 Multiple Unfrozen Water Fractions in Biological Tissues: Freezing Point and Size
Multiple Unfrozen Water Fractions in Biological Tissues: Freezing Point and Size
Cameron IL1*, Haskin CL2 and Fullerton GD3
1Department of Cellular and Structural Biology, University of Texas Health Science at San Antonio, San Antonio, Texas 78229-3900
2School of Dental Medicine, University of Nevada Las Vegas, Las Vegas, Nevada 89106
3Department of Radiology, University of Colorado, 12700 E. 19th Ave. Aurora, Colorado 80045-2507
*Correspondence E-mail: cameron@uthscsa.edu
Key Words: Water, Freezing, Tissues, Collagen
Received July 18th, 2012; Accepted May 13th, 2013; Published May 30th, 2013; Available online June 5th, 2013
Abbreviations Guide: SHM: stoichiometric hydration model, g/g: grams water per g dry mass, s: seconds, OUR: osmotically unresponsive, NMR: nuclear magnetic resonance, ESR: electron spin resonance, DSC: differential scanning colorimetry, TMJ: temporalmandibular disk, SEM: standard error of mean, ˚C: degrees centigrade.
Abstract
This minireview deals with multiple freezing temperatures and sizes (in gwater/g dry mass) of individual unfrozen water fractions in biological specimens.Data were compiled from animal, plant and microcrystalline cellulose reports. Multiple freezing points (FP) were reported in all eight of the samples surveyed. The freezing points of water fractions occurred most frequently at temperature intervals of -6.5,-15.0, -30.4, -74.0 and -96 ˚C. Little or none of the total sample water content froze at the -6.5 ˚C interval. The largest fraction of tissue water froze at about -15˚C, the next largest size fraction, of about 0.72 g/g,remained unfrozen until -30.4˚C. Lesser sized fractions of waters remained un frozen at variable lower temperatures. However,below a FP of -74˚C a distinct unfrozen water fraction of 0.20 to 0.28 g/g was observed in all eight of the samples surveyed. Five mechanisms are offered to help explain the water fractions that remain unfrozen at the different subzero temperatures.
Article Outline
- Introduction
- Skeletal Muscle Water of Hydration Freezing Fractions
- Size of the Multiple Water of Hydration Fractions and Their Freezing Temperature in Baboon Temporolmandibular Joint (TMJ) Disk and in Cow Tendon Collagen
- Water Fraction Freezing Temperatures in Plant Tissues
- Proposed Mechanisms to Help Explain the Multiple Unfrozen Water Fractions at Subzero Temperature
- Conclusions
- Acknowledgements
- References
- Discussion with Reviewers
Introduction
This is a minireview on the freezing/melting properties of water fractions in biological materials. The materials reviewed include: skeletal muscle, temporolmandibular disk, tendon/collagen, nerve fiber, plant xylem ray, flower bud and seed tissues as well as granulated and ungranulated microcrystalline cellulose. The focus of the review is on freezing temperature, size (in g water per g dry mass) and number of multiple water freezing/melting fractions. Mechanisms to help explain the multiple subzero water freezing fractions are presented.
The results of the review are applicable to: 1) cold storage and preservation of food, cells, embryos, seeds and body tissues, 2) freezing tolerance in plants at subzero temperatures and 3) understanding mechanisms that explain the multiple unfrozen water fractions at different subzero temperatures.
Skeletal Muscle Water of Hydration Freezing Fractions
Burgaard (2010) has recently reported on the freezing of skeletal muscle water in fish using NMR, ESR and DSC measures. Her main measurement tool was differential scanning calorimetry (DSC) done on cod fish (Gadus morhua). The muscle water content varied between 78 and 83% and averaged 4 g water/g dry mass and the protein content varied from 15 to 19% and fat from 0.1 to 0.9%. Thus protein constitutes between 80 and 84% of the total dry mass. The first freezing water fraction in fresh and previously frozen cod fish muscle occurred as the temperature was lowered to between -14˚ and -16˚C. This occurred in both fresh and in preciously frozen muscle but the fraction of unfrozen water was significantly greater (p<0.05) in fresh vs. previously frozen muscle (23.9±1.6% vs. 19.5± 1.7% amounting to 0.96 g/g to 0.78 g/g respectively). There was no indication of ice crystallization at temperatures of -10˚ to -14˚C regardless of time of annealing from 2 to 240 min but evidence of ice crystallization did occur at -15˚C and at lower temperatures. Further lowering of the temperatures from -20˚C to -60˚C did not decrease the size of this frozen water fraction of the fresh or previously frozen cod muscle.
