Pre-treatment of Culture Media with an Extremely Low-Frequency Magnetic Field Alters Daily Response of Dinoflagellate Cultures to Space Weather Variables Similar to Direct Magnetic Exposure
Paulo Vale
The Portuguese Sea and Atmosphere Institute, I.P. (IPMA, IP), Sea and Marine Resources Department (DMRM) R. Alfredo Magalhães Ramalho, 6. 1495-165 Algés. PORTUGAL
Email: pvale@ipma.pt
ORCID ID: 0000-0002-2524-4453
Keywords: Gymnodinium catenatum; Alexandrium pacificum; extremely low frequency magnetic field; water memory; mycosporine-like amino acids; saxitoxins; hormesis
Submitted: April 6, 2022
Reviewed: October 10, 2022
Accepted: November 1, 2022
Published: January 27, 2023
Abstract
The dinoflagellates, Gymnodinium catenatum and Alexandrium pacificum, have been previously studied regarding daily variations in solar-terrestrial interaction, known as space weather (SPW). To perform in vitro simulation of geomagnetic pulsations, an external extremely low-frequency magnetic field was employed previously for the study of their growth and metabolite production. Here, the same field was “informationally” transferred through a prior 24 h exposure of a culture media aliquot, the so-called “water memory” effect. To understand the effect of the magnetically treated culture media (MTCM), results had to be interpreted in light of the hormesis dose-response curve regarding natural fluctuations in SPW. The MTCM reduced growth at the hormesis optimum for selected SPW parameters. In these cells with reduced growth, the ultraviolet -photoprotective pigments mycosporine-like amino acids (MAAs) and the paralytic shellfish poisoning toxins (PSTs) increased in concentration. G. catenatum was more affected by MTCM than A. pacificum, with a generalized reduction trend in growth and MAA concentration. These pigments, besides having a UV-protection role, can also function as osmolites and antioxidants in microalgae. The use of MTCM elicited the same effect as direct exposure to an external extremely low-frequency magnetic field.
1. Introduction
Gymnodinium catenatum, Pyrodinium bahamense, and several marine microalgae of the genus Alexandrium are producers of paralytic shellfish poisoning toxins (PSTs), also known as saxitoxin analogues. These potent neurotoxins cause an acute human neurological syndrome through ingestion of transfer vectors (mainly coastal and estuarine bivalve molluscs, such as mussels, clams, cockles, oysters, etc.) that feed on these microalgae (FAO, 2004). On the Atlantic Iberian coast, bivalve contamination episodes attributed to G. catenatum can be recurrent on consecutive years or can be absent for several consecutive years (Vale et al., 2008; Vale, 2019). The worst PSTs’ episodes on the Portuguese coast between 1986-2021 coincided with the last four periods of minimal activity of the 11-year solar cycle (Vale, 2013, 2019; IPMA, 2022a). In this period, the longest intra-annual absence – a decade of absence – was between 1995 and 2005 (Moita et al., 2006).
This decadal-like periodicity has prompted research on the influence of solar activity (SA) and geomagnetic activity (GMA) on cultures of this toxic microalgae. Another microalga occurring at the Iberian coast was chosen for physiological comparison: Alexandrium pacificum. It has in common a chain-forming habit and production of the same PSTs: C1+2 (N-sulfocarbamoyl Gonyautoxins 2 + 3) and B1/GTX5 (Gonyautoxin 5). These studies revealed differential responses to shock, such as hypo-osmotic shock or addition of oxidizing agents (hydrogen peroxide), depending on the occurring SA and/or GMA at the time of the experiments (Vale, 2017a, 2017b, 2018a, 2018b). The response to space weather (SPW) followed the hormetic dose-response model on numerous occasions (reviewed in Vale, 2020b). For GMA, the interval around 7-20 nT, representing optimal growth, was common on multiple instances.
Geomagnetic storms (GMSs) are a superposition of slow changes in the geomagnetic field (GMF) and geomagnetic pulsations. These low-frequency fluctuations of the GMF range from 0.001 to 5.0 Hz (Jacobs et al., 1964). Oscillations with quasi-sinusoidal waveforms were called pulsations continuous (Pc), while more irregular waveforms were called pulsations irregular (Pi). Pc waves were subdivided into five ranges, starting with Pc1 in the 0.2-5.0 Hz range (Jacobs et al., 1964). Some studies reported biological effects in experiments where weak extremely low-frequency magnetic fields with a constant frequency and strength close to natural geomagnetic disturbances were used (Zhadin, 2001; McKay et al., 2003; Dupont et al., 2004, 2005; Lednev et al., 2008; Hu et al., 2010; Belova et al., 2010; Krylov et al., 2014).
