 Deactivation of Radiation from Radioactive Materials Contaminated in a Nuclear Power Plant Accident
Deactivation of Radiation from Radioactive Materials Contaminated in a Nuclear Power Plant Accident
Sugihara S1,2*
1Kanagawa University, Yokohama, 221-8686, Japan
2Sugihara Institute of Science and Technology, Yokohama, 236-0046, Japan
*Correspondence E-mail: natsuyama41@gmail.com
Key Words: Half-life, Radioactive decontamination, Cesium nucleus, Stable elements, The long-wavelength synthesis, Active water
Received April 16th, 2013; Accepted September 24th, 2013; Published October 27th, 2013; Available online November 15th, 2013
Abstract
Many methods for reducing radioactive contamination have been proposed, most of which rely on the use of materials such as zeolites or plants to adsorb radionuclides. In such processes, the absorbents become radioactive and require subsequent disposal, usually by long-term burial. Deactivation of the radionuclides would constitute a far superior solution to the problem of radioactive contamination. A method is described for deactivating radionuclides independently of their half-lives. The theory underlying the method encompasses two ideas: the interaction of photons from radioactive cesium with Infotons (hypothetical particles generated by specially processed water with a certain energy) and the application of group theory to Infotons to show that stable elements can be generated from radioactive cesium. In a six-month trial on samples of contaminated soil, we confirmed that measured levels of radioactivity (β- and γ-rays) were reduced by treatment with processed water containing Infotons. The radioactivity of the contaminated soils was reduced by 60% after 42 hours of treatment, and this reduction persisted during six months. Another specimen of soil showed a 90% reduction in radioactivity after six months. Furthermore, analyses by inductively coupled plasma/mass spectrometry and X-ray fluorescence analysis showed that the treated soils and their extracts contained barium, lanthanum, and cerium ions in amounts that corresponded closely to those predicted by the theory.
Article Outline
- Introduction
- Materials and Methods
- Results
- Discussion and Interpretation
- Conclusions
- Acknowledgements
- References
- Discussion with Reviewers
Introduction
As stated, many methods for reducing radioactive contamination have been proposed, most of which involve the use of bacteria, plants, or materials such as zeolites. Such methods rely on adsorption of the radionuclides and they require subsequent disposal of the contaminated adsorbent, usually by long-term burial. Although our method, which involves the use of water treated at high pressure to disrupt its hydrogen bonds, deviates from the model of radioactivity that is currently accepted in physics, it has been repeatedly shown to be effective in small-scale (25–1000 g) experiments on contaminated soil and rice, and the active water has been shown to have an effect in reducing the half-lives of radionuclides that extends beyond mere shielding. How is it possible to reduce the half-life of a radioactive substance? The accepted view among the scientific community is that this is not possible. This is because the accepted view involves the consideration of the energy of nucleus, which is extremely high. The un-precedented phenomenon that I describe can be explained by considering an extended particle, similar to other elementary particles (Yukawa, 1950), in conjunction with the theory of light propagation in an anomalous dispersion proposed by Brillouin (1960) and Dirac theory (Dirac, 1938); taken together, these suggest that the interior of the electron is a region of space through which a signal can be transmitted faster than light in a medium. Another view of the mechanism for the way that an extended particle can access an atomic nucleus, such as that of cesium, is that the potential around the nucleus and the particle is in the form of a spherical surface possessing a Gaussian curvature (Bleecker and Wilson, 1978; Shima et al., 2009) in which a reaction can occur within a very short time and in a very small space. In consid
ering these ideas, I propose a mechanism for the reduction of the radioactivity of contaminated substances through the inter-action of Infotons with cesium nuclei. I also provide experimental results to confirm this mechanism, including follow-up data collected over a six-month period.
Materials and Methods
Samples of contaminated soil were collected on May 5th through May 9th, 2011, from the grounds of a primary school located 50 km from the Fukushima nuclear power plant from which radioactive materials were released following the earthquake and tsunami of March 11th, 2011. Samples of soil were also collected from a farm 23 km from the plant. The contaminated soil samples (25–1000 g) were placed in a specially treated pot together with sufficient water to cover the surface of the soil. The treated pot was fabricated as follows. Pellets of acrylic–styrene resin were energized by immersion in water processed by pressurization (~3 MPa) following repeated detonation, and the pellets were then injection molded to form the pot as shown in Figure 1 (Hatanaka, 1990, 1991, 1993). The radiation was measured for 1000 s for each sample by using a Geiger–Müller (GM) counter (Inspector; S.E. International Inc., Summertown, TN, USA) capable of detecting β- and γ-rays. We also used a second GM counter (GAMMA-SCOUT GmbH & Co. KG, Schriesheim, Germany), also capable of detecting β- and γ-rays. For comparison, the contaminated materials were also placed in a nonactivated pot as a control. Furthermore, the full range of energy emitted by the radioactive sub-stances was analyzed at Kobe University by using a high-purity germanium detector (HPGe; GC3019; Canberra Industries, Inc., Meriden, CT, USA). The elements produced by decay of the radionuclides were analyzed by inductively coupled plasma / atomic-emission spectrometry (ICP-AES), inductively coupled plasma/mass spectrometry (ICP-MS), and X-ray fluorescence analysis (XRF).

Figure 1: Activated pot (1.9 L) containing contaminated soils (left) or withered grasses (right) together with water.
In the field test, we carried out to set many activated bricks (20×15×5cm approx.) in the field so that the water and soil in the rice field can be activated from plating rice (June) to harvest (late September). We checked the activation of water using FTIR, NMR, Terahertz spectroscopy and Superconducting Quantum Interference Device (SQUID) indirectly. Some of methods were discussed in the reference (Sugihara, 2008 and 2009) as well as discussion below.