Fresh tuna fish muscle subjected to decreasing temperature appears to have two temperature exotherms one at between -11˚C and -21˚C and the second at about -74˚C (Orlien et al. 2003 a,b). Burgaard suggest that the exotherm at between -11˚ to -21˚C may cause some melting but the second exotherm at -74˚C may be caused by a glass transition event. One can question if this glass transition at -74˚C is a water glass transition or a solute glass transition? Burgaard reported that the ESR of a spin probe in fresh and previously frozen fish muscle revealed a decrease in mobility and increase in viscosity down to -21˚C then no further change was observed at lower temperatures. The DSC results showed clear evidence of ice crystallization by -21˚C. Such ice crystallization is expected to result in protein folding and aggregation accompanied by the observed significant decrease in the unfrozen water fraction from 23.9 to 19.5% or from 0.96 to 0.78 g/g in fresh and previously frozen fish respectively. The previously frozen fish may have already experienced irreversible protein folding and aggregation changes accompanied by a decreased unfrozen water fraction. It seems likely that this decrease may be due to a decrease in protein surface area and decrease in the protein unfreezable water associated with a decrease in protein surface area directly available for water molecule interaction.
Burgaard also surveyed literature and reported (2010) on the percent of total skeletal muscle water from several fish species that remained unfrozen at subzero temperature of -40˚C to -80˚C. The fish unfrozen water values range from: 9.5% at -40˚C (cod), 11% at -40˚C (haddock), 37% at -90˚C (king fish), and 24% from -40˚C to -80˚C (cod). Burgaard’s measures indicate that extent of unfrozen skeletal water remains almost constant from -16˚ to -80˚C in cod. The percent of unfrozen skeletal muscle water at temperatures of -40˚C to about -80˚C appears to depend on species and ranged from 9.5% to 37%. Based on an assumed average total muscle total water content value of 4.25 g/g DM the size of this unfrozen water fraction ranges from 0.40 to 1.57 g/g. Burgaard suggested that this wide range of values may be due to measurement methods and assumptions used in these different studies.
Peemoeller et al. used proton NMR T2 relaxation decay curve deconvolution an-alysis on the skeletal muscle of mice at nonfreezing and freezing temperatures. The relaxation times of the water of hydration fractions were: extremely slow 0.2-0.5s, slow 0.045-0.12s, intermediate 0.001-0.1s and too fast to measure. They report on three freezing fractions; one did not freeze at -4˚C but was frozen at -16˚C, the second was frozen at below -35˚C and a final fraction was not frozen at -103˚C. The size in g/g of the freezing fractions was not reported.
Size of the Multiple Water of Hydration Fractions and Their Freezing Temperature in Baboon Temporolmandibular Joint (TMJ) Disk and in Cow Tendon Collagen
Freezing data on the baboon TMJ disk (which contains 2-5% cells by volume, cartilage, type 1 collagen, proteoglycans and glycosaminoglycan GAG’s such as the highly charged sulfated GAS’s) are presented in Figures 1 and 2 and summarized in Table 1 (Haskin et al. 2006). No tissue water freezing occurred until -14˚C. The largest fraction of the total tissue water of 2.49 g/g or 81% froze at about -14˚C. The remaining unfrozen water fraction of 0.48 g/g of the total tissue water remained almost level from about -20˚C to -42˚C. Another small decrease in size, to a mean of 0.318 g/g, occurred between -45 to -72˚C. Another decrease in amount of unfrozen water then starts to occur at temperatures of about -76˚C and the size of this unfrozen water fraction decreased to a mean level of 0.227 g/g at between -80 to -96˚C. The amount of unfrozen water then underwent another drop to a non-equilibrium value of 0.192 g/g at a temperature of -98˚C (the lowest temperature measured). These TMJ data indicate the subzero freezing points of five non-bulk water fractions and the size in g/g of four of these fractions.
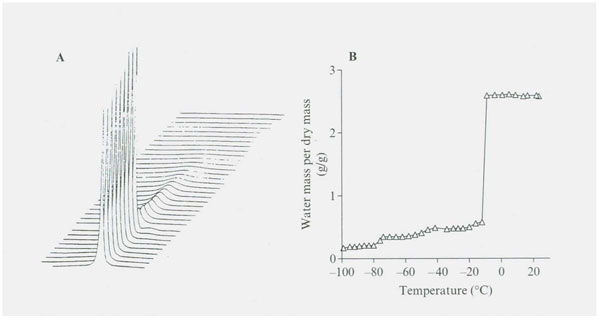
Figure 1: (A) (B) NMR proton spectra as a function of temperature for adult baboon’s TMJ joint disc. Proton spectra were recorded using a 300 MHz spectrometer and were measured over a temperature range of +20˚ to -98˚C. Above 0˚C, the peak consists of a single Lorientation component with a little shoulder on the upfield side of the spectrum. The proton signal shows a reduction upon freezing at -12˚C. The stepwise reduction in amount of water signal in each hydration fraction is summarized in Figure 2.