For these two dinoflagellates, a weak extremely low-frequency magnetic field (ELFMF) with a 0.5 Hz frequency and central amplitude of 7 µT was previously employed to perform in vitro simulation of geomagnetic pulsations. Short-term exposure (hours) to this ELFMF increased relative cell growth around 10 nT of naturally occurring GMA. Relative growth outside these intervals gradually approached 0% or was negative for G. catenatum. Differential survival to a subsequent shock was inversely related to growth, and minimal survival coincided with the same 10 nT interval (Vale, 2020a). Relative growth and survival displayed opposite hormetic curves toward GMA: inverted U-shaped for growth, and J-shaped for survival. Long-term exposure (days) increased relative growth in A. pacificum but reduced in G. catenatum when low GMA was taking place (Vale, 2020a).
These alterations in growth were both associated with a reduction in the cellular pool of nitrogen-containing ultraviolet-photoprotectors, the mycosporine-like amino acids (MAAs). Some of these molecules can have an antioxidant role as well (Dunlap and Yamamoto, 1995). MAAs that are more prone to oxidation (presenting double bonds) were more reduced than those less susceptible, highlighting that an ELFMF can act by increasing cellular oxidative stress status (Vale, 2018a, 2018b, 2020a). This was an expectable result, according to the best explanation for the interaction of weak magnetic fields that is postulated to occur through the radical-pair mechanism, increasing oxidative stress (Barnes and Greenbaun, 2015; Binhi and Rubin, 2022). Other small nitrogen-containing metabolites, the PSTs, increased +16% in G. catenatum and +12% in A. pacificum after exposure to the ELFMF described above. A similar increase was achieved by exposure to the common oxidant H2O2: +50% in G. catenatum and +10% in A. pacificum (Vale, 2022).
Electromagnetic fields elicit a wide diversity of effects in biological systems, such as changes in Ca2+ regulation, channel activity, enzyme activity, DNA and RNA synthesis or growth behavior (Novikov et al., 2010). Also, a wide range of frequencies as well as a wide range of power densities have been observed to evoke biological effects. It is unlikely that biological systems can possess receptors for such a wide range of frequencies (Fesenko and Gluvstein, 1995). It has been suspected that the primary receptor for such effects may be water. Irradiating water with a given electromagnetic field can elicit similar responses as exposing living systems to the same electromagnetic field (Fesenko and Gluvstein, 1995; Fesenko et al., 1995).
Water presents unique hydration properties toward biological molecules, determining their three-dimensional structures, and its own structuring can inform other processes over significant distances (Chaplin, 2001). In addition, molecular information can be stored in water after multiple steps of ultrahigh dilutions and shaking (Davenas et al., 1987; Endler et al., 1994; Rey, 2003). Molecular information can also be transferred into water via electric fields (Thomas et al., 2000; Jerman, 2005). In the second case, water can be informed even without any direct molecular contact with the donor substance, which is not the case with ultrahigh dilutions. The properties of infrared and ultraviolet absorptions, Raman scattering and X-ray diffraction were greatly changed in water under the action of a magnetic field relative to those of pure water (XiaoFeng and Bo, 2008).
This research is aimed at understanding if an extremely low-frequency magnetic field imprinted into water could elicit bioeffects similar to the direct exposure of microalgal cells to that same type of artificial magnetic field. Besides growth, the concentration of small nitrogen-containing molecules typical of microalgae, the MAAs, and of toxin-producing dinoflagellates in particular, the PSTs, was accessed. These metabolites were chosen because these are routinely screened in a marine biotoxin laboratory (PSTs), where the author works or can be easily screened with the equipment available (MAAs).
2. Materials and Methods
2.1 Strains and Culturing Techniques
he G. catenatum strain nº IPMA_GYMN1_L5_19 was collected on 26/07/2019 at Costa da Caparica coast, SW of Portugal (approx. 38.6º N, -9.2º W). The A. pacificum strain nº EXT-1653 was collected at Tarragona coast, on the Catalonian coast of Spain (approx. 41.1º N, 1.3º E). It was from the Laboratorio de Control de Calidad de los Recursos Pesqueros culture collection (LCCRRPP, Andalucía, Spain).
