from weak, complex magnetic fields and pharmacological interactions. Pharm. Biochem. Behav. 78, 1219-1224.
McFadden J (2002) Synchronous firing and its influence on the brains electromagnetic field: evidence for an electromagnetic field theory of consciousness. J. Conscious. Stud. 9: 23-50.
McFadden J (2007) Consciousness Electromagnetic (CEMI) Field Theory. Neuroquantol. 3: 262-270.
Murugan NJ, Karbowski LM, Lafrenie RM, Persinger MA (2013) Temporally-patterned magnetic fields induce complete fragmentation in planaria. PLOSone 8(4): e61714. (a)
Murugan NJ, Karbowski KM, Persinger, MA (2013) Dose-dependent comparisons of morphine, naloxone and/or weak pulsed magnetic fields on planarian behaviour: revealing receptor subtype affinities and non-specific effects. In submission (b)
Persinger MA (2010) 10-20 J as a neuromolecular quantum in medicinal chemistry: an alternative approach to myriad molecular pathways? Cur. Med. Chem. 17(27): 3094-3098.
Persinger MA (2013) Experimental evidence that Hubble’s Parameter could be reflected in local physical and chemical reactions: Support for Mach’s Principle of Imminence of the Universe. Int. Lett. Chem. Phys. Astron. 11 ,86-92.
Persinger MA, Belanger-Chellew G (1999) Facilitation of seizures in limbic epileptic rats by complex 1 microTesla magnetic fields. Percep. Mot. Skil. 89: 486-492.
Persinger MA, Koren SA (2007) A theory of neurophysics and quantum neuroscience: implications for brain function and the limits of consciousness. Int J. Neurosci. 117(2): 157-175.
Persinger MA, Koren SA, Lafreniere GF (2008) A neuroquantological approach to how human thought might affect the universe. NeuroQuantol. 6:267-271.
Persinger MA., Lavallee CF (2010) Theoretical and experimental evidence of macroscopic entanglement between human brain activity and photon emissions: implications for quantum consciousness and future applications. J. Consc. Explor. Res. 1(7): 785-807.
Persinger MA, Lavallee CF (2012) The ∑n=n concept and the quantitative support for the cerebral-holographic and electromagnetic configuration of consciousness. J. Conscious. Res. 19(11-12) 128-153.
Persinger MA, Saroka KS, Koren SA, St-Pierre LS (2010) The electromagnetic induction of mystical and altered states within the laboratory. J. Consc. Explor. Res. 1(7): 8-8-830.
Persinger MA, Saroka KS (2013) Minimum attenuation of physiologically-patterned 1 microTesla magnetic fields through simulated skull and cerebral space. J. Electromagn. Anal. Appl. 5: 151-156. (a)
Persinger MA, Saroka KS (2013). Comparable proportions of classes of experiences and intracerebral consequences for surgical stimulation and external application of weak magnetic field patterns: implications for converging effects in complex seizures. Epilep. Behav. 27: 220-224. (b)
Pollack GH (2003) The role of aqueous interfaces in the cell. Adv. Colloid Interface Sci. 103: 173-196.
Pollack GH, Figueroa Z, Zhao Q (2009) Molecules, water and radiant energy: new clues to the origin of life. Int. J. Mol. Sci. 10: 1419-1429.
Radin D, Hayssen G, Emoto M, Kizu T (2006) Double-blind test of the effects of distant intention on water crystal formation. Explore 2(5): 408-411.
Richerson GB (2004) Serotonergic neurons as carbon dioxide sensors that maintain pH homeostatsis. Nature Rev: Neurosci. 5, 449-461.
Ryskin G (2009) Secular variation of the earth’s magnetic field: induced by ocean flow? New J. Phys. 11, 1-23 (063015).
Saroka KS, Persinger MA (2013) Potential production of Hughling Jackson’s “parasitic consciousness” by physiologically-patterned weak transcerebral magnetic fields: QEEG and source localization. Epilep. Behav. 28: 395-407.
Schwiening CJ, Thomas RC (1998) pH consequences of calcium regulation. In Kaila K, Ransom BR (eds) PH and brain function. Wiley-Liss, N.Y., pp. 277-286.