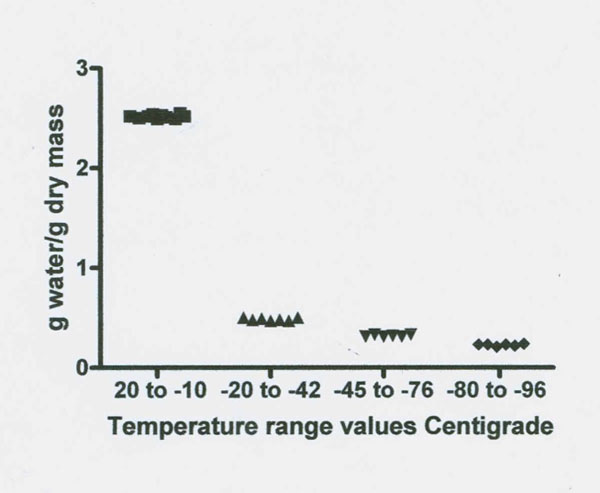
Figure 2: Illustrates the stepwise decrease in NMR proton spectra signal intensity as temperature decreases from 20˚ to -98˚C in the TMJ disk. Four significantly different unfrozen water fractions are revealed (see Table 1).
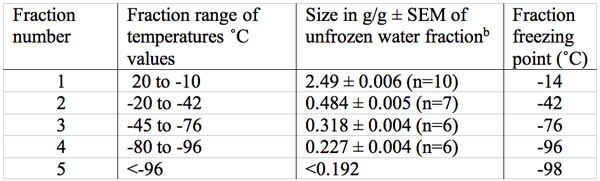
Table 1: Size in g water /g dry mass (g/g) and freezing temperatures of multiple unfrozen water fractions in adult baboon TMJ disk over the temperature range of 20˚C to – 98˚Ca.
aNone of the initial water content of 2.52 g/g froze at the temperature expected of a bulk water fraction.
bThe size of the first four fractions are all significantly different (p<0.001).
Figures 3 and 4 and Table 2 illustrates the results of stepwise loss of the total water proton NMR signal of beef Achilles tendon as the temperature was decreased. The method used was done using the same condition as described by Haskin et al. (2006). The figure indicates the tendon signal from two tendons at 28 temperature points for each tendon. Thirty minute intervals were allowed for equilibrium at each temperature.
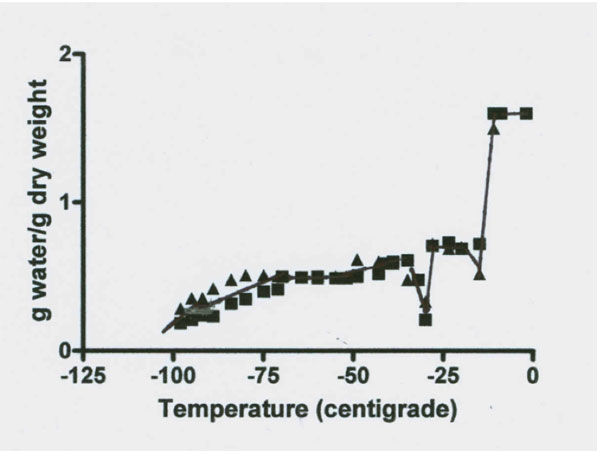
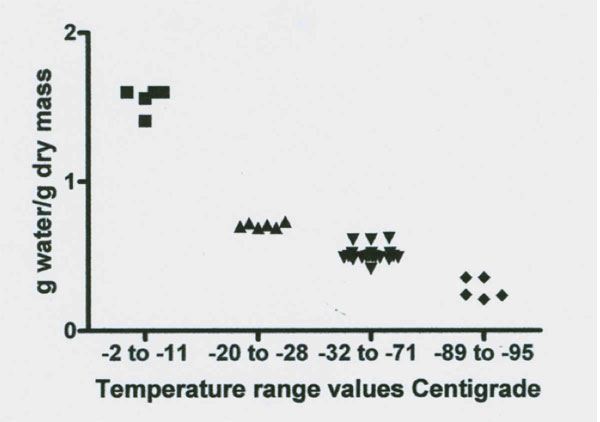
Figures 3 and 4: NMR proton spectra signal intensity measured over temperature range of 0˚ to -98˚C of fresh bovine Achilles tendon. The data from two cows indicate the presence of four signal intensity plateaus with two apparent rebound drops (one at -18˚ and one at -30˚C) between the first three plateaus. Data on each plateau’s temperature range signal intensity values (expressed in g water/ g dry mass) are illustrated in Figure 4 and the results of statistical analysis of these data for significant difference between the four plateau values are summarized in Table 2.