The culture medium was prepared by filtering seawater collected from Cascais Bay on a 20 µm sieve. Salinity was measured with a visual hand-held refractometer (Index Instruments, Ramsey, UK) and adjusted to 35 ± 1 psu with demineralized water as required, enriched with f/2 nutrients, with extra 10-7 M selenium and no silica (UTEX, 2022). The medium was not autoclaved but sterilized in an oven at 80ºC for 1 hour.
DuranR GL45 borosilicate bottles with the respective caps were used for maintaining stock cultures. The inoculates for the experiments were maintained in semi-continuous culturing conditions in 1000 mL bottles (> 950 mL of culture). The culture was replenished with fresh medium at the end of each week with an approximately 1:1 dilution, maintaining the culture in a permanent mid-exponential phase. During experiments, the 250 mL GL45 bottles were covered with a polystyrene petri-dish half allowing light to pass through vertically while minimizing evaporation and avoiding airborne contamination.
The lighting facility was placed in a dark room without air-conditioning, located at sea-level altitude in Algés (Lisbon periphery; 38.70º N, 9.23º W) and synchronized in a 12:12 hour light:dark cycle (7 am until 7 pm, wintertime). Water temperature was measured once in the morning. During wintertime, artificial heating was supplied by an electrical heater placed at least 1 m apart from the cultures.
Two identical LED illumination sets, one for controls and another for magnetic testing (CON and MAG locations, respectively), were each equipped with one overhead warm-white LED spot (OSRAM, Germany; model LED STAR PAR16 36º, 8 W equivalent to 520 lumens, color 2700 K, GU10 adaptor), mounted on a wood frame attached on top of wooden tables to avoid ferromagnetic materials creating static magnetic fields close to the cultures (Fig. S1). Distances of spots to the shelves were 57 cm. The distance between the CON and the MAG spotlight centers was 80 cm.
Photosynthetic active radiation (PAR) was measured at the shelf level with a Li-Cor light meter (Li-Cor, Nebraska, USA). UV-A radiation was measured with a UVA-BTA probe, interfaced with Go!®Link and data acquisition was performed by Logger Lite software (Vernier Software & Technology, Beaverton, USA). With white cardboard reflectors placed on the edges (30 x 30 x 30, d x w x h), PAR was 44 µmol m-2 s-1 at the center of the spot focus and vertical UV-A radiation (20 cm above shelf level, or maximal exposition for surface swimming cells in a 1 L bottle), was <0.5 mW/m2.
2.2. Natural exposure to space weather
Growing conditions were not specifically shielded from electric or magnetic fields and were naturally exposed to oscillations in SPW (Fig. S2). The aa Geomagnetic Index values were obtained from the British Geological Survey (BGS, 2022). The aa Index is expressed in nanoTesla (nT) and is available with a 3-hour resolution (a total of eight daily periods), starting the first period daily at midnight Universal Time (UT) (Fig. S2a). For calculating recent GMA, the activity from the 8th period of day-1 was averaged with the 1stt, 2nd and 3rd periods from day-0. Solar activity parameters were retrieved from NOAA’s SWPC Anonymous FTP Server (NOAA, 2022a). The solar radio flux and the number of solar flares (categories C+M+X) from day-1 were used for calculating recent SA.
The static geomagnetic field in Lisbon was +43,912 nT by 2020-September-01 slowly drifting to 43,935 nT by 2021-June-30 (NOAA, 2022b). Indoor and outdoor artificial electromagnetic fields were kept to a minimum as much as possible. Extremely low-frequency magnetic fields (from power lines, transformers, adapters, etc) in the 10–2000 Hz range were below 0.01 µT (EMFields Professional, EMFields, UK). Average microwave fields from telecommunications in the 200–8000 MHz range were below 1 µW/m2 (Acoustimeter, RF Meter model AM-10, EMF fields, UK). To keep 50 Hz electric fields to a minimum, electric cables were kept at a minimal distance from bottles of 50 cm.
