 The Changing State of N: P: Si Stoichiometry in Ganga River: Possible Implications for Production and Fate of Phytoplankton Biomass
The Changing State of N: P: Si Stoichiometry in Ganga River: Possible Implications for Production and Fate of Phytoplankton Biomass
Pandey J* and Yadav A
Ganga Research Laboratory, Environmental Science Division, Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi- 221005, India
*Correspondence E-mail: jiten_pandey@rediffmail.com
Key Words: Dissolved organic carbon, Ganga River, Nutrients, Phytoplankton, Stoichiometry
Received June 1st, 2014; Accepted Jan 11th, 2015; Published Jan 30th, 2015; Available online Feb 20th, 2015
Abstract
Nutrient loading to surface waters has increased over recent decades while silicon loading has remained relatively constant or declined. A shift in N: P: Si stoichiometric ratios due to anthropogenic influences may become a feature of changing biogeochemistry in surface waters. We studied the changing state of NO3–, NH4+, PO43-, Si4+, dissolved organic carbon (DOC), chlorophyll a (Chl a) biomass and gross primary productivity (GPP) along a 35 km long stretch of Ganga River at Varanasi (India). Concentrations of NO3–, NH4+, PO43 increased downstream characterized by anthropogenic influences, while silica showed an opposite trend. On a seasonal scale, unlike other nutrients, Si concentration was lowest in winter. The N: P stoichiometry showed a declining trend downstream indicating N limiting condition. The Si: N and Si: P ratios also showed deviation from classical Redfield ratios and indicated Si limitation as influenced by excessive N and P inputs. Chl a biomass and GPP followed a trend similar to nutrients. This relationship was also evident in DOC. Statistically significant differences in Chl a biomass and GPP as well as in N: P: Si and C: Chl a ratios indicated the changing state of stoichiometric coupling of essential elements with possible implication on ecological status of Ganga River.
Article Outline
Introduction
In recent years an unprecedented increase in human population coupled with economic growth has dramatically altered the stoichiometric coupling of essential elements and in turn, the ecosystem functioning and climate change drivers (Gruber and Galloway, 2008; Pandey et al., 2014). A specific stoichiometric ratio of N, P and Si, often called the Redfield ratio (N: P: Si: 16: 1: 16), is important the for balanced growth of phytoplankton (Turner et al., 2003). A deviation from this ratio associated with the deviation in the level of particular nutrients influence phytoplankton growth, community composition and quality of food for consumers (Turner, 2002) and consequently, has potential effect on food web structure (Elser et al., 2000a; Turner, 2002; Dodds et al., 2004).
The concentration of nutrients in rivers depend on their loading from the catchment as influenced by catchment characteristics, such as human activities, climate change, biogeochemistry and the river’s own assimilation potential. Human activities have potentially influenced the biogeochemical cycles of many elements, specifically carbon (C), nitrogen (N) and phosphorus (P) (Caraco, 1993; Turner et al., 2003; Howarth, 2008). In aquatic ecosystems, the turnover of N and P is relatively higher (Downing and McCauley, 1992; Downing, 1997), while the cycle of silica is relatively slower and stable (Sommer, 1988). The concentration of dissolved silica is largely controlled by runoff volume. The theory of resource competition among algal communities, consumer driven nutrient recycling and food chain dynamics based on the stoichiometric ratios of N: P: Si suggests a long term shift in trophic cascades and ecosystem resilience when such stoichiometric ratios are skewed in favour of one element relative to other (Teubner and Dokulil, 2002; Elser et al., 2009). For instance, in phytoplankton assemblages, diazotrophic cyanobacterial dominance declines when nutrient supply leads to higher N: P ratio (Guildford and Robert, 2000; Elser et al., 2000b). Similarly, a Si: N ratio below 1: 1 leads to reduced proportion of diatoms in phytoplankton communities and, by implication, a shift in higher trophic level structure. Although diatoms support herbivorous zooplankton–coupled fish production, they reduce the hatching success of copepod eggs (Kristiansen and Hoell, 2002). Changes in the Chl a: N: P ratio reflect a shift in phytoplankton production as a function of N and P. The turnover of pelagic phytoplankton regulates the dissolved organic carbon (DOC) and, consequently, the microbial loop in aquatic habitats. The overall DOC in receiving waters depends on the coupled effect of allochthonous input and autochthonous C-pool. The proportion of these two sources of C however, regulates the nutritional status of detrivores (Elser et al., 2000b). Most of the studies so far on N: P: Si stoichiometry have focused mainly on oceans and lake ecosystems (Granelil et al., 1999; Turner et al., 1998; Kristiansen and Hoell, 2002). Data on the changing state of such stoichiometric relationships especially in major rivers of India are very scarce (Pandey et al., 2013; 2014).