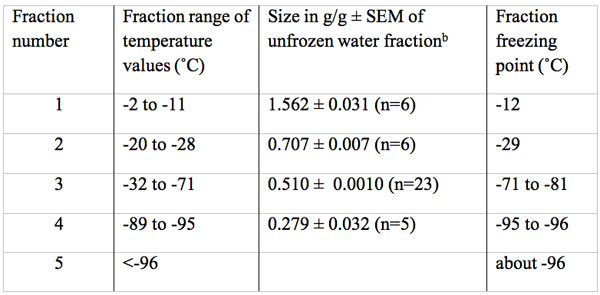
Table 2: Size in g water/g dry mass and freezing temperature of multiple unfrozen water fractions in fresh bovine Achilles tendona.
aThe total water content of fresh tendon was 1.6 g/g, none of which froze at the temperature expected of a bulk water fraction.
bThe size of the first four fractions are all significantly different (p<0.001).
Figures 3 and 4 reveal a major loss of signal at a temperature of -12˚C. This drop was followed by a rebound increase in signal size to a level of 0.707 g/g (Table 2). The total unfrozen water signal then remained level between -20˚ to -28˚C. At -29˚C the signal again dropped followed by a second rebound to a new level of 0.510 g/g between -32 to -71˚C. This new level of 0.51 g/g is 67% below the total water signal. The signal remained almost level from -32 to between -71 to -81˚C. At about -80˚C the signal began to steadily decrease to a mean level size of 0.279 g/g or a 82% below the total water signal and at -96˚C the signal appears to again decrease. Thus there are at least four freezing fractions based on these observations.
Water Fraction Freezing Temperatures in Plant Tissues
Data on plant and animal tissue water freezing temperature points are summarized and referenced in Table 3. The data indicates two tissue freezing points in plants. One freezing point is in the -4 to -7˚C range, which may be bulk water with enough solutes to lower the freezing point of bulk water and a second much lower freezing point fraction that appears to vary with the season of the year. The freezing of the second freezing point fraction is lowest in the winter and higher in the spring and summer (Ashworth 1990, Fujikawa et al. 1997, Hong et al. 1980). The seasonal difference is reportedly linked to the development of known freezing tolerance seasonal changes. Fujikawa et al. (1997) proposed that xylem parenchyma cells have the ability to tolerate and survive dehydration caused by growth in size of extracellular ice crystals and lose this ability in summer. Large species differences are known to exist in tolerance/survival upon exposure to subzero temperatures (Williams and Williams 1976). For example, Williams and Williams report that the flowering southern dogwood Cornus floride survives in winter at -25 ˚C but survives to only -10 ˚C in the late spring while its northern relative Cornus stolonifera fully tolerates winter freezing temperatures of -125 ˚C.
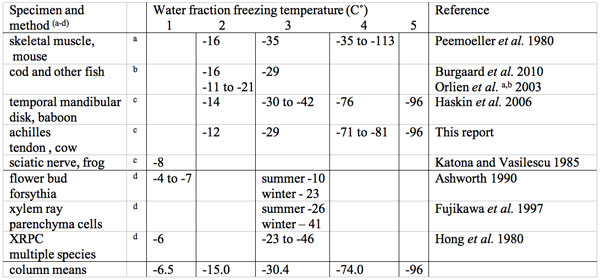
Table 3: Multiple water fraction subzero centigrade freezing point temperatures in animal and plant tissues.
aNMR proton T2 decay curve deconvolution.
bScanning colorimetry (DCS) and Electron spin probe (ESR).
cNMR proton spectroscopy.
dDifferential thermal analysis (DTA).
Unfortunately, plant tissues (because they consist of both cell and cell wall fractions) make it difficult to identify the location and size in g/g of frozen and unfrozen water fractions in the cell proper. There appears to be little available information of freezing or unfrozen water fractions in plants at temperatures in the sub -50 ˚C range putting a limit on determination of unfrozen water fractions below -50 ˚C.