2.3. Indirect exposure to a low-frequency magnetic field
A miniaturized extremely-low frequency magnetic field was produced by a common alarm clock drive (Timex, West Germany), powered by a single AA-type battery (Fig. S3a). The field reversed polarity every second, generating a frequency of 0.5 Hertz (Fig. S3b). The intensity of the field between 1 and 5 cm from the clock solenoid was detailed in Figure S3c. This equipment was chosen due to its ability to produce fields near the sub-Hertz range, which are typical of geomagnetic storms (Jacobs et al., 1964). Also, its long autonomous functioning (low consumption, off-grid battery operated) avoided proximity to electrical wires that could introduce higher frequencies, such as the electric grid at 50 Hz and its higher harmonics. In the absence of an expensive specific wave generator, this is a piece of low-cost and easily accessible equipment.
Circa 3-4 hours after the onset of the light phase, 10 mL of culture media aliquot was dispensed into two 30-mL flat-bottomed clear polystyrene tubes. These tubes were placed 80 cm apart on an acrylic shelf (Fig. S1). One of the tubes was placed in close contact with the clock solenoid (MAG, magnetically exposed tube) for 24 hours. The amplitude at the center of the flask and 1 cm height was 30 µT (detailed in Fig. S3b). To keep 50 Hz electric fields to a minimum, power line cables were kept at a minimal distance from these tubes of 15 cm. The tubes were used immediately after the 24-hour exposure.
Circa 220 mL of microalgae culture, aged 5-7 days after the last media replenishment, were removed from stock 3-4 hours after onset of the light phase and thoroughly homogenized. Triplicate 4 mL sub-aliquots were taken for cell counting and 100 mL aliquots were transferred into two 250 mL GL45 bottles. One microalgae bottle was placed in the control location (CON) and the other at the magnetic test location (MAG) (Fig. S1). The CON bottle received the non-exposed culture media aliquot. The MAG bottle received the magnetically exposed aliquot. Both bottles were gently homogenized by rotating ten times, kept for 24 hours, homogenized and sampled: triplicate 4 mL sub-aliquots (dispensed in 10 mL PP tubes) for cell counting and triplicate 30 mL sub-aliquots (30 mL PP tubes) for cell harvesting.
For cell counts, sub-samples were immediately preserved with Lugol’s iodine solution (10 µl/mL). 50 µl-aliquots were counted in 96-well microplates (1 aliquot for A. pacificum and 2 aliquots for G. catenatum) using a stereomicroscope (model MZ7s, Leica Microsystems, Wetzlar, Germany). Homogenization was performed by gentle hand flipping to preserve information of chain composition. The coefficient of variation between triplicate tubes was kept below 10% for A. pacificum and 15% for G. catenatum.
For cell harvesting, after centrifugation at 11 min for 1500 x g (Rotofix 32 A, Hettich, Germany), culture media was discarded and the cell pellet was suspended with 2.0 mL 0.05% acetic acid and kept frozen until metabolite extraction. MAAs and PSTs were extracted by a double freeze-thaw cycle and a final 15 min in a 110 Watt ultrasonic bath (Selecta, Spain). Supernatants were obtained by centrifugation at 2500 x g for 12 min, transferred into HPLC vials and frozen. Metabolite analyses were performed within less than one month after cell harvesting.
This experiment was repeated thirty times for both A. pacificum (run between October 2020 and March 2021) and G. catenatum (run through March and July 2021) (Fig. S2a). Experiments took place during solar minimum between Solar Cycles 24 (ending December 2019) and 25 (NOAA, 2022a).
2.4. Analysis of MAAs
High-performance liquid chromatography (HPLC) analyses for MAAs were detailed in Vale (2016) and were carried out using an Agilent (Palo Alto, USA) system comprising 1260 series modules: a quaternary pump with integrated degasser; a refrigerated autosampler (kept at 8ºC); a thermoregulated column compartment; a diode array detector (DAD) and a control software OpenLAB CDS (Rev. C.01).
MAAs were separated on a 125 mm x 3 mm column packed with 5 µm Nucleosil® 100-5 C18 (Macherey-Nagel, Duren, Germany), and protected with a C18 guard column (SecurityGuard™, 4 x 3 mm, Luna, Phenomenex, USA). The mobile phases were as follows: A: 0.2% aqueous formic acid; B: acetonitrile. The column was equilibrated with 100% eluent A. The following elution conditions were applied: 0% eluent B from 0 to 3 min, 0% to 10% eluent B from 3 to 6 min; hold at 10% B from 7 to 10 min, return to 0% B at 10.1 min and hold until 15 min before the next injection. The flow rate was set at 0.5 mL/min, and the run time at 16 min. The column temperature was kept at 30ºC and 10 µl of the extracts were injected into the column.
