 Sodium Potassium Exclusion by a Non-bulk Like Water Fraction in Hen Egg White
Sodium Potassium Exclusion by a Non-bulk Like Water Fraction in Hen Egg White
Cameron IL1* and Fullerton GD2
1Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, TX 78229-3900 USA
2Department of Radiology, University of Colorado, 12700 East 19th Ave., Aurora, CO 80045-2507 USA
*Correspondence E-mail: cameron@uthscsa.edu
Key Words: Water solvency, Ion distribution, Egg white, Solute exclusion
Received June 7th, 2012; Accepted April 23rd, 2013; Published May 20th, 2013; Available online May 30th, 2013
Abstract
Hen egg white thick gel and thin sol fractions are naturally adjacent membraneless but non-miscible fractions in the egg. Their sodium and potassium concentration was measured by flame photometry. Sodium and potassium were in higher concentration in the thick gel than in the thin sol. Centrifugal force of the thick gel forced its particle rich birefringent domains towards the bottom of the centrifuge tube and a particle poor sol fraction formed at the top of the tube. With time of centrifugation the concentration of sodium and potassium continued to increase in this top particle poor sol fraction. This finding is attributed to the partitioning of sodium and potassium into a more solvent water fraction. The greater extent of protein aggregation and decrease in solvent (water) accessible surface area in thick gel vs. thin sol is proposed to decrease the solute (ion) excluding non-bulk like water fraction towards the bottom of the centrifuge tube and increase the solute solvent water fraction towards the top of the centrifuge tube. This proposed mechanism explains the heterogeneous distribution of ions between thick and thin egg white and is probably applicable to other biological systems.
Article Outline
Introduction
This report presents the results of experiments designed to get at: 1) the role of ion exclusion from the non-bulk water of hydration fraction on proteins present in a protein rich biological specimen (hen egg white) and 2) the possible role of ion accumulation by the thick hen egg white protein fractions.
Background information is offered to explain the choice of hen egg white for this study. Hen egg white placed on a sieve allows separation into a thin sol and a thick gel fraction (Cameron 2010). Thick gel has large birefringent domains rich in microscopic particles (Cameron and Fullerton 2010). Thin sol has no such particle rich domains. The fragmentation of the large gel domains allowed the increased mobility of particles previously entrapped in the large relatively immobile domains to move more rapidly and lead to an overall shorter proton NMR T1 relaxation time due to the fragmentation (Cameron and Fullerton 2010).
The selective exclusion of the vital dye, methylene blue, by the relative immobile and viscous thick albumen gel and the loss of gel behavior and the loss of dye exclusion due to gel agitation indicates that dye exclusion is linked to the, macroscopic viscosity decrease and mobility increase (Cameron 2010). The molecular docking of proteins in the particle rich birefringent domains in the thick gel is proposed to lead to a decrease in proteins solvent (water) accessible surface area (SASA) compared to the more abundant monomeric proteins in the thin particle poor sol fraction.
The difference in waters physical properties between thick and thin hen egg white fractions (Cameron, 2010; Cameron and Fullerton, 2010) has led to the question of how these water properties might impact the distribution of Na+ and K+ between adjacent but non-miscible membraneless thin and thick egg white fractions. To answer this question measures were made on 1) the concentration of Na+ and K+ in the thick gel and in the thin sol egg white fractions and 2) the effects of centrifugal separation of the thick gel into protein particle enriched and into a protein particle poor sol fraction that could be used for ion concentration measures. The change in Na+ and K+ concentration in the protein poor sol particle fraction with time of centrifugation can provide information on the possible binding/accumulation potential of the protein particle enriched gel fraction.
Materials and Methods
Freshly laid hen eggs were collected and stored at 4.0˚C for 4-6 days prior to use. The procedure for collecting thick and thin egg white (albumen) was done using a gravy sieve filter separation as previously described (Cameron, 2010).
The sodium (Na+) and potassium (K+) concentration measures of the thick and thin were done at room temperature of 22˚C using a Jenway model PFP7 flame photometer purchased from Bibby Scientific Essex U.K.
Ion concentration was measured and reported in mM. The water content of thick was 7.311 g water/g dry mass and thin was 9.627 g/g (Cameron, 2010).
The centrifugal force procedure used on the thick gel albumen fraction was as previously described (Cameron, 2010). Briefly two cm long stoppered volume calibrated and marked centrifuge tubes were loaded with thick gel, placed in the centrifuge and subjected to a centrifugal force of 14,000 x g. At intervals, the centrifuge was stopped and tubes were removed for ion concentration measures. The samples for ion analysis were taken 2-3 mm below the albumen surface in the centrifuge tube. This centrifugation procedure has been previously shown to cause separation of a dye excluding thick gel albumen fraction at bottom of the centrifuged tube from a non-dye excluding sol fraction towards the top of the centrifuged tube (Cameron, 2010). An increase in centrifugation time resulted in an increase in non-dye excluding fraction at the top of the centrifuge tube (Cameron, 2010). The dye including fraction at the top of the centrifuged tube but not the dye excluding fraction at the bottom of the centrifuged tube could be poured off the top of the centrifuged tube. This finding demonstrates a gel to sol transition of a portion of the original totally thick gel by application of centrifugation.
Statistical analysis were carried out using Graph pad software programs.
Results
The results of the flame photometric measures of the sodium and potassium concentration, expressed in mM in thin and in thick hen egg white fractions that were not centrifuged are summarized in Figure 1.