A report by Sun (2000) deals with red oak (Quercus ruba) seeds that were equilibrated to various water content levels over saturated solutions. Dehydration, using a thermally stimulate current method, revealed three dielectric water dispersions: 1) loosely-bound and small polar groups, 2) tightly bound and large (macromolecular) polar groups and 3) glassy state. Changes in dielectric and thermodynamic water properties at different seed dehydration levels were recorded. The presence of seven discrete water content levels of 0.08, 0.13, 0.21, 0.31, 0.41, 0.55 and 1.40 g/g dry mass corresponding to -1.5, -8, -11, -14, -24, -74 and -195 MPa respectively were identified. Based on such findings it has been proposed that dehydration <0.10 g/g allows no metabolic activity due to high viscosity while hydration from 0.2 to 0.3 g/g allows restricted metabolism and hydration from 0.4 to 0.6 g/g allows conventional metabolism (Sun 2000, Pagnotta and Bruni 2006). This indicates that tolerance to freezing may be governed by the cells ability to tolerate dehydration to a level that vitrification occurs. In plant cells this degree of dehydration may be aided by the formation and growth of extracellular crystals before growth of intracellular ice crystal size becomes lethal (Kuroda et al. 2003).
Tissue Pore Size on the Freezing/Melting Temperature of Water
The main component of the cell wall of higher plants is cellulose. The freezing characteristics of purified microcrystalline cellulose were studied and reported by Luukkonen et al. (2001). Microcrystalline cellulose (MCC) is made from wood pulp by acid hydrolysis, then washed with water and spray dried to from a MCC powder. This process removes dissolved fiber parts like lignin and hemicellulose. Ungranulated MCC (ug MCC) is made by adding water to the dry powder in a morter. Granulated MCC (g MCC) was obtained from a manufacturer. The mean diameter of the granules/particles was 60 nm. As the particles swell water pores develop and increase in size.
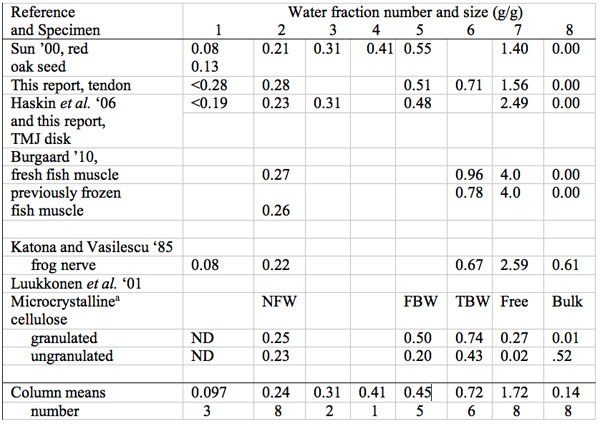
Table 4: Summary of size in g water per g dry mass (g/g) of multiple water freezing/melting fractions in biological materials: plant, animal and cellulose.
aNFW, non-freezing water at -35 ˚C, FBW, Free Bound Water, TBW, Total bound water, Free but non-bulk, Bulk water (see text), ND not detected
The aim of the study was to measure water fraction properties in granules over a wide moisture content range. Measures were specifically made to determine: the number, the size in g/g and the freezing properties of the multiple water fractions as well as the calculated pore size diameter distribution of the wet g MCC.
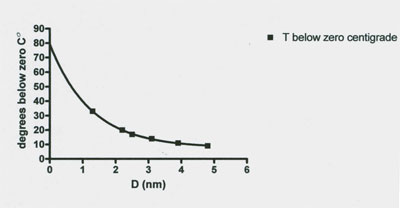
Figure 5: Graph of the relationship between the thermoporosimetry melting temperature of granulated microcrystalline cellulose and the calculated pore size distribution. The data indicate the smaller the pore size diameter (D) the lower the subzero melting point value. Statistical analysis of the data points demonstrates a highly significant exponential fit (r2 0.999). Data from Table 1 of Luukkonen et al. (2001).
The measurement methods used were thermoporosimetry (isothermal step melting) and solute (dextran polymer) exclusion. Two main finding from this study seem especially relevant to understanding the occurrence of multiple freezing and melting points of water fractions in biological tissues and cells. One of the two main findings was evidence of four distinct water fractions. The names and size of the fractions are summarized in Table 5.
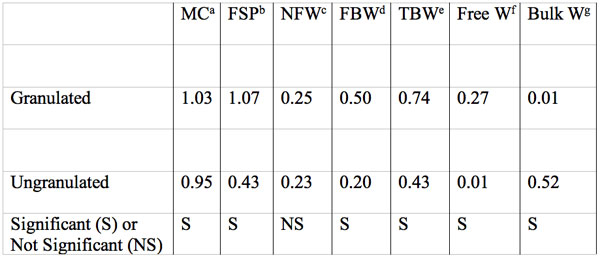
Table 5: Moisture content, fiber saturation point, and size in g water per g dry mass of water fractions of MCC samples as measure by isothermal step melting. Data summarized from table 1 of Luukkonen et al. 2001. Each value is the mean of three grades of MCC. Significant differences between granulated and ungranulated MCC samples are indicated.
aMoisture content by weighing.
bFiber saturation point.
cNonfreezing water.
dFreezing bound water.
eTotal bound water = NFW+FBW.
fFSP-TBW, when MC<FSP+MC-TBW
gMC-FSP.