The present study was an effort to quantify the changing state of the N: P: Si stoichiometric ratio to explore possible implications on the ecology of Ganga River, the second largest river in the world, as far as the amount of water discharge is concerned. The study addresses emerging trends in phytoplankton nutrient limitation and associated shift in Chl a biomass and productivity in the study river stretch. The major goal was to test the hypothesis that the changing state of the N: P: Si stoichiometry of the Ganga River in response to anthropogenic activities would lead to a shift in phytoplankton production in the river.
Materials and Methods
Study Area
The present study was conducted from March 2013 to February 2014 at 3 selected stations along a 35 km long stretch of the Ganga River at Varanasi (25º18’ N lat and 83º1’ E long). Site selection was based on sub-catchment characteristics, land use pattern, and anthropogenic disturbances and nutrient input. The climate of the region is tropical with distinct seasonality. The year can be divided into a hot and dry summer (March-June), a humid rainy season (July- October) and a cold winter season (Nov-February). The mean annual rainfall varied between 870-1130 mm, relative humidity between 27 and 83% (summer) and 58 and 99% (rainy season). More than 90% of the annual rainfall occurs during rainy season. During summer day temperature varied between 29 to 46ºC and during winter night temperature at rare occasions drops below 4ºC. The soil is alluvial fluvisol. The Ganga River is about 2,525 km long and its basin area in India is about 8, 61,404 km2. The Ganga basin is surrounded by Himalaya from north and Vindhyas from south. The river system covers cool upland streams and warm water stretches together with deltaic habitats. Due to availability of water, fertile soil and suitable landscape, the Ganges basin is the most heavily populated river basin in the world. Water of this sacred river in India is used for drinking, clothes washing, bathing of domestic animals and irrigation.
The river receives pollutants from sewage, cremation of dead bodies, agricultural and urban runoff, atmospheric deposition and land use change. Station 1 was relatively natural and Station 3 was the most human disturbed. Among the selected stations, three sites (urban side, mid stream and offsite) were selected at each station. The samples were taken in replicate (n=3), in presterilized acid rinsed plastic containers, directly below the surface (15-30 cm depth) at monthly interval. The distance between replication sampling was about 50 m.
Measurements
The experimental design consisted of two tiers of study that include water chemistry and phytoplankton biomass. A total of 108 analyses were carried out (12 variables in 9 samples). The chemicals used for the analysis were Merck analytical grade.
Water Chemistry
Nitrate and ammonia together comprises dissolved inorganic nitrogen (DIN). Nitrate-N was quantified using brucine sulphanilic acid method (Voghe, 1971) and ammonia-N using Nesseler’s reagent (Maiti, 2001). Dissolved reactive phosphorous was quantified as orthophosphate using ammonium molybdate method (Mackereth, 1963). Dissolved reactive silica was measured following Dienert and Wandenbulke’s (1923) method. Dissolved organic carbon (DOC) was estimated using KMnO4 digestion procedure (Michel, 1984). For this purpose, a known volume of filtered water was mixed with acidified N/80 potassium paramagnet and incubated at 37º C. Organic carbon was estimated by titrating to quantify oxygen after 4 hours of incubation (APHA, 1998).
Phytoplankton Production
Phytoplankton biomass measured in terms of chlrophyll a (chlorophyll a comprises about 1.5% of algal dry organic matter (APHA, 1998). For this purpose, water samples collected in acid rinsed plastic containers from each replication point, from directly below the surface (15-30 cm depth) were filtered by Whatman No. 42. From the filter paper the sample was extracted using acetone extraction procedure (Maiti 2001) and measured spectrophotometrically. The primary productivity was measured by light and dark bottle method (APHA, 1998).
Statistical Analysis
Significant effect of site and time was tested using analysis of variance (ANOVA). The study considered a well defined protocol with balanced experimental/sample collection designs which validate the use of ANOVA for the analysis. To give an indication of the uncertainty of the mean, arithmetic means are accompanied by ±1SE. The Mann-Kendall test and Sen’s slope estimator (XLSTAT 2014) were used for detecting the trend direction and magnitude of change in seasonal data sets. Principal components analysis (PCA) was used to evaluate the overall spatial and temporal profile of nutrients, OC, productivity for the study period. SPSS package (version 16) was used for statistical analysis.