The 21% mean higher sodium concentration in thick gel compared to thin sol is significantly different (p<0.01). The 34% mean higher potassium concentration in thick compared to thin is almost significantly different (p<0.07).Ion concentration results of centrifugation of the thick egg white gels using a g force of 14000 x g for periods of time from zero to 60 minutes, are plotted in Figure 2.
The concentration of both sodium and potassium sampled at 2-3 mm below the top surface of the egg white both demonstrated a significant (p<0.01) linear increase with time of centrifugation. The slope of increase of potassium in mM per minute of centrifugation (0.232 ± 0.072) is not significantly different from the slope of sodium (0.403 ± 0.104).
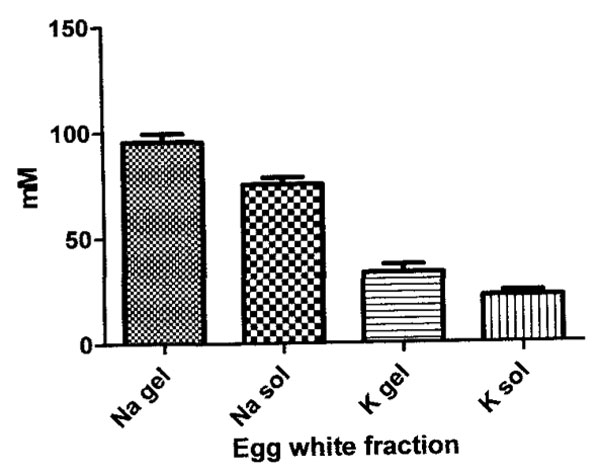
Figure 1: Graph of the concentration (in mM) of sodium (N) and of potassium (K) in thick gel and thin sol egg white fractions. Note the concentration of both a sodium and potassium is higher in thick gel vs. the thin sol. The results of statistical analyses of these data are summarized in the text.
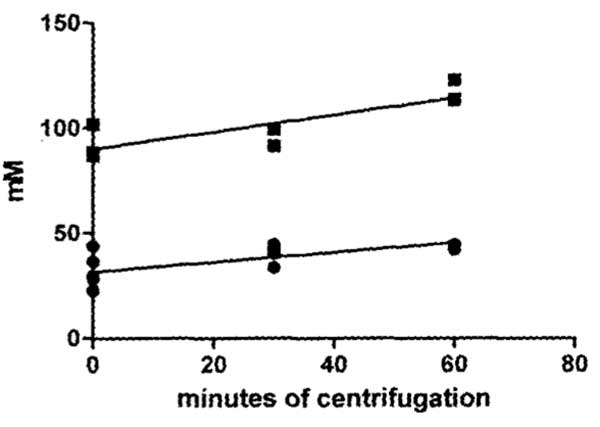
Figure 2: Graph plotting results of time of centrifugation at 14,000 x g of thick egg white on the concentration of sodium (Na+) and of potassium (K+) sampled 2-3 mm below the top surface of the 2 cm column of thick egg white in the centrifuge tubes. The positive slope of both sodium and potassium from this top surface sample site demonstrates a significant linear increase in concentration with time of centrifugation.
Results and Discussion
Mechanisms to explain the non-homogeneous distribution of sodium and potassium between thin and thick hen egg white albumen fractions.
The higher concentration of sodium and potassium in the thick vs. thin egg white fractions (Figure 1) has two possible explanations: 1) the exclusion of sodium and potassium from a greater proportion of sodium and potassium excluding water of hydration in the thin egg white proteins than in the thick egg white proteins and 2) the accumulation of sodium and potassium by binding to or entrapment within the protein birefringent particle rich domains in the thick egg white fraction. The accumulation possibility is discussed first.
If sodium or potassium is bound to or entrapped in the thick gel proteins then they should be concentrated of the centrifuge tube towards the bottom by centrifugal force. Centrifugation should therefore result in a decrease concentration of these elements at the top of the centrifuge tube. This is opposite to what occurred (Figure 2). What occurred was a significant linear increase in concentration of both Na+ and K+ at the top of the centrifuge tube with increase in duration of centrifugation. That both Na+ and K+ increased at the top of the centrifuge tube shows that neither Na+ nor K+ was concentrated by the centrifugal force that is known to concentrate particles (protein aggregates or crystals) towards the bottom of the centrifuge tube. These centrifugal force results indicate that binding or entrapment of neither sodium nor potassium in the thick egg white protein precipitate accounts for the observed higher concentration of Na+ and K+ in thick egg white gel (Figure 1).
A decrease in proportion of Na+ and K+ excluding water in the thick gel egg white, due to protein aggregation with loss of solvent water accessible surface area is therefore offered to explain the naturally higher content of Na+ and K+ in the thick gel (Figure 1).
Conclusion
Protein aggregation and/or tighter folding in the thick gel is proposed to decrease the solvent (water) accessible surface area and thereby proportionally increase the proportion of water available to act as solvent for solutes like sodium and potassium as compared to the thin sol albumen fraction. If follows that protein aggregation and folding are likely, but under recognized, mechanisms involved in ion in other biological systems.
References
Cameron I.L. Dye exclusion and other physical properties of hen egg white. Water 2010; 2: 83-96.
Cameron I.L., Fullerton G.D. Evaluation of hen egg white as a model of cytoplasmic pumping mechanisms. Water 2010; 2: 97-107.