The thermoporosimetry measures, for the first time, made possible a second finding which is that the pore size distribution of wet g MCC masses and the differentiation of frozen water depressed melting temperature from that of free and bulk water. Finally they report that as pore diameter decreases in g MCC the frozen water melting temperature becomes exponentially lower (Figure 5).
Here is a summary of the Luukkonen et al. (2001) reported results.
1. At -35 to -45˚C with a moisture content of 0.23 to 0.25 g/g there was a nonfreezing water fraction (NFW) in both g and ug MCC.
2. At 0.74 g/g moisture they reported a total freezing bound water fraction (TBW) in g MCC.
3. Subtraction of NFW from TBW in g MCC revealed a freezing bound water fraction of 0.50 g/g with a depressed melting temperature.
4. The mean total water content (MC) of g MCC and ug MCC at full hydration was 1.03 and 0.9 g/g respectively by gravimetric measure. The MC-TBW yields a water fraction that can be subdivided by use of a separate measurement method i.e. the fiber saturation point (FSP) by solute exclusion. Any difference between MC and FSP allows division into a free water fraction within g MCC particles and a bulk water fraction located between particles.
5. Granulated MCC when fully hydrated (MC) had a free water fraction of 0.27 g/g but had little if any bulk water. Thus the FSP and the MC values were nearly equal.
6. Ungranulated MCC had considerable bulk water (about 0.52 g/g but little or no free water (0.001 g/g).
7. A pore diameter decrease caused a significant exponential decrease in the thermoporosimetric melting temperature (Figure 5).
8. Thermoporosity and solute exclusion measures allowed distinction of four water fractions in MCC (non-freezing, freezing bound, free and bulk, Table 5). An NMR report by Froix and Nelson (1975) gave evidence that the non-freezing fraction has two subfractions. Thus giving a total of six water fractions.
9. Granulation of MCC increased volume of micropores and formed macropores which contain free water thus g MCC has more water inside the particles while ug MCC has none.
What is the relevance of the g MCC pore size findings to the freezing fractions in cells and tissues? Biological tissues and cells are known to have relatively small distances between macromolecular surfaces. Some examples are: porous cellulose (as described above), tightly packed collagen fibers in tendon and basement membranes, and in the cracks, channels, crevices, cavities and pores of proteins and other macromolecules in cells and tissues. The water molecules associated with macromolecular surfaces are known to differ in their physical properties (i.e. motion, freezing/melting temperature, osmotic activity, solvency, density, etc.) from those of bulk water. Thus the numerous microscopic and submicroscopic crevices, curved surfaces, channels and pores in tissues can help explain the reduced freezing point of water of hydration fractions associated with the large surface area to water volume ratios in tissues and cells.
Proposed Mechanisms to Help Explain the Multiple Unfrozen Water Fractions at Subzero Temperature
The following is a list of five mechanisms:
1. Addition of solutes to bulk water lowers the freezing point. This is ascribed to the binding of some of the water molecules to the solute which lowers the osmotic freezing point. The addition of small solute molecules, such as salts and sugar, is generally reported to lower the freezing point a few degrees, i.e. to between -1 and -8˚C. Some tissue freezing points in this range are listed in fraction 1 of Table 3. For small solutes the freezing point depression is due to the entropy of mixing and is proportional to the number of solute molecules.
2. The freezing point of a fraction of water is also depressed by the presence of macromolecules, membranes and other hydrophilic surface structures in tissues. This unfrozen water of hydration fraction does not freeze even in the presence of ice crystals (Wolfe et al. 2002). The prime factor for freezing point depression by large hydrophilic surface area structures can be attributed to the low energy level of water molecules near the hydrophilic surface. The unfrozen water associated with such macromolecular surfaces can remain unfrozen at tens of ˚C below the freezing temperatures of a bulk water solution even in the presence of ice crystals. Data on the TMJ Disk in Tables 1 and 3, indicate as much as 2.49 g/g does not freeze until -14˚C and may be due to presence of a large macromolecular surface area.