Sharp FR, Bernaudin M (2004) H1Fi and oxygen sensing in the brain. Nature Rev. Neurosci. 5: 437-448.
Thomas AW, Kavaliers M, Prato FS, Ossenkopp KP (1997) Antinociceptive effects of pulsed magnetic fields in the land snail. Neurosci. Lett. 222: 107-110.
Discussion with Reviewers
Anonymous Reviewer 1: The authors did not study mechanisms involved with frequency-modulated weak electromagnetic fields.
Murugan NJ, Karbowski LM and Persinger MA: This particular pattern has been shown, with maintained exposure in combination with another field, to dissolve planarian after five days of a few hours of exposure per day. This pattern produces reliable analgesia in mice, rats, and invertebrates. This same pattern also retards several types of cancer cell lines but does not affect normal cell lines. Hence we were more focused upon how this particular field would affect pH, given the role of very small changes in pH around the membrane, in subtle electrical changes in membranes. Indeed there are changes within the gases contained within the spring water that contributed to the small shift in pH. However that was not the thrust of the study.
Reviewer: Since the author utilized a frequency – modulated magnetic field, the pattern of pH might depend upon the frequency. If the authors used some other frequency, they might get a difference change.
Murugan, Karbowski and Persinger: As stated above, the critical feature was the patterned field because of its potency of effects on so many different biological systems. The issue of a “magnitude” dependence, which we agree is a classic means of establishing relationships, such as the dose-dependence curves in radiation research, was accommodated by the Δt experiment where pH shifts collected at different increments of time showed that the 20 to 40 ms increment was most optimal to demonstrate the effect. This is an important result because theoretical work from Sommerhoff to Edelman and the empirical work from Llinas to our research have demonstrated the central role of this temporal parameter in the recursive reconstitution of cerebral cortical fields involved with consciousness, not to mention microstates.
Reviewer: The authors presented only a simple experimental result on frequency-modulated magnetic field induced pH shift in exposed water, but too much is made of the results.
Murugan, Karbowski and Persinger: On the contrary, the results of this study converge quantitatively with what is known about water from a traditional physical chemistry perspective as well as the applications within physiological systems. Discussion sections and applications of results to more generalized contexts, assuming the quantification is accurate, allows Science to move forward.
Reviewer: Why was Spring Water employed?
Murugan, Karbowski and Persinger: Its constituents more closely simulate the electrophysiological condition of cells.
Anonymous Reviewer 2: The article is interesting but the discussion about relationship between spring water and brain function is not obvious, even less so about states of consciousness.
Murugan, Karbowski and Persinger: The “spike” durations of the pH shifts were within the range associated with recursive recreation of transcerebral fields associated with consciousness as noted in the text. In addition, the movement of proton’s within the water matrix (which includes the ground substance) within which neurons and glial cells are immersed, is congruent with the 20 to 40 ms range of pH spike duration. The quantity of momentum can be derived, according to de Broglie, from hλ-1 where h is Planck’s constant and λ is wavelength resulting in units of kg m s-1. For a proton (or electron) with a radius in the order 10-15 m, the momentum becomes ~2.35 10-19 kg m s-1 and when multiplied by the classic rostra-caudal bulk velocity of cerebral cortical voltage waves (4.5 m s-1, which is the velocity required for the traversal of rostral-caudal length, 11 cm, to occur in 25 ms), the energy is within the range of 10-20 J.
This is the quantum unit associated with action potentials as well as the energy associated with the separation (about 10 nm) between K+ ions spread as a thin sheet over the membrane to whose (inside-outside) disparity of charge is attributed to the source of the resting membrane potential. (However as Pollack and others have clearly indicated, interfacial water against many types of surfaces can produce a shell of protons and a potential difference that approximates the resting membrane potential). In addition the solution v=√(E∙m) for a proton exposed to this increment of energy is remarkably approximate to the velocity of phonons (sound pressure waves in water).
Finally even if one applied the specific intensity that we employed, based upon previous effective values and theory, the results are congruent. Assuming an average of 8 µT and knowing that Hz=(kg A-1s-2 ) A∙s kg-1, the solution for 8∙10-6 T times 1.6∙10-19 A s and the inverse of the mass of a water molecule (inverse of 30∙10-27 kg) is ~40 Hz, i.e., the 25 ms increment.