Results and Discussion
The concentration of nutrients increased downstream, and there was an over 1.4 fold increase in NO3–, a 1.2 fold increase in NH4+ and an over 3.4 fold increase in PO43- at Site 3 ( Rjht) compared to Site 1 (Adpr) (Figure 1). Dissolved organic carbon (DOC) also followed a similar trend. In contrast, the concentration of silica decreased downstream from Adpr to the Rjht site. Gross primary productivity and Chl a biomass increased downstream by about 1.6 and 1.9 fold respectively. Productivity variables showed distinct synchrony with nutrients (Figure 1). Seasonally, the concentrations of DOC and silica were highest in monsoon season and lowest in summer. Unlike DOC however, concentration of silica was recorded higher in summer in comparison to winter season. Chl a biomass and productivity was highest in winter season and lowest in monsoon. On a spatial scale, the concentration of river nutrients was highest at Rjht and lowest at the Adpr site (Figure 1). Seasonal (p<0.01) as well as spatial (p<.05) variations in these variables were significant (ANOVA). The Mann-Kandall test with Sen’s slope statistics revealed a positive rising trend across site for NO3–-, NH4+, PO43-, DOC and productivity. In case of silica however, a decreasing trend was noticed (Figure 1).
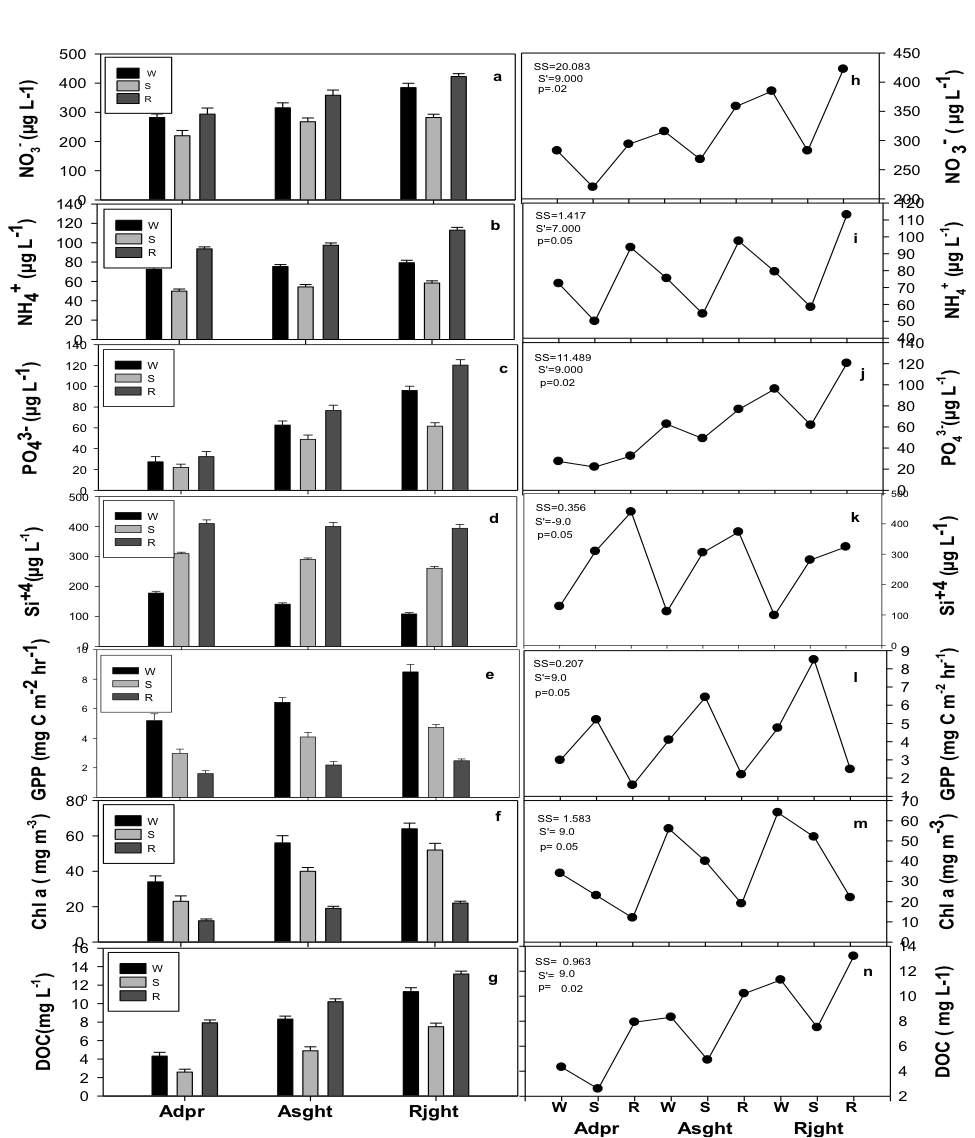
Figure 1: Seasonal trends and Mann-Kendall test with Sen’s slope statistics for NO3– (a,h), NH4+ (b,i), PO43- (c,j), Si4+ (d,k), GPP (e,l), Chl a (f,m) and DOC (g,n) at three study sites. S’, Sen’s estimate; SS, Sen’s slope; GPP: gross primary productivity; Chl a: chlorophyll a
The N: P: Si stoichiometric ratio declined downstream from Adpr to the Rjht site (Figure 2). The N: P, Si: N and Si: P ratios were highest at Adpr and lowest at the Rjht site. Seasonally, N: P: Si stoichiometry was highest in summer and lowest in winter season. Chl a: DOC ratio also showed a similar trend from Adpr to the Rjht site and was highest in summer season (Figure 2).
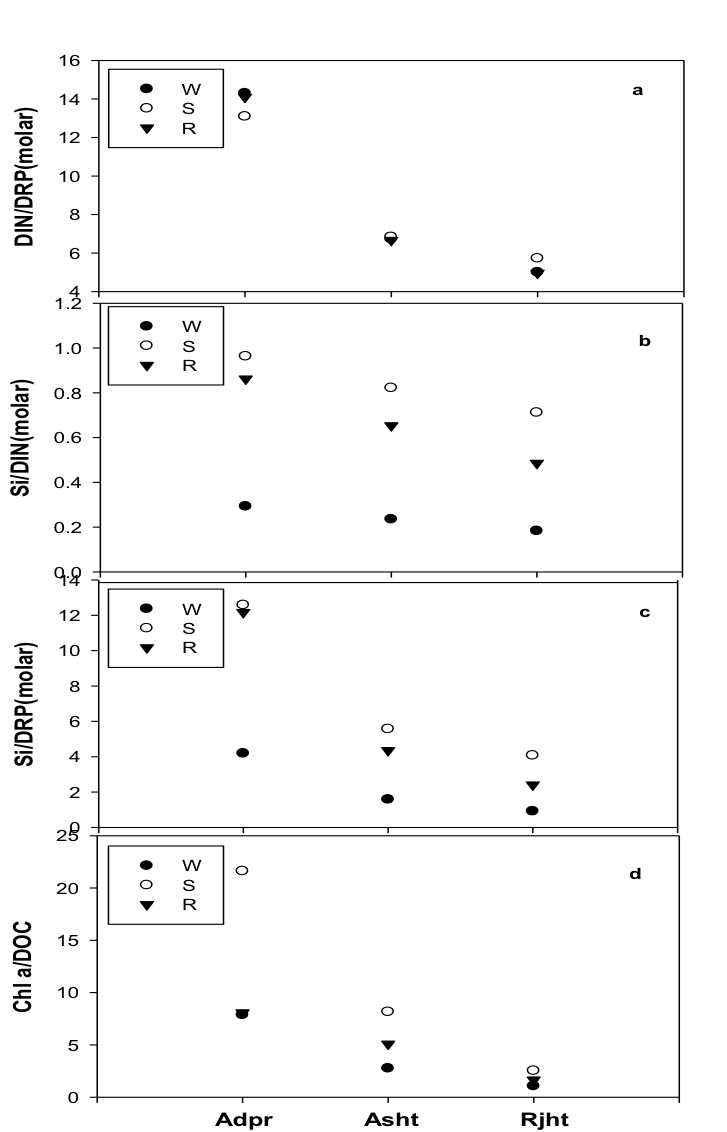
Figure 2: The seasonal trend in DIN: DRP (a), Si:DIN (b), Si: DRP (c) and Chl a: DOC ratios at three study sites
Principal components analysis (PCA) separated Adpr, the least anthropogenically influenced site, from rest of the sites demarcating water quality characteristics including nutrients and productivity variables (Figure 3).