Theory
Radioactive Decay
According to a report by the Japanese government, 25 of the 32 radionuclides released as a result of the disaster at the Fukushima Nuclear Power Plant on March 11, 2011, have short half-lives of less than one year; almost all these radionuclides have low decay energies of less than 100 keV and emit β- and γ-rays. We calculated the temporal decay status against time for all the radionuclides except for the isotopes 238Pu through 241Pu and elements possessing half-lives of less than the order of an hour.
The half-life is defined as the time required for the level of radioactivity to fall to half the initial level. The rate of radioactive decay can be calculated by using the following differential equation: (1)
dN/dt = –λN(t)
Here, N(t) is the quantity of radioactive substance at time t, and λ is the decay constant:
∫(dN/N(t)) = –λ∫dt
Solving Equation 1, we have N = N0e-lt, where N0 is the initial amount of radioactive substance. The half-life (T1/2) is defined as the time required for N to become half the value of N0 and it is related to λ as follows:
Tv2 = ln2/λ = 0.693/λ
For example, for 134Cs, the half-life is 2.1 years, so that:
λ = 0.693/2.1 = 0.33
Therefore,
N/N0 = e-0.33*2 = 0.517
In other words, 51.7% of the radionuclide 134Cs remains after two years. This is a general and familiar method for calculating how much radioactivity remains after a given period of time. Graphs showing the decay status for radionuclides possessing a half-life of more than a year are shown in Figure 2a. In this figure, the decay mode 106Ru is by emission of β-particles (with no γ-emissions) and its decay energy is 39 keV. Approximately, 50% of 134Cs was present at the time two years after the radioactive release in 2011. Figure 2b shows that, other than 144Ce, almost all the radionuclides that had half-lives of less than 300 days, had decayed by mid-2013. Furthermore, radionuclides with half-lives of less than 30 days had also decayed by mid-2013 (Figure 2c).
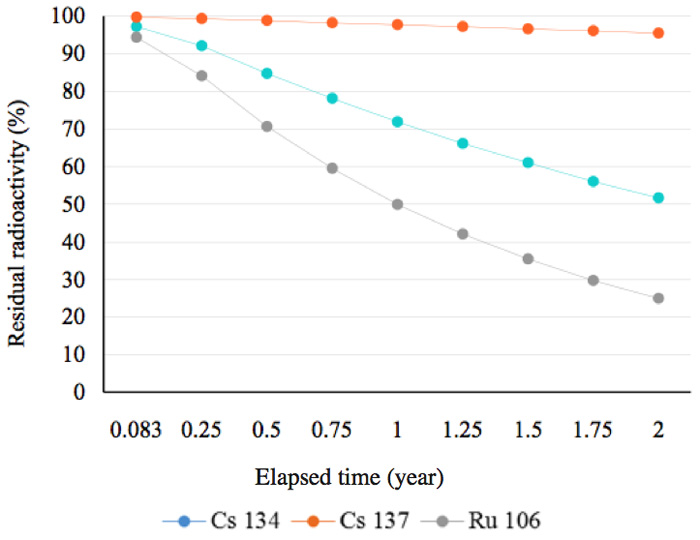
Figure 2a: Residual radioactivity calculated from half-lives. These nuclides posses half-lives of more than one year.
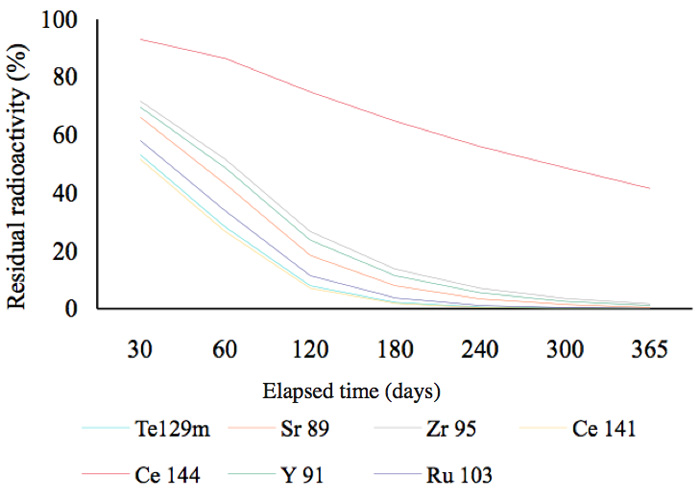
Figure 2b: Residual radioactivity calculated from half-lives. These nuclides posses half-lives of less than 300 days.
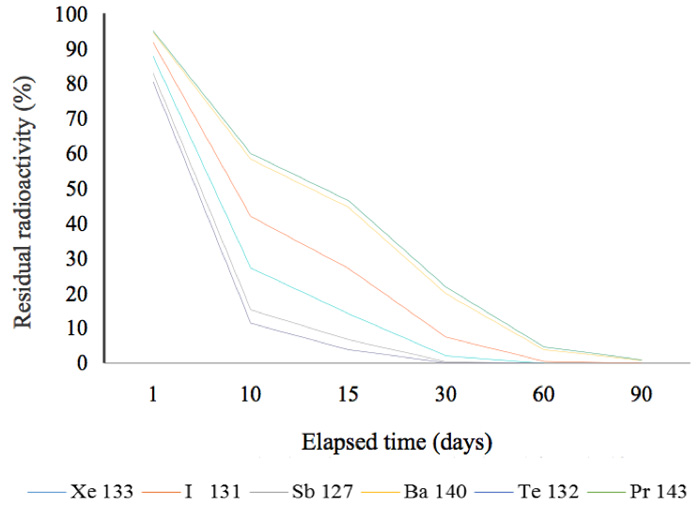
Figure 2c: Residual radioactivity calculated from half-lives. These nuclides posses half-lives of less than 30 days.