3. Highly concentrated water solutions, like those that occur in some seeds (Pagnotta and Bruni 2006, Sun 2000), can have high enough viscosity that it slows the growth of ice to mm per century resulting in a highly viscous (vitreous or glassy state) of the unfrozen water (Wolf et al. 2002). Burgaard (2010) using fish skeletal muscle suggest that the freezing exotherm that occurred at -74˚C resulted in an unfrozen water fraction of about 0.23 g/g which may be caused by a water glass transition. Data in Tables 3 and 4 indicates that freezing in the -74˚C range results in 0.2 to 0.3 g/g level of unfrozen water. Does this low g/g value have biological relevance? In response to this question Pagnotta and Bruni (2006) reported that a glass state of acorn seed axes exists at about 0.3 g/g and the such seeds remain dormant but are germinal by rehydration above this level. At this same level of hydration the brine shrimp embryo cyst remains non-metabolically active (Clegg 1991). In cold hardy hardwood plants it may be that dehydration of cells to a glassy state, due to growth of extracellular ice crystals and to a very slow growth of intracellular ice crystals (Kuroda et al. 2003), is a cold tolerance mechanism. The glassy state may act to arrest metabolism at very low temperatures or dehydration.
4. A fourth subzero ˚C unfrozen water fraction mechanism is the presence of submicroscopic cracks, pores and crevices in biological specimens. This mechanism is discussed in the tissue pore size section above. In brief the findings indicate that tissue porosity at the submicroscopic level, with pores of the 1.3 nm to about 5.4 nm exponentially increases the melting temperature of frozen water in granulated cellulose from -33˚C to -0.8˚C. This is a pore, crevice, crack and cavity size range expected to decrease the freezing temperature of water in the crowded cytoplasm of cells and porous tissues.
5. Another possible mechanism to explain the multiple water freezing fractions is that tendon/collagen, globular proteins and cells have also been demonstrated to have multiple water of hydration fractions as measured by multiple different methods. A model has been proposed to explain these multiple water fractions. The model was developed based on the know amino acid composition of collagen and was later applied to globular proteins (Fullerton and Cameron 2007, Cameron et al. 2011). The model is based on the electrostatic interaction of polar water molecules with electric fields between fixed oppositely charged pair groups on the protein backbone. The distance between the fixed oppositely charged pairs permits formation of either a single water molecule bridge or a double water bridge for every three amino acids. The sum total size of these two water bridge fractions is 0.26 g/g. Dielectric water clusters form over the remaining polar-hydrophilic protein surfaces (0.54 g/g) and a water cluster covers over the hydrophobic surface of the protein (0.8 g/g) to complete a monolayer of water coverage over the protein water accessible surface area. The sum size of the three hydrophilic surface fractions is 0.8 g/g and the size of hydrophobic surface fraction is 0.8 g/g.
This gives a calculated water monolayer of 1.6 g/g. Multiple different measurement methods confirm the size of these calculate water of hydration compartments for tendon/collagen (Cameron and Fullerton 2011). The molecular model is referred to as the stoichiometric hydration model (SHM). Notice that the size of three of the unfrozen water fractions in tendon/collagen of 0.28, 0.71 and 1.56 g/g (Table 4) closely match the size of the water of hydration fractions predicted by the SHM, but there is an additional unfrozen water fraction of 0.51 g/g not found by the other ten measurement methods.
What accounts for this extra 0.51 g/g fraction? Mechanism number 4, above, may help explain this additional unfrozen water fraction. Given the close regularly spaced distance between collagen molecules in hydrated tendon of 0.6-0.7 nm it seems likely that water molecules in this space might have an unfrozen water fraction that could account for this additional unfrozen water fraction of 0.51 g/g found in the temperature range between -32 to -71˚C (Tables 3 and 4). Except for this one additional unfrozen water fraction the size in g/g of the other unfrozen water fractions closely match the size predicted by the SHM.
Conclusion
The results of a general survey of unfrozen water fraction sizes and freezing temperatures in plant and animal tissues is presented. Multiple unfrozen freezing points were found in all eight of the samples surveyed. Mechanisms to explain the findings are offered. This report appears to be the first to review the subject of multiple subzero freezing fractions over a range of animal and plant tissues.
Acknowledgements
The assistance of Kerri Glaspie with manuscript preparation is gratefully acknowledged. The helpful review of the manuscript by Dr. Burgaard is acknowledged.
References
Ashworth EN. The formation and distribution of ice with Forsythia flower buds. Plant Physiol. 1990; 92:718-725.
Burgaard MC. Effect of frozen storage temperature on quality-related changes in fish muscle. PhD Thesis, Technical University of Denmark www.food.dtu.dk; 2010.
Cameron IL, Short NJ, Fullerton GD. Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type 1 collagen. Cell Biol Int 2007; 31:351-9.