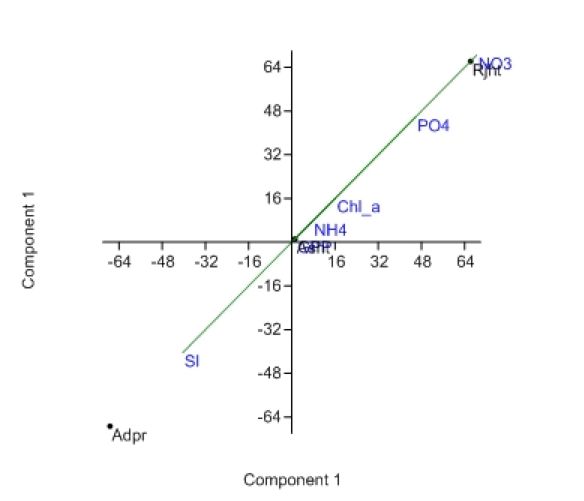
Figure 3: Principal Component Analysis (PCA) showing the position of three sampling sites based on water quality characteristics. At the center of the axis, variable GPP overlaps with site Asht.
Enhanced nutrient loading is seen as the key factor in enhancing excessive algal growth and other problems associated with eutrophication and the poor ecological status of fresh waters. The results of this study indicate that the concentration of nutrients in river increased in response to changes in site characteristics and season. Increases in nutrient concentration could be a combined effect of natural input from weathering and erosion and anthropogenic activites such as land use changes, sewage input and other sources. In addition, air-borne materials also add nutrients to waterways either through direct deposition on water surfaces or indirectly through catchment deposition-coupled surface runoff (Pandey et al., 2014). In this study, Site 1 (Adpr) is a comparatively natural site whereas Site 3 (Rjht) represents downstream urban core with strong influence of industrial and sewage outlets and atmospheric deposition. Thus, the downstream rising trend in concentrations of nutrients and DOC indicates strong influence of urban core. This effect was clearly evidenced in PCA results wherein Adpr (Site 1), with low level of nutrients and DOC, is grouped away from rest of the sites experiencing local controls (Figure 3).
Results showed marked seasonality in concentrations of N, P and Si wherein storm water runoff appeared to be the major determinant for high concentrations in the rainy season. In the aquatic systems, especially in rivers, a major part of silica comes from weathering and erosion of lithogenic materials which is accelerated by rainfall. The influence of surface runoff has been shown to be one of the most important causations regulating seasonality in nutrient concentration in Ganga River (Pandey et al., 2014).
Changes in surface water quality are most often reflected in primary production and, if persist for long time, in the trophic cascades. Productivity variables such as GPP and Chl a biomass increased consistently downstream and showed synchrony with the concentration of nutrients. The variation in the N: P: Si ratio in the river is the result of net change in the concentration of these nutrients caused mainly by anthropogenic factors. A classical study by Redfield (1958) has revealed the importance of N: P: Si (16: 1: 16) ratios for phytoplankton growth. In the present study, the N: P ratio ranged from 4.96 to 14.29. Since, the N: P stoichiometry below 16:1 shows N limiting condition, the trend observed in N: P ratios downstream indicate N limitation as we move from Adpr to the Rjht site. Lower N compared to P downstream could be due to excess P addition from anthropogenic sources, or loss of N to the atmosphere through denitrification. Similar to N: P, we found a clear deviation in the Si: N and Si: P ratios. The former varied between 0.18 and 0.96 and the latter between 0.91 and 12.58. Lower Si: N ratio than the classical Redfield ratio of 1: 1 merits attention especially for growth of diatoms (Ittekkot et al., 2000; Gilpin et al., 2004). Low DSi: DIN ratios cause potential Si limitation, especially during diatom blooms in human impacted waters (Viaroli et al., 2013). Further, lower Si: P stoichiometry than 16: 1, resulting possibly from high P input through sewage etc. has concern for phytoplankton growth, especially for diatoms.
Competition for resources (N: P: Si) in fresh water is often used to explain the structure of phytoplankton communities (Tilman et al., 1982; Elser et al. 2000b). Variation in N: P: Si ratio observed in present study could influence phytoplankton growth and community composition especially with silicious algal groups. For instance, silica, a major component of the cell wall of diatoms, is required for the balanced growth of diatoms.