Infoton Theory and Energy Transfer
When a hydrogen bond is broken, the associated proton and electron, rather than forming hydronium ion or hydroxyl ions, remain inside the water molecule and retain energy in the form of spin and momentum, resulting in the emission of weak forms of radiation such as terahertz radiation. We have reported computer simulations, made by using a molecular-orbital method, of two water molecules after the breaking of a hydrogen bond (Sugihara and Hatanaka, 2009). We call such a fragment an “Infoton.” The Infoton is an elementary particle that do not decay like radioactive material; instead, it has a stable existence, carrying energy in a water molecule. Unfortunately, we are currently unable to detect Infotons directly, although we have found circumstantial evidence for their existence by using NMR, terahertz, and FTIR spectroscopy and by SQUID and isotopic analyses (the ratio of 2H to 1H), too.
The discussion of Infotons involves two aspects, one in which the Infoton is considered to function as a particle (Sugihara, 2008) and one in which it is considered to function as long-wavelength electromagnetic wave. If we introduce a new player for long-wave length synthesis, namely the reaction between Cs and Infotons, the characteristics of Infotons can be as described by Equation 2:
n/2H2O → n <H+– e–>p/e (n=1,2,3,4)
Here, the subscript p/e refers to the proton and electron in the fragment <H+– e–>p/e, which exists as a plasma state in the treated water molecule. We call these fragments “Infotons.” The Infoton is an extended particle that rotates in the axis of <H+– e–> with a spin of 1, and also vibrates in the longitudinal direction. We believe that the Infoton itself cannot be transferred to another substance; instead, the energy of the Infoton transfers to another substance in the form of an electromagnetic wave.
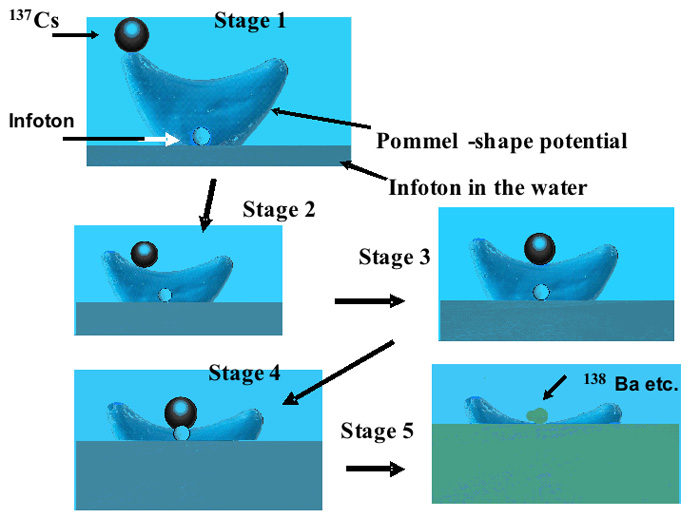
Figure 3: Reaction between Cs and an Infoton that ascends the pommel-shaped potential with energy provided by 137Cs (661.6 keV portion) and/or 134Cs (563–1365 keV portion) (not shown). Furthermore, if the mass of the Infoton is 1.676 × 10 -27 kg and it is traveling at 5% of the speed of light, its kinetic energy is calculated to be about 1170 keV, corresponding to the decay energy of 137Cs.
The Infoton <H+– e–>p/e plays a role in internal functions such as the internal spin and angular momentum of H+ and e–. Another type of Infoton, <H+– e–>e, functions only in relation to the electrical charge on an element. Four Infotons <H+– e–>p/e together do not interact with the nucleus because, together, they are too large, and it takes time to transfer information when they approach the nucleus one-by-one. The reactions of Infotons occur in a very small space (of the order of picometers) and within a very short time (of the order of picoseconds). If we assume that the potential should be hyperboloid, where the Gaussian curvature K(p) < 0 and the length of the circuit in the spherical surface of radius r is Lp(r), we obtain Equation 3:
K(p) = lim r→10 3/Π (2Πr-Lp(r)/r3)
The distance between the nucleus and the Infoton <H+– e–>p/e is probably of the order of picometers. The curvature increases with decreasing distance, and the energy of the Cs nucleus is reduced through emission of photons.
Results
We have proposed the existence of a pommel -shaped potential between an Infoton and a radionuclide, such as radioactive cesium, as shown in Figure 3; this implies that a group velocity of greater than c, the velocity of light in a vacuum, is possible in an anomalous dispersion medium such as water, resulting in the Infotons having large energies (corresponding to 1170 keV at 5% of light velocity, which is close to the photon energy from Cs).
We have obtained results from many experiments with samples from various locations, collected at various sampling dates. First, Figure 4 shows the results for measurements after the soil samples had been kept for 92 h in the activated pot. Figure 5 shows the short-term changes in the radioactivity of soil samples collected from various locations, some measured by an independent organization, indicating that decay of 137Cs proceeds logarithmically in accord with its half-life (30 years). Soil samples (1 kg each) were placed in activated pots, as shown in Figure 1, and water was added to just cover the surface of the sample. A drastic reduction of radioactivity was apparent, even if the decay of many nuclides with shorter half-lives is taken into account, as shown in Figures 2b and 2c.
We also subjected other soil samples from the same site to an identical process to that shown in Figure 4, but we monitored them for 6 months, as shown by the logarithmic scale in Figure 6.
The soils were sample from the same area as those for which results are shown in Figures 4 and 5. The measurements were performed by an independent organization, and the radioactivity changes are shown in Figures 7a (137Cs) and 7b (134Cs); the half-lives are also shown for comparison with the calculated results for the follow-up period of two years between June 2011 and June 2013.