Peaks were detected with the diode array detector set at 260 (for ruling out interferences), 310 (mycosporine-glycine [MYGL]), 319 (palythine, M-320), 330 (shinorine [SHIN], porphyra-334 [PORP], M-333, M-331, M-335/360) and 360 (palythene [PALE]; M-370) nanometres, each with a 5 nm bandwidth. Individual peaks were confirmed by online absorption spectra, retention time and relative retention times comparison with qualitative standards. Qualitative standards were prepared from extracts of Porphyra yezoensis (farmed in Kyushu, Japan, distributed by Clearspring, UK), providing shinorine, porphyra-334 and the isomeric pair usujirene/palythene.
The cellular concentrations of MAAs were obtained using the molar extinction coefficients reported by previous studies (Gröniger et al., 2000). The instrument’s optical path was calculated with a Certified Reference Material of domoic acid (CRM-DA-g, National Research Council of Canada). Extinction coefficients for unknown MAAs were derived from molecules with closest maxima: M-333, M-331 and M-335/360, from shinorine (44 700 M-1cm-1), M-320 from palythine (36 200 M-1cm-1), M-370 from palythene (50 000 M-1cm-1).
2.5. Analysis of PSTs
Oxidation products from PSTs were obtained by reaction with alkaline hydrogen peroxide at room temperature (detailed in Vale et al., 2021). Their HPLC analysis was carried out using an Agilent (Palo Alto, USA) system comprising: a quaternary pump with a built-in degasser and a refrigerated autosampler kept at 8ºC (1290 Infinity series); a column oven and a fluorescence detector (1260 Infinity series) and a control software OpenLAB CDS (Rev. C.01).
The separation was performed on a Supelcosil™ LC-18, 150 x 4.6 mm, 5 µm column (Supelco, USA) equipped with the same guard column as above. Mobile phase A was made of 0.1 M ammonium formate, pH= 6 and mobile phase B was made of 0.1 M ammonium formate in 5 % acetonitrile, pH = 6. The flow rate was 1 mL/min at 100% A, with the following gradient during the run: 5% B at 0.25 min, 10% B at 4.0 min, 90% B at 9.0 min, 10% B at 11 min, 0% B at 13.0 min. The run time was 13 min, with 1.5 min post-run. The column was maintained at 30ºC, and 40 µL were injected.
Detection wavelengths were set at 340 nm for excitation, 395 nm for quantitation and 430 nm for confirmation. Toxins were identified by comparison of retention times with standards and wavelength ratios. For toxin quantification, Certified Reference Materials from NRC of Canada were used: CRM-C1&2 and CRM-GTX5 (B1). The method had been previously in-house validated, presenting a LOD of 0.02 and 0.004 µM for C1+2 and B1, respectively (Vale et al., 2021).
2.6. Data Management and Analysis
The relative daily growth was calculated by the cell concentration formula: (CMTCM − CCON )/CCON x 100, where C is the concentration in cells/mL and MTCM is the magnetically treated culture media exposure. Daily metabolite variance was calculated by the same formula, where C is the respective metabolite concentration in pg/cell (MAAs) or fmol/cell (PSTs).
Statistical analyses were carried out in KyPlot ver. 6.0 (Kyenslab Inc.). For multivariate analyses, the non-parametric multiple comparison test – Steel-Dwass Pair-wise comparison test – was used. Boxplots were drawn in KyPlot, and represent the median and interquartile range in boxes, min. and max. ranges in whiskers. The number of symbols on top of each box denotes increasing significant differences at 0.05, 0.01 and 0.001 levels, respectively. When no symbols were presented, no significant differences were found.
3. Results
Experiments took place during a solar minimum period (NOAA, 2022a). For A. pacificum experiments, recent geomagnetic activity varied between 2.0-45.5 nT, solar radio flux between 70-104 F10.7 units and solar flares between 0-5 (comprised only of C-class flares, no M-class or X-class flares). For G. catenatum experiments, GMA varied between 2.0-42.0 nT, solar radio flux between 70-93 F10.7 units and solar flares between 0-3 (comprised only of C-class flares, no M-class or X-class flares). Water temperature was, respectively, 20±1ºC and 21±1ºC during A. pacificum and G. catenatum experiments. These parameters were reported in detail in Figures S4 and S5.