A consistent and long-term increase of N and P at low Si may cause dramatic shift in the phytoplankton community with dominance of green or blue-green algae (Tilman et al., 1982; Teubner and Dokulil, 2002). Experimental evidence suggest the dominance of diatoms at high Si: N ratios and that of non-silicious flagellates at low Si: N ratio in marine ecosystems (Sommer, 1994). Further, low N: P ratio favours the dominance of diazotrophic cyanobacteria (Havens et al., 2002; Smith and Schindler, 2009). Thus, along a N-limiting gradient, as may be the case of present study, the contribution of cyanobacterial population, especially N-fixing species to overall phytoplankton biomass would be higher. Similarly, the Si- limiting condition, as observed spatially toward downstream, could slowly replace diatoms (Sommer et al., 1986). Additionally, during eutrophication, biogeochemical feedbacks further increase the supply of P, but decrease the availability of silica favouring the formation and persistence of harmful algal blooms (Howarth et al., 2011). In fresh waters, cyanobacteria commonly bloom and fix N when the availability of N relative to P is low (Howarth et al., 2011).
The increase in Chl a biomass and GPP downstream indicate enhanced growth of phytoplankton associated with nutrient enrichment. The concentration of DOC also indicate an increasing trend due to the coupled effect of autochthonous C- pool and allochthonous C input. The Chl a/ DOC ratio is the measurement of autochthonous C in the river. In this study, the Chl a/ DOC ratios indicated major contribution of phytoplankton in winter season. Despite enhanced phytoplankton development however, Chl a/ DOC ratio declined at downstream sites indicating the loading of organic carbon in the river at a rate faster than the rate of phytoplankton turnover.
This indicates the influence of the loading of organic input from terrestrial sources (Pandey et al., 2014). This has relevance for the long term shift in trophic cascades since the nature of C available in surface waters regulates the nutritional status of consumers.
Conclusions
Anthropogenic loading of nutrients in surface waters has substantially increased over recent decades. This may lead to a shift in the N: P: Si stoichiometric elemental coupling and, by implication, the production and fate of phytoplankton in surface waters. Our study, conducted along a 35 km stretch of the Ganga River, showed a shift in N: P: Si stoichiometric ratios in the river at Varanasi. Such changes are expected to be the result of anthropogenic release coupled with biogeochemical feedbacks, including denitrification and sediment efflux regulating N and P levels in the river water. Since the N: P: Si ratio for phytoplankton growth is specific, a long-term shift in N: P: Si stoichiometry may induce a dramatic change in the ecological dominance of the phytoplankton community in major rivers experiencing anthropogenic controls.
Acknowledgements
The authors thank the Coordinator, Centre of Advanced Study in Botany, Banaras Hindu University for facilities. One of the authors (AY) is grateful to Banaras Hindu University for funding support.
References
American Public Health Association (1998). Standard methods for the examination of water and wastewater. APHA, Washington, DC.
Caraco NF (1993). Disturbance of the phosphorus cycle: A cast of indirect effects of human activity. Trends in Ecology and Evolution 8: 51–54.
Dienert F, and Wandenbulcke F (1923). Sur Ie dosage de la silice dans les eaux. C.R. Acad. des Sciences, Paris 176: 1478-1480.
Dodds WK, Martí E, Tank JL, Pontius J, Hamilton SK, Grimm NB, Bowden WB, McDowell WH, Peterson BJ, Valett HM, Webster JR, Gregory S (2004).Carbon and nitrogen stoichiometry and nitrogen cycilng rates in streams. Oecologia 140: 458–467.
Downing JA (1997). Marine nitrogen: Phosphorus stoichiometry and the global N:P cycle. Biogeochemistry 37: 237–252.
Downing JA, McCauley E (1992). The nitrogen: phosphorus relationship in lakes. Limnol and Oceanogr 37: 936-945.
Elser JJ, Andersen T, Baron JS, Bergstrom AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO (2009). Lake stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326: 835–837.
Elser JJ, Fagan FW, Denno RF, Dobberfuhl DR, Folarian A, Huberty A, Interlandi S, Kilham SS, Mc Cauley E, Schulz KL, Siemann EH, Sterner RW (2000a). Nutritional constraints in terrestrial and fresh water food webs. Nature 408: 578–580.