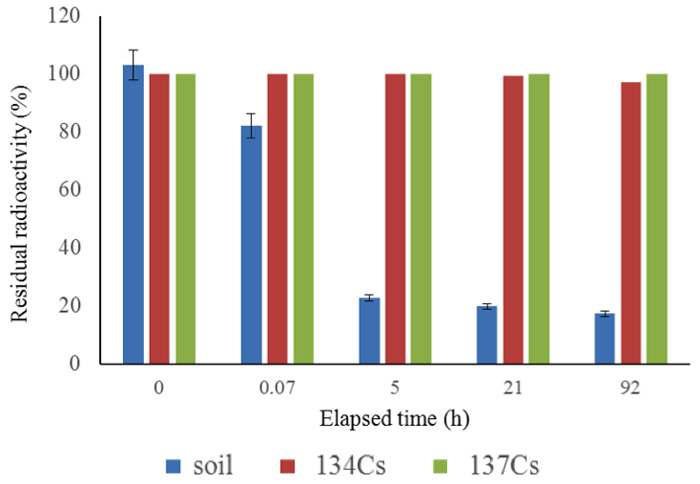
Figure 4: 92-hour follow up for radiation reduction. Soils were sampled in the hot spot, 23 km from the nuclear power plant, on April 3rd, 2011.
Table 1 shows the long-wavelength synthesis of elements by reaction of 137Cs with Infotons, <H+– e–>p/e and/or <H+– e–>e, calculated using the group theory multiplication. As a result, we obtain the following predicted values: Ba, 52%, La, 32%, Ce, 16% from 137Cs and Ba (84%), La (16%), and Ce (0%) from 134Cs (these are abbreviated in the table). According to our theoretical calculations, the overall composition of the elements formed should be as follows: Ba, 68%; La, 24%; Ce, 8%.
We also carried out an analytical investigation. The aqueous extracts of soils were analyzed by inductively coupled plasma/mass spectrometry (ICP/MS) and by inductively coupled plasma/atomic absorption spectrometry (ICP/AES), and the soils themselves were analyzed by x-ray fluorimetry (XRF). The results showed that the radioactive materials were converted into Ba, La, and Ce; the detected levels of these elements were markedly different from those usually found in soil. The amounts of the elements were as follows: Ba, 63.3%; La, 21.3%; and Ce, 15.4%.
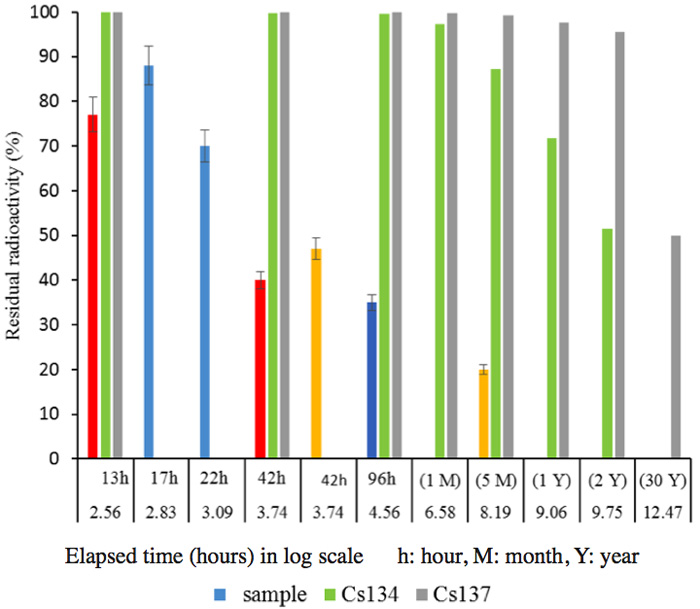
Figure 5: Residual radioactivity for various locations for periods of up to 5 months, compared with Cs changes corresponding to the decay status calculated from a half-life. Red and blue; samples in different spots, and measured after 17, 22, 42 and 96 hours.Orange; measured by a different organization (42h and 5M).
In addition to the many experiments that we performed at the scale of the activated pot (~1 kg), we also performed large-scale tests on a rice field. We have added these results to show the reduction in radioactivity of unprocessed rice and in samples of rice subjected to such processes as unhulling and cooking (Figure 8). We analyzed the vapor during cooking, but we could not detect any radioactivity; this study is ongoing.
Discussion
There are two ways to reduce radioactivity; one is by shielding using concrete, lead, or water for γ-rays and or electron beams; these function by absorbance and/or scattering of the radiation. The second is to rely on adsorption of the radionuclides by materials such as zeolites, as mentioned in the introduction. These methods do not, however, reduce the total amount of radio-activity. We have found the radioactivity can, however, be reduced by treatment with activated water or with an energized substance exposed to activated water. Of course water itself can function as a radiation shield, as noted above; however, we have shown that the effect of activated water on reducing radiation is greater than the mere shielding effect, as shown in Figures 4-6; these show results for short follow-up periods of up to two years. Before we discuss why this reduction in radiation occurs, we need to look at the decay curves in Figure 2a; at the time of the measurements, ruthenium was still present in an amount of about 25% 10644Ru , which has a half-life of 374 days, transmutes into 10645Ru through emission of β-particles in approximately 30 s; this nuclide, in turn, transforms into 10646Pd which is stable, and analysis showed this was present in an amount of about 17 ppm. The three elements shown in Figure 2a did not play any role in the marked reduction in radiation within the short elapsed time.
Next, we will discuss the seven nuclides with half-lives of less than 300 days shown in Figure 2b. When these elements decay, some of them participate in isomeric transitions; these are transitions between nuclides whose atomic numbers and numbers of neutrons are the same, but which have different stable or metastable energy states.