Cameron IL, Lancot AC, Fullerton GD. The molecular stoichiometric hydration model (SHM) as applied to tendon collagen, globular proteins and cells. Cell Bio Int 2011; 35: 1205-1215.
Clegg JS, Drost-Hansen W. On the biochemistry and cell physiology of water (1991) in Biochemistry and molecular biology of fish. Eds, Hochachka and Mommsen. pp 1-23 Elsevier NY.
Froix M, Nelson. The interaction of water with cellulose from nuclear magnetic resonance relaxation times. 1975; Macromol. 8:726-730.
Fullerton GD, Cameron IL. Water compartments in cells. Methods in Enzymol 2007;428:1-28.
Fujikawa S, Kuroda K, Ohtani J. Seasonal changes in dehydration tolerance of xylem ray parenchymal cell of Stylax obassia twigs survive freezing temperatures by deep supercooling. 1997; Protoplasma 197: 34-44.
Haskin CL, Fullerton GD, Cameron IL. Freezing, flow and proton NMR properties of water compartments in the temporal mandibular disk. 2006. Water and the Cell. Springer press, Berlin. 341-52.
Hong SF, Sucoff E, Lu-Stadelman OKY. Effect of freezing deep supercooled water on viability of ray cells. 1980; Bot. Gzz. 141: 464-468.
Katona E, Vasilescu V. State of water in peripheral nerve. In New Trends in the study of water and ions in Biological systems. In V. Vasilescu and C.F. Hazlewood. Proc. Romanina American Workshop, Bucharest. 1985; 177-190.
Kuroda K, Kasuga J, Arakawa K, Fugikawa S. Xylem ray parenchyma cells in boreal hardwood species respond to subzero temperatures by deep supercooling that is accompanied by incomplete desiccation. Plant Physiol. 2003; 131:736-744.
Luukkonen P, Maloney T, Rantanen J, Paualapuro H, Ybruusi J. Microcrystalline cellulose-water interactions-a novel approach using thermoporosimetry. 2001; Pharm. Res. 18: 15662-1569.
Orlien V, Andersen ML, Jouhtrimaki S, Risbo J, Khibsted LH. Effect of temperature and glassy state on molecular mobility of solute in frozen tuna muscle as studied by electron spin resonance spectroscopy with spin probe detector. 2003; J. Agri and Food Chem. 63:63-67.
Orlien V, Risbo J, Andersen ML, Skibsted LH. The question of high-or-low-temperature glass transition in frozen fish. 2003; J. Agri Food Chem. 2003; 51:211-217.
Pagnotta SE, Bruni F. The glassy state of water: a stop and go device for biological processes. 2006; Water and the Cell 341-351 eds GH Pollack, IL Cameron and DN Wheatley, Springer, Berlin.
Peemoeller H, Pintar MM, Kydon DW. Nuclear magnetic resonance analysis of water in natural and deuterated mouse muscle above and below freezing. 1980; Biophys J. 29:427-435.
Sun WQ. Dielectric relaxation of water and water-plasticized biomolecules in relation to cellular water organization, cytoplasmic viscosity, and desiccation tolerance in relacitrant seed tissues. 2000; Plant Physiol. 124:1203-1214.
Williams JM, Williams RJ. Osmotic factors of dehardening in Cornus florida L. 1976; Plant Physiol. 58: 243-247.
Wolf J, Bryant C, Koster KL. What is unfreezable water, how unfreezable is it and how much is there? 2002; Cryo Letters. 23, 157-166.
Discussion with Reviewers
Anonymous Reviewer: Please discuss if there is any difference between the unfrozen mechanism in plants and animals.
I. L. Cameron, C. L. Haskin and G. D. Fullerton: Plants differ from animals by having both a cell membrane and an extracellular cell wall often with an extracellular compartment between these two structures. Water in this extracellular environment can start to form ice crystal at a higher subzero temperature than it does in the intracellular cytoplasm. The extracellular ice crystal can continue to grow by recruitment of cytoplasmic water resulting in cytoplasmic dehydration. This process lowers the cytoplasmic freezing point enough to avoid intracellular ice crystal formation and ice crystal growth that would otherwise cause lethal cell damage. Thus plant cells may tolerate a degree of dehydration aided by formation and growth of extracellular ice crystals before formation and growth of intracellular ice crystal size becomes lethal.
Reviewer: Are there any implications associated with the listed five mechanisms?
Cameron, Haskin and Fullerton: That subfactions of tissue water freeze at different subzero temperature and these water fractions sizes call for explanations. The five mechanism offered to explain the different subzero degree freezing points and sizes, offered in this report, is an attempt to explain the reported findings. The five mechanisms offered may not be all inclusive but they do give reasonable explanations of the findings reviewed in this report.