Reviewer: How could this particular field affect spring water, which is bulk water, and what is the role of gas within the water?
Murugan, Karbowski and Persinger: In other experiments involving photon spectrophotometry we found that “pure” water, that is double distilled water, did not display the shift in pH as a function of duration exposed to this patterned magnetic field. Although we know that “air” or gaseous mixtures within the spring water can affect the shift in pH over time, the critical feature of the present experiments is that the sham-field (control) condition involving the same water did not display the shift associated with the magnetic field exposures.
Reviewer: The relationship between “spring water” and “physiologically patterned” bra-in magnetic fields is not obvious. Can you elaborate particularly within the context of consciousness?
Murugan, Karbowski and Persinger: The physiologically patterned magnetic fields employed in this study are the ones that have evoked clear changes in nociceptive thresholds in mice and rats and altered movement in planarian. When applied strategically to human brain, s_LORETA (standardized Low Resolution Electromagnetic Tomography) shows the types of changes in current density that are associated with altered consciousness. Several approaches suggest that the nature of water movement within brain space, as indicated by Diffusion Tensor Imaging (DTI) in schizophrenic vs reference brains (Alba-Ferrara and de Erausquin, Frontiers in Integrative Neurosicence, 2013) and the penetration of photons through the cerebral volume (Persinger et al., World Journal of Neuroscience, 2013) are a powerful correlate of consciousness. It is well known that H+ (proton) channels exhibit powerful influence upon the dynamics of the cell membrane and that a major source of these protons is intrinsic to water (the hydronium ion) itself. Rapid extracellular alkalinization is frequently measured at the onset of neuronal activity in the cerebral cortices, the presumed substrate for consciousness (Eder and DeCoursey, Progress in Neurobiology, 2001). In the medulla oblongata a 0.2 pH unit decrease results in an immediate increase in Ca++ across the entire field of astrocytes.
Reviewer: The water of “spring water” is very different from intracellular water of cells. Indeed “spring water” is more similar with bulk water without interfacial structure (no membrane). Can you explain your arguments for the similarity of “spring water” with cellular and extracellular fluids of the brain?
Murugan, Karbowski and Persinger: Both intra- and extra-cellular fluids within the brain tissue contain ions as does spring water. The shared major ions are sodium, potassium, calcium, chloride and bicarbonate. In addition the primary range of pH in both our solutions and brain water are remarkably similar.
Reviewer: Have you exposed spring water with other external magnetic fields?
Murugan, Karbowski and Persinger: Yes, we have exposed spring water to greater intensity static magnetic fields; those results were published in this journal by Gang, St. Pierre and Persinger. The senior author has also exposed spring water employing a similar paradigm to a variety of physiologically patterned magnetic fields. There is clear “intensity” dependence for the rate at which the asymptote is achieved.
Reviewer: How do you explain that static magnetic fields also modify pH?
Murugan, Karbowski and Persinger: Given the range of configurations and intensities of magnetic fields one would not expect a single mechanism to be the only means by which they modify pH. Although one could appreciate the importance of the 50,000 nanoTesla static geomagnetic field and the approximate Liboff cyclotron resonance frequency of 45 Hz for Ca++, the primary frequency band associated with “consciousness”, there are many likely mechanisms. This was discussed in our previous experiments with strong static magnetic fields (Gang, St-Pierre and Persinger, 2012, this journal).
Reviewer: Were the pattern and intensities of the physiologically patterned magnetic fields specific to modify pH of the exposed spring water?
Murugan, Karbowski and Persinger: We do not know if this pattern was “specific”. It was selected because of its powerful impact on cancer cells, “pain” threshold in rodents, and capacity to alter human brain cerebral activity as well as the “sensed presence”, a type of “other self” frequently reported by patients with electrical foci in the temporal lobes.
Reviewer: Have you measured the electric fields during your experiments?