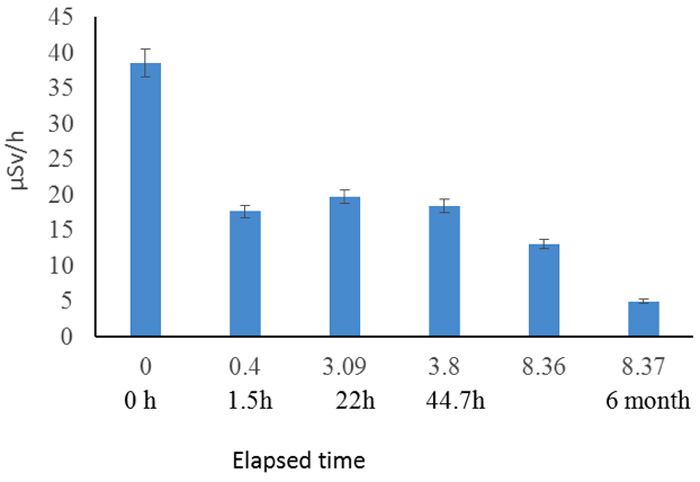
Figure 6: Follow up of radiation reduction from soil for 6 months (5/22/2011 – 11/16/2011). Elapsed time (h)/ log scale and real.
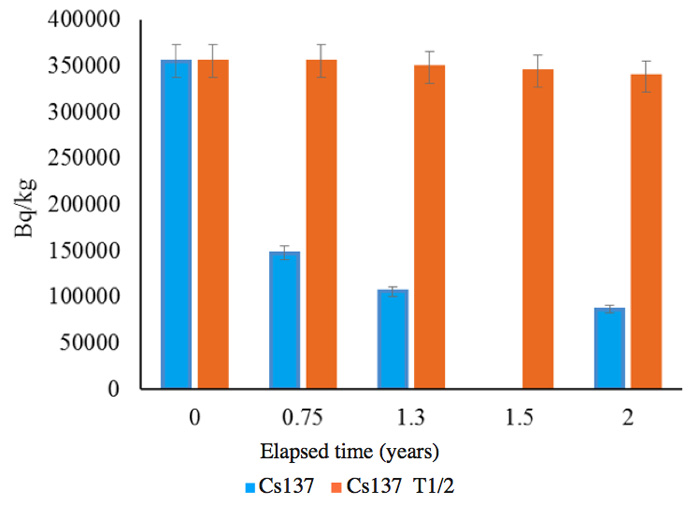
Figure 7a: Follow up of radioactivity reduction from soil for 2 years. 0: June 2011, 0.75: March 2012, 1.3: October 2012, 2: June 2013. 1.5 shows only the calculation based on the half-life T1/2 for Cs-137.
For example, 9541Nb, a metastable radionuclide with a half-life of 87 hours, is converted into the stable molybdenum nuclide 9542Mo by an isomeric transition with a low energy of 240 keV. The second group of elements (Figure 2c) might have played a role in the marked reduction of radiation during six months (Figure 6), but their energies are smaller. We cannot therefore deny the contribution made by nuclides with short half-lives to the marked reduction in the initial period following the contamination incident, but we can recognize that the radiation changed in the follow-up periods of more than six months and two years. For example, the radiation in a rice plant from the contaminated rice field recorded in June (generally) fell to that recorded in unhulled rice (in September), and finally to that in cooked rice, as shown in Figure 8.
To provide a scientific explanation of these phenomena, we applied two methods, including a purely theoretical one developed by using group theory. Furthermore, we performed chemical analyses to detect elements in soil samples subjected to our special treatment to confirm the changes in cesium levels. The reduction in radioactivity is regarded as being due to novel synthesis by Infotons and their associated long-wavelength radiation. Thus, a half-life can be drastically reduced due to interaction of Infotons with cesiums as we showed in the case of 134Cs and 137Cs, although the following decay mode is regarded in general;
13755CS → meta stable 137m56Ba (95% of the mode) and (T1/2 =30y) β ray (512 KeV) (T1/2 =2.55 m) → stable 13756Ba (5% of the mode), γ ray (661.6 KeV) which is one of the stable 7 elements of Ba.
If we adopt another quantum view, the wave function provides an effective way of achieving a qualitative understanding of this process. We can recognize that the drastic reduction in radioactivity involves mechanisms pertaining to interactions between cesium nuclei and Infotons, which generate long-wavelength (0.03–1 mm) radiation (Sugihara, 2009); these wavelengths correspond to frequencies of the order of 1011 to 1013 Hz, namely those in the far-infrared and terahertz regions.
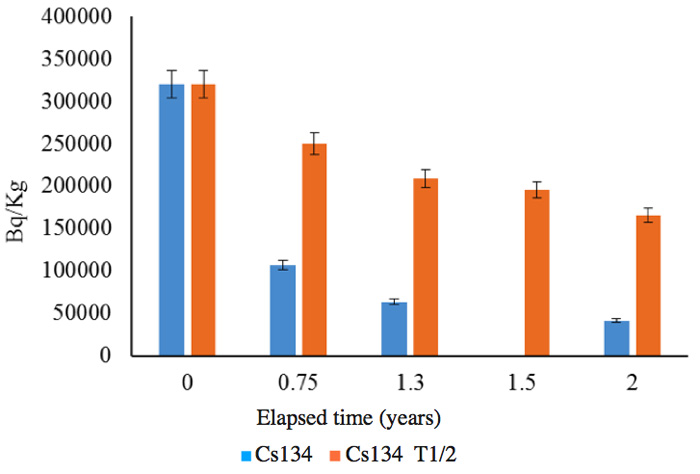
Figure 7b: Follow up of radioactivity reduction from soil for 2 years. 0: June 2011, 0.75: March 2012, 1.3: October 2012, 2: June 2013. 1.5 shows only the calculation based on the half-life T1/2 for Cs-134.