Daily median growth observed for A. pacificum was 115.6% and 114.5%, in the control and exposed to magnetically treated culture media (MTCM), respectively (Fig. 1a). In only 2/30 and 3/30 experiments population numbers were below 100% (i.e., no growth took place; Fig. S4). Daily median growth observed for G. catenatum was 108.1% and 105.4%, in the control and MTCM exposed, respectively (Fig. 1b). In 7/30 and 8/30 experiments population numbers were below 100% (i.e., no growth took place; Fig. S5).
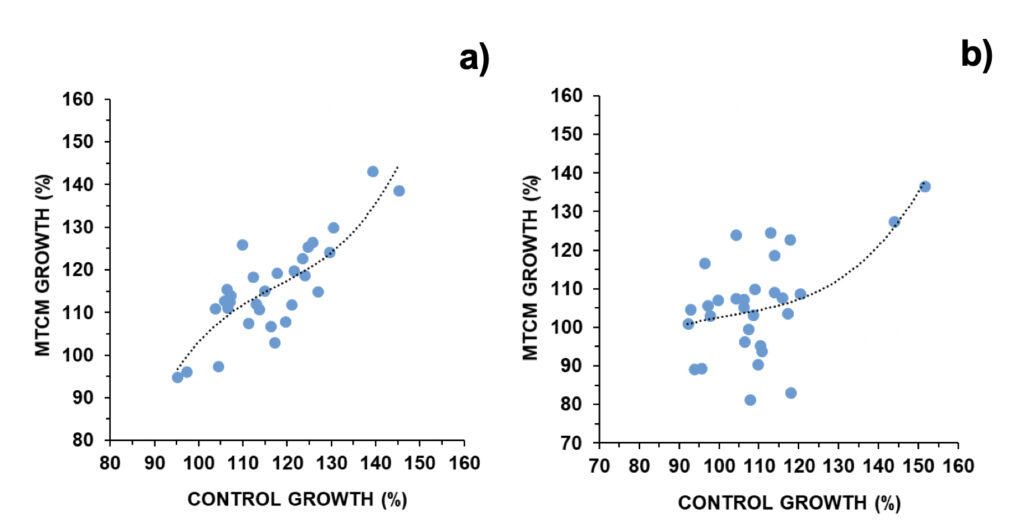
Figure 1. Daily growth in control cultures versus exposed to magnetically treated culture media (MTCM) in a) A. pacificum and b) G. catenatum (N=30). Regression parameters: a) R=0.827; p < 0.001; b) R=0.559; p < 0.05.
The relative difference in cell concentration observed between the control and the MTCM-exposed cells was next compared with daily occurring GMA and solar activity parameters. For A. pacificum, cell concentrations were inferior to control when solar radio flux was between 76-83 F10.7 units or GMA was comprised between 9-13 nT: 94.5% and 96%, respectively (Fig. 2). In contrast, cell concentrations were above control when these parameters fell outside these windows, ranging between 101% and 106% (Fig. 2).
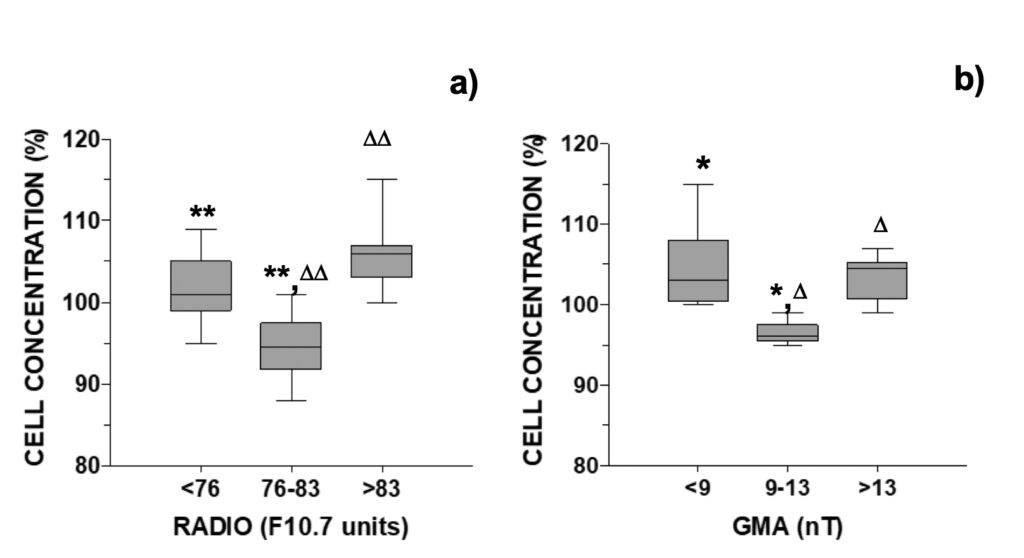
Figure 2. Relative growth of A. pacificum versus control observed 24-hour after addition of
culture media pre-treated with a magnetic field distributed accordingly with the daily a) RADIO flux (N=30) or b) GMA activity (N=18, data selected for days when RADIO <76 or >83 F10.7 units).