Elser JJ, Sterner RW, Galford AE, Chrzanowski TH, Findlay DL, Mills KH, Paterson MJ, Stainton MP, Schindler DW (2000b). Pelagic C:N:P Stoichiometry in a Eutrophied Lake: Responses to a Whole-Lake Food-Web Manipulation. Ecosystems 3: 293-307.
Gilpin LC, Davidson K, Roberts E (2004). The influence of changes in nitrogen : silicon ratios on diatom growth dynamics. Journal of Sea Research 25: 21-35.
Granelil E, Per Carlsson, Jefferson T, Turne er, Patricia A, Tester CB, Dawson R, Funari E (1999). Effects of N: P: Si ratios and zooplankton grazing on phytoplankton communities in the northern Adriatic Sea. I. Nutrients, phytoplankton biomass, and polysaccharide production. Aquatic Microbial Ecology 18: 37-54.
Gruber N and Galloway JN (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451: 293-296.
Guildford SJ and Heeky RE (2000). Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnol Oceanography 45(6): 1213-1223.
Havens KE, James RT, East TL, Smith VH (2002). N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environmental Pollution 122: 379-90.
Howarth R, Chan F, Conley DJ, Garnier J, Doney SC, Marino R and Billen G (2011). Nutrient Coupled biogeochemical eycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Frontiers in Ecology and the Environment 9: 18-26.
Howarth RW (2008). Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmfull Algae 8: 14-20.
Ittekot V, Humborg C, Schafer FP (2000). Hydrological alterations and marine biogeochemistry: a silicate issue? Bioscience 50: 776-782.
Kristiansen S and Hoell EE (2002). The importance of silicon for marine production. Hydrobiologia 484: 21-31.
Mackereth FJH (1963). Some methods of water analysis for limnologists. Freshwater Biologists Association for Scientific Publication, 21, Ambleside, London.
Maiti SK (2001). Handbook of methods in environmental studies. Water and wastewater, vol 1. ABD, Jaipur.
Michel P (1984). Ecological methods for field and laboratory investigation. Tata McGraw–Hill Publ Co. Ltd., New Delhi.
Pandey J, Pandey U, Singh AV (2014). Impact of changing atmospheric deposition chemistry on carbon and nutrient loading to Ganga River: integrating land–atmosphere–water components to uncover cross-domain carbon linkages. Biogeochemistry 014:9957-2.
Pandey J, Singh AV, Singh A, Singh R (2013). Impacts of changing atmospheric deposition chemistry on nitrogen and phosphorus loading to Ganga River (India). Bull Environ Contam Toxicol 91: 184-90.
Redfield AC (1958). The biological control of chemical factors in the environment. Am Sci 46: 205-222.
Smith V and Schindler DW (2009). Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24: 201-7.
Sommer U (1988). Growth and survival strategies of planktonic diatoms. In: Sandgren CD (ed.) Growth and reproductive strategies of freshwater phytoplankton. Cambridge University Press, Cambridge.
Sommer U (1994) Are marine diatoms favoured by high Si:N ratios? Mar Ecol Prog Ser 115: 309-315.
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986). The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106: 433–471.
Teubner KD, Dokulil M (2002). Ecological stoichiometry of TN : TP : SRSi in freshwaters : nutrient ratios and seasonal shifts in phytoplankton assemblages. Arch Hydrobiol 154: 625-646.
Tilman D, Kilham SS, Kilman P (1982). Phytoplankton community ecology: The role of limiting nutrients. Ann Rev Ecol Syst 13: 349-372.
Turner RE, Qureshi N, Rabalais NN, Dortch Q, Justić D, Shaw RF Cope J (1998). Fluctuating silicate:nitrate ratios and coastal plankton food webs. PNAS 95: 13048-51.
Turner RE, Rabalais NN, Justic’ D, Dortch Q (2003). Future aquatic nutrient limitations. Marine Pollution Bulletin 46: 1032-4.
Turner RE (2002). Element ratios and aquatic food webs. Estuaries 25: 694-703.
Viaroli P, Nizzoli D, Pinardi M, Rossetti G, Bartoli M (2013). Factors affecting dissolved silica concentrations, and DSi and DIN stoichiometry in a human impacted watershed (Po River, Italy). Silicon 5: 101-114.
Voghe AI (1971). A text book of quantitative inorganic analysis, 4th Edn. The English Language Book Society, Longman, Essex.