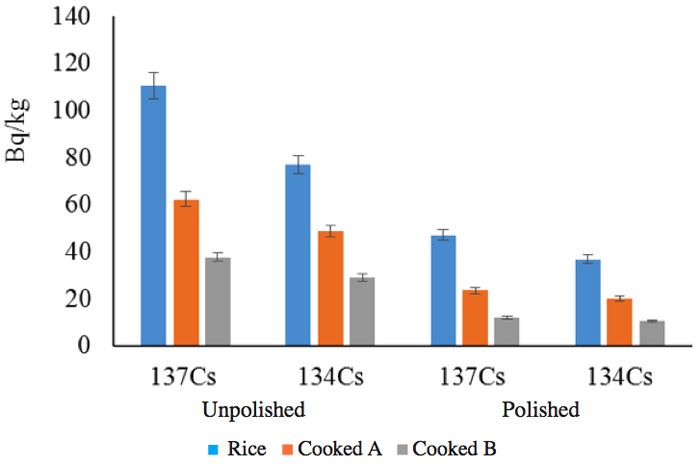
Figure 8: Radiation reduction in ride depending on treatment.
Cooked A: Cooked with ordinary tap water.
Cooked B: Cooked with activated water.
Reduction rate in polished/unpolished ratio:
1) Rice: 137Cs = 57.4%, 134Cs = 52.1% (in total, 55.2%)
2) Cooked A: 137Cs = 62.3%, 134Cs = 58.6% (in total, 60.7%)
3) Cooked B: 137Cs = 68.0%, 134Cs = 63.6% (in total, 66.1%)
As discussed above, photons of energy 563 to 1365 keV are emitted from 134Cs and photons of energy of 661.6 keV are emitted from 137Cs; the Infotons receive part of this energy, which increases their momentum and spin. Meanwhile, the calculated values of the kinetic energies of the Infoton (T = 1/2mv2), where m is the mass of the Infoton and v is its velocity in water) correspond to the energies emitted from Cs, provided that the Infoton has even 1% of the speed of light.
Thus the interaction can be considered as illustrated in Figure 3, in which the Infoton and Cs present in water form a pommel-shaped potential and cesium loses energy by emitting photon(s); the Infoton regains energy from Cs, causing it to climb the potential slope, leading to the formation of the stable element; this process occurs within a very short time and in a very small space, as mentioned previously. As a result, the potential of Cs decreases along the larger Gaussian curvature, whereas the potential of the Infoton increases toward the Cs nucleus in a very short time, facilitating interaction with the nucleus.
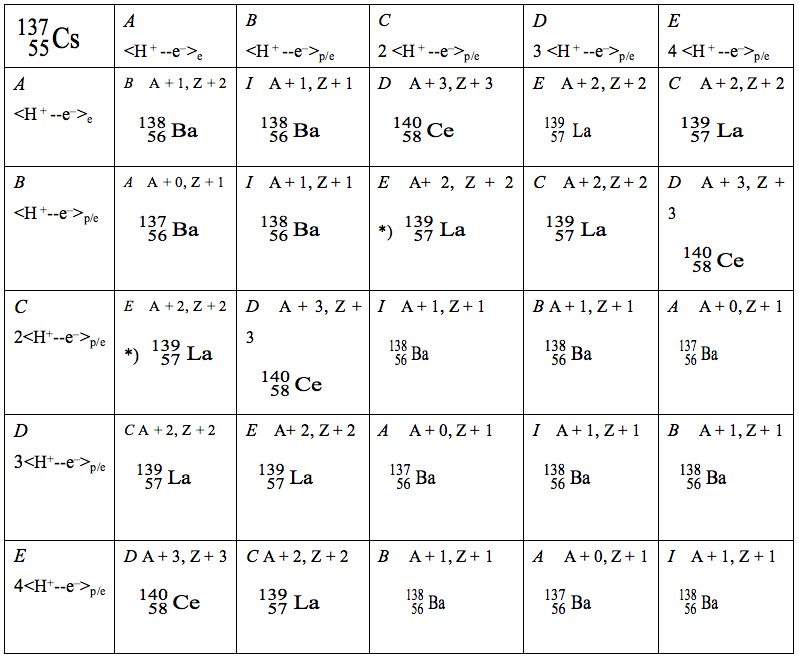
Table 1: Long-wavelength synthesis of elements by reaction of 137Cs with Infotons <H+– e–>p/e calculated by using group theory. The group consisted of six action elements (I, A, B, C, D, E in italic); for example, (B×D)=C. C means an element of 2<H+– e–>p/e. A; <H+– e–>e functions only in the valence shell of the element and does not react with the nucleus. E; 4<H+– e–>p/e means 2 <H+– e–> + 2 <H+– e–>.
As a result, we obtain the predicted values Ba=52%, La=32%, and Ce=16% from the Table.*) 4<H+– e–>p/e → 2 <H+– e–>p/e + 2 <H+– e–>p/e is defined on page 73.A + 2 = 139, Z + 2 = 57 make La, A + 3 = 140, Z + 3 = 58 make Ce, and so on, in which A and Z mean a mass and an electric charge, respectively.
We are still continuing our attempts to confirm the existence of long-wavelength synthesis, although it is difficult to elucidate the mechanism of deactivation of radionuclides qualitatively, because so many kinds of radioactive nuclides were emitted from the nuclear power plant and some of them decayed with shorter half-lives to generate stable nuclides, whereas others changed into other radionuclides that emitted β- and γ-rays or, in one rare case, α-rays.
It has been shown, both in the short term and over longer periods, that radiation (γ- and β-rays) levels of contaminated soil show effects not only of shielding but also of transformation of radioactive substances, such as cesium radionuclides, into stable elements.