These observations were the reverse of those observed for normal growth. Median growth in the control was higher in the intermediate intervals of 76-83 F10.7 units and 9-13 nT: 121% and 124%, respectively (Fig. S6). Outside these central intervals, median growth was only 110% in all cases (Fig. S6). Both growth curves presented an inverted-U shaped curve typical of hormesis dose-response.
For analysis of UV-photoprotective pigments in A. pacificum, MAAs were grouped into those with an additional relevant role as osmolytes (shinorine, porphyra-334, M-333, M-331) or antioxidants (mycosporine-glycine, M-320, M-335/360, palythene). The same SA and GMA intervals used above to study growth were used to study the variation of MAAs with space weather. Distribution of MAA variance in the MTCM-exposed cells followed an inverted-U shaped curve (Fig. 3). For solar activity, MAA’s concentration increased above control in the lowest and middle intervals, and were similar to control in the highest interval (Figs. 3a-b). For GMA, MAAs increased above control in the middle and highest interval and decreased below control in the lowest interval (Figs. 3c-d). When relating MAAs with another proxy for solar activity, the number of solar X-ray flares, MAAs increased in the absence of solar flares of C-level or higher but remained similar to control when 1 or more flares occurred (Fig. 4a).
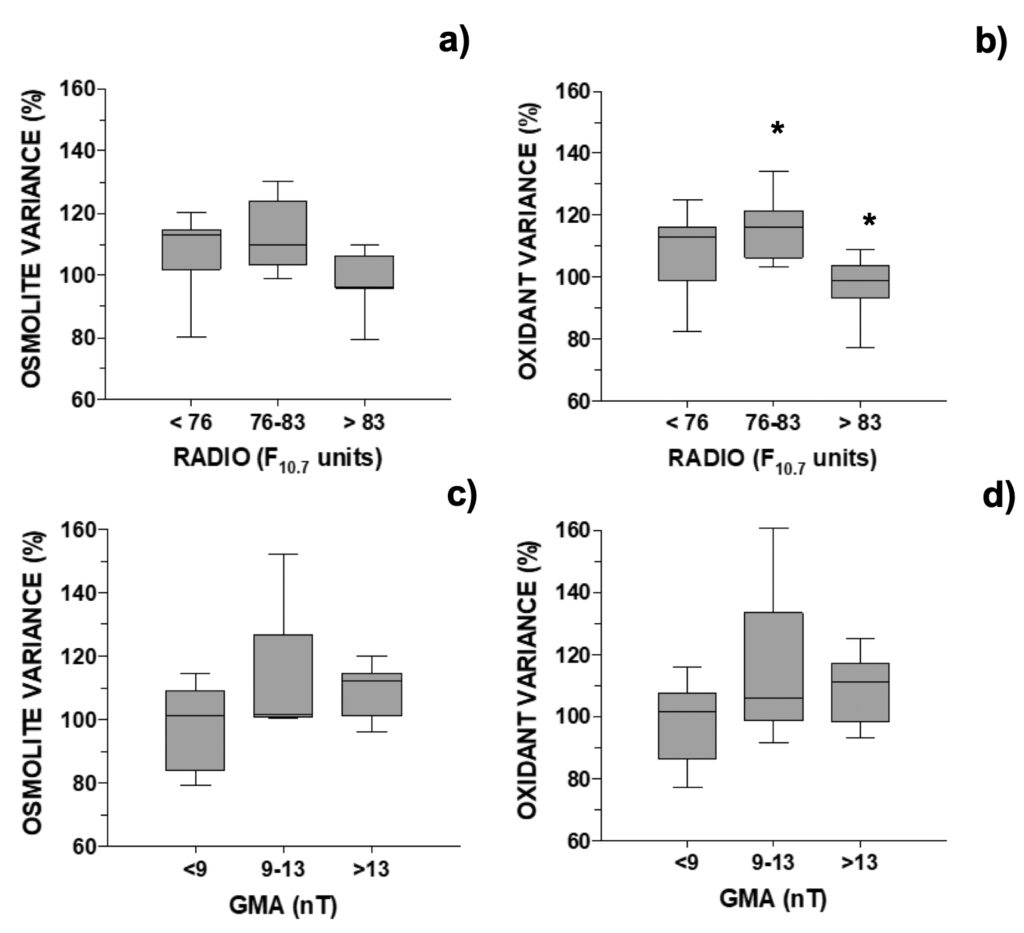
Figure 3. Changes in MAA’s concentration relative to control in A. pacificum distributed