Murugan, Karbowski and Persinger: We have not measured electric fields from the water. We employed magnetic fields because of the ease of penetrability through water. The senior author observed the futility and frustration of measuring and penetrating electric fields through water and glass while he was involved with the Overhead Power Lines Project for the State of New York. Low frequency magnetic fields are more easily controlled and measured.
Reviewer: The field is four times weaker than the earth’s magnetic field and weaker than kT. How do you explain the time-dependence of the external magnetic field?
Murugan, Karbowski and Persinger: The kT boundary has been shown, by Cifra and others, not to be as relevant or limiting as traditionally considered. In addition the magnetic energy within the field is certainly sufficient to affect the energies involved with hydrogen bonds as shown in the manuscript. The repeated application of the same magnetic field to the same flask of water over time appears to allow an intrinsic ordering to occur that we think may be analogous but not equivalent to the ordering of ions that defines the ground substance between cells. This must still be examined.
Reviewer: You make the statement “The repeated application of the same magnetic field to the same flask of water”; how did you make sure that you had the same spring water for each experiment?
Murugan, Karbowski and Persinger: The water for all containers was obtained from the same source (large bottles) containing the same ID number and purchased from the same stores.
Reviewer: Have you studied the role of gas in the water, or out-gassed the spring water before magnetic field exposure?
Murugan, Karbowski and Persinger: The senior author studied the opposite, over-bubbling air into the water samples before applying the field. The net effect is the shift to a more basic pH occurs almost immediately and the baseline values begin at much higher values than following removal from the jugs of spring water. We had noted that samples taken from the same jug of spring water as the jug becomes emptier showed initially higher and higher (these are small changes) pH values from the beginning. However the magnetic field effect was still much greater and larger.
Reviewer: The beakers are opened and not sealed; moreover the duration of the measurement is 12 hours. Have you prepared the water in a glove box to ensure controlled atmosphere? How do you know the change is not due to alkaline compounds from the beakers?
Murugan, Karbowski and Persinger: We have not prepared the samples in a glove box. Remember that the critical feature is that the magnetic field increased the pH shift in very specific ways that were quite different from the non-field samples. If there were contaminants in the beaker they would have leached similarly in both conditions. Finally, the important feature here is the temporal characteristics of the pH shifts within the msec range for the magnetic field exposed samples only.
Reviewer: Have you reversed the containers of exposed water and reference water? The release of compounds from the beakers could be different according to the used beaker.
Murugan, Karbowski and Persinger: Yes, we reversed and counterbalanced the containers (beakers or flasks) that were employed for the different field conditions. In addition all of the glass containers were washed before each experiment.
Anonymous Reviewer 3: Would the changes occur also with dyes? Sometimes pH measuring instruments can be influenced in some way that is not identical to the dyes.
Murugan, Karbowski and Persinger: We have not pursued dye mixtures or colloidal suspensions, which are the next phase of the experiment. Clearly we are interested in pursuing the mechanisms that maintain and create the interfacial water boundaries.
Reviewer: I believe the Discussion section is too far fetched. The similarity between some characteristics of the presented experimental results and brain neurophysiologic features may be accidental or superficial.
Murugan, Karbowski and Persinger: We agree that care must be taken with quantitative analyses. However, what we present here, in terms of the biophysics of brain physiology and function is relatively well known in modern neurophysiology. It is an extension of the seminal work of Ruch and Patton. The major emphasis for interdisciplinary research and integrated perspectives across sciences is to show the relationships, e.g., the commonalities of the Schumann resonance and cerebral activity. By applying what we learned from our experimental work with spring water, presented here in this paper, we can systematically investigate the macro- and micro-levels of discourse in order to a have a unified perspective.
If as Pollock implies the “intrinsic properties of water” are coupled to all of the unusual physical features, e.g., absorption and emissions spectra, correlated with interfacial water then this type of exploration should show convergences between scientific areas. We would not be surprised if the extraordinary degrees and unusual physical properties of water will ultimately explain the most fundamental properties of membranes, consciousness and the integration between organisms and their environment. The critical discovery we made is that by applying appropriately patterned weak magnetic fields whose energies match those at the quantum levels within water quantitatively predictable changes occurred.
Thank you for the questions and the opportunity to answer them.