Conclusions
We have shown that samples of contaminated soil from areas near the Fukushima nuclear power plant showed a rapid decrease in radioactivity in both the short term and over a period of up to 2 years when placed in plastic pots treated with activated water. We estimated theoretically which stable elements should be formed from radioactive materials by using the group theory multiplication method, and the calculated results (which suggest the formation of Ba, La, and Ce, among other elements) agreed well with our experimental results. A plausible mechanism for this process involves the reaction of 134Cs and 137Cs with Infotons n<H+– e–>p/e in the treated water, which emit long-wavelength radiation. This long-wavelength radiation resonates with the potential on the spherical surfaces of the nuclei of the cesium radionuclides. Larger-scale studies (with a 100-L container) to confirm this phenomenon have been ongoing in the Fukushima area since January 2012 and, in November 2012, we found a reduction in radioactivity in a rice field and in rice harvested from the field.
Acknowledgments
We express our gratitude to Mr. K. Hatanaka of MCM for water treatment to produce the activated devices, and also to Mr. S. Katanahara of Alps Engineering Co. for the 100-L facility for transporting activated water and for the instrumentation for measuring radioactivity (GM counter Inspector). We also thank Senator Mr. G. Satoh for providing a warehouse and a rice field for the large-scale test, and Mr. Y. Nagasaka (Brighton Inc.), N. Iwamoto (CSR), and K. Hatanaka (MCM) for sampling contaminated soil etc. and for performing measurements. We express our gratitude for measurements of radioactivity and energy (HPGe ) to Professor A. Kitamura of Kobe University, and we thank Mr. K. Hagiwara of Kanagawa University ICP/MS measurements and Mrs. M. Shishido of ACR Co. for ICP/AES measurements. Finally, wish to express our gratitude to Mr. F. Emoto of MassTech Corporation for providing a GM counter (γ-Scout).
References
Bleecker D, Wilson L (1978). Stability of Gauss maps. Illinois J Math 22: 270–289.
Brillouin L (1960). Wave Propagation and Group Velocity. Academic Press, New York. NY, 12.
Dirac PAM (1938). Classical theory of radiating electrons. Proc R Soc A 167: 148–169.
Hatanaka K. (1990) European Patent 0421563. (1993) Japanese Patent 1786552. (1991) US Patent 5034138.
Shima H, Ono S, Taira H. (2009) Quantum mechanics on a curved surface and its application to materials science. J Surf Sci Soc Jpn 30: 652–658 (in Japanese); and papers cited therein.
Sugihara S. (2008) Infoton: Japanese Trademark Right. June 13, 2008.
Sugihara S, Hatanaka K. (2009) Photochemical removal of pollutants from air or automobile exhaust by minimal catalyst water. WATER Journal 1: 92–99.
Yukawa H. (1950) Quantum theory of non-local fields. Part I. Free fields. Phys. Rev. 77: 219–226.
Discussion with Reviewers
Anonymous Reviewer: It is unclear for this reviewer whether the “Infoton” theory is only a theoretical concept, or if it can actually be used to calculate and thus predict the accelerated decay.
S. Sugihara: When a hydrogen bond is broken, the proton and electron remain inside the water molecule, which retains the energies associated with spin and momentum; this results in emissions of weak energy, in the form of terahertz (THz) radiation, instead of the formation of hydronium ions or hydroxyl ions. We have previously reported [S. Sugihara et al., WATER Journal 1, 92, (2009)] computer simulations using a molecular-orbital method of two water molecules with a broken hydrogen bond. We call this ‘fragment’ the Infoton. The Infoton is similar to an elementary particle in that it does not decay and that it exists as a stable entity in a water molecule. Unfortunately we cannot detect it directly yet, although we have found circumstantial evidence for its existence by using NMR, terahertz, and FTIR spectroscopy and superconducting quantum interference device (SQUID) and isotope analyses. In 2009, we showed that the lowest energy level was the breaking state of bonding in part of water molecule after breaking of a hydrogen bond. Furthermore, by analyzing the relaxation time using NMR we found that the presence of an Infoton causes a decrease in the size of the water molecule, and we also detected a transmittance of water in the terahertz region by using terahertz measurement (0.6–20 THz) and FTIR. In SQUID studies on activated plastic pellets, we detected hysteresis changes in the magnetic moment of up to 5 Tesla, to which the spin of the Infoton may contribute.
Reviewer: The method itself is not clear to this reviewer: What is the “activation” process of the pot? How is this “Infoton” supposed to be generated, and why does this only happen in the “activated” pot? Is there any (be it hypothetical) possibility to directly measure “infotons”?
Sugihara: The following statement is provided in the text. “The treated pot was fabricated as follows. Pellets of acrylic–styrene resin were energized by immersion in water processed by pressurization (~3 MPa) following repeated detonation, and the pellets were then injection molded to form the pot [Hatanaka K.; EP 0421563 (1990); JP 1786552; (1993); US 5034138; (1991)].» Energy from activated water can be transferred as follows. A proton and electron (which we call an Infoton <H+– e–>) can possess energy in the form of spin and momentum after breaking of a hydrogen bond. We believe the Infoton itself cannot be transferred to another substance. Instead, the Infoton’s energy transfers in the form of electromagnetic waves to another substance; this is demonstrated by an energy shift in the X-ray absorption near-edge structure (XANES) spectrum of amino acids, the transmittance of terahertz waves and infrared radiation in water, and changes in the magnetic moment of glass, gums, and plastics, as shown by SQUID analysis. Any liquid besides water or any solid that interacts with this energy will be defined as ‘activated’. As there are no exceptions, we can produce the ‘activated’ pot, some characteristics of which we have previously examined by terahertz spectroscopy and SQUID analysis. The energized pot can subsequently transfer its energy to ordinary water and/or soils that are present inside it. We confirmed this by the following experiment.
Four ‘activated’ seals (plastic)
Ordinary empty commercial PET bottle
Tap water (150 mL)
Four activated plastic seals were attached to a polyester (PET) bottle in the region above the water level. After one day, we analyzed the water by the methods described above, and we showed that the energy from the seals was transferred to the water in the lower part of the bottle.
Unfortunately we cannot yet directly detect Infotons, although we have found circumstantial evidence for their existence by using NMR, THz, and FTIR spectroscopy and SQUID, XANES, and isotope analysis.
Reviewer: What makes the material of the “activated pot” so special? Why do the “Infotons” form in this pot – and not just in any pot? Where does the energy come from for these hypothetical particles to form, and how does its origin relate to the pot?
Sugihara: The important issue is not that the material itself is special. As we showed in WATER Journal in 2009 and 2011, Infoton-activated copper-plated plastic can be used to reduce emissions of automobile exhaust gases and Infoton-activated polyethylene film can be used to keep foods fresh. In our recent tests in a rice field, activated plastic pipes measuring 60 cm long by 6 cm in diameter with numerous 5-mm-diameter holes were charged with pieces of activated plastic; 50 of these pipes were then stood in the rice field (30 × 30 m). The water in the field was able to pass into and out of the pipes so that the energy from the pipes and the inserted plastic pieces was transferred to the water in the field, and from the water to the rice plants. Furthermore, we also performed tests in which 50 activated 6-cm-diameter stainless-steel balls contained in small ceramic pieces (50 g per ball) were hung on a rope set in the rice field.
With regard to the second part of the questions, we will repeat the answer that we gave to a previous reviewer’s comments. The Infoton itself behaves like an elementary particle in that do it does not decay and that it exists in a stable energy state in the water molecule.
In 2009, we showed that the lowest energy level was the breaking state of bonding in part of the water molecule af-ter hydrogen-bond breaking. Furthermore, by NMR studies, we found that the water molecules become smaller as a result of the presence of an Infoton. We also found that it affects the transmittance of water in THz region (0.6–20 THz); this could also be observed by FTIR spectroscopy. SQUID studies showed hysteresis changes of up to 5 Tesla in the magnetic moments of activated plastic pellets, to which Infotons may contribute. We also confirmed the existence of a change in the deuterium (2H) content in water through isotope analysis.
The energy of Infotons is considered to be derived from the spin of the electron and/or the Infotons <H+– e–> themselves and their momentum. These are related to the electromagnetic energy in the terahertz and far-infrared regions; this energy can transfer through and change other substances.
Reviewer: The author says that “…When a hydrogen bond is broken, the associated proton and electron, rather than forming hydronium ion or hydroxyl ions, remain inside the water molecule…”. So the autoprotolysis obviously stops at some intermediate state: the intramolecular H-OH breaks – and instead of forming H+ and OH–, the “infoton” is formed. The author is certainly aware of the fact that there is not sufficient energy in liquid water for this bond to actually break and that this is a quantum tunnel effect that is only possible in the bulk of the liquid state. My question is thus: How does the infoton relate to this tunneling effect? It seems to me that the proton would have to literally “stop half way”, which it can not – or can it?
Sugihara: The reviewer’s comment seems to me to be correct. Furthermore, “…this is a quantum tunnel effect that is only possible in the bulk of the liquid state — ” Accordingly, instead of H+ and/or OH– escaping outside the water, they exist as Infotons inside water in a plasma state, as discussed in the text. This means that the wave functions of all the Infotons inside a water molecule can overlap, resulting in a tunneling effect, although the energy can transfer in space, as we described above. If we consider energy transfer by Infotons in space in addition to that in the bulk of the liquid state, as the reviewer has suggested, we can propose a mechanism arising from the Aharanov–Bohm effect; this is a quantum mechanical phenomenon, that occurs because the Infoton is an electrically charged particle and is affected by electromagnetic fields, despite being confined in a region where both the magnetic field and the electric field are zero. The underlying mechanism is the coupling of the electromagnetic potential (such as the pommel shape shown in the text) with the complex phase of the wave function of a charged particle. However, we need further research to detect the Infoton itself, for example, the detection of an elementary particle, or the direct detection of something like a terahertz wave that is directly related to the Infoton, although we continue to discover Infotons and/or Infoton-like objects.
Reviewer: If the process he discovered accelerates the radioactive decay, then it must also increase the radioactivity during the treatment significantly – all the alpha, beta and gamma radiation that would normally be free over a couple of years should then be set free in a couple of days. So in other words, although the outcome of the treatment is something desirable (less radioactivity), the treatment process itself must be very, very dangerous – and cause secondary radioactivity of the shielding material of the device. Whereas this reviewer dearly hopes that the author has protected himself from this radiation – did he also measure it? Does he have any suggestions how to deal with this issue, especially when thinking about large scale applications?
Sugihara: As discussed above, “…photons of energy 563 to 1365 keV are emitted from 134Cs and photons of energy of 661.6 keV are emitted from 137Cs; the Infotons receive part of this energy, which increases their momentum and spin. Meanwhile, the calculated values of the kinetic energies of the Infoton (T = 1/2mv2, where m is the mass of the Infoton and v is its velocity in water) correspond to the energies emitted from Cs, provided that the Infoton has even 1% of the speed of light. Thus the interaction can be considered as illustrated in Figure 3, in which the Infoton and Cs present in water form a pommel-shaped potential and cesium loses energy by emitting photon(s); the Infoton regains energy from Cs, causing it to climb the potential slope, leading to the formation of the stable element; this process occurs within a very short time and in a very small space, as mentioned previously. As a result, the potential of Cs decreases along the larger Gaussian curvature, whereas the potential of the Infoton increases toward the Cs nucleus in a very short time, facilitating interaction with the nucleus.”
Namely, the energies of cesium provide to Infotons so that they can get into the cesium nucleus. This is very important characteristic to reduce a half-life in our theory.