accordingly with the daily: a) and b) RADIO flux (N=24; data selected for days when GMA <9 or >13 nT) or c) and d) GMA activity (N=17, data selected for days when RADIO <76 or >83 F10.7 units). One outlier was removed, see Fig. 6.
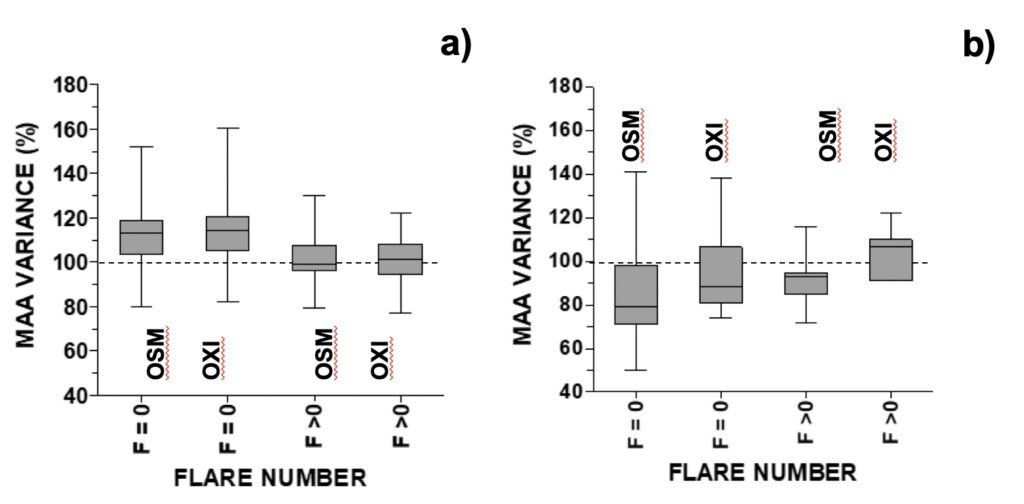
Figure 4. Changes in selected MAA’s concentration (OSM=osmolyte; OXI=anti-oxidant) relative to control distributed accordingly with the daily number of X-ray flares in: a) A. pacificum (N=25, selected for days when GMA< 9 or >13 nT), b) G. catenatum (N=21, selected for days when GMA <10 or >16).
These trends were opposite to those found when observing cell numbers (Fig. 2). The relative MAA concentration was then inversely related to relative growth. Examples were provided in Figure 5: shinorine, one MAA with an additional role as an osmolyte, and mycosporine-glycine, one MAA with an additional role as an antioxidant. In the case of an MAA with an additional role as an antioxidant, its relation with relative growth was stronger (p<0.001) than in the case of an MAA with an additional role as an osmolyte (p<0.01) (Fig. 5). Selected outliers were removed from regression analysis. One outlier presented an 82% reduction in the osmolyte’s concentration and a 54% reduction in the antioxidant’s concentration relative to control. It coincided with a relevant earthquake that took place the next day of the experiment starting on February 12, 2021, with its epicenter located circa 80 km north of the experimental location, and a magnitude of M3.6 on the Richter scale (IPMA, 2022b).
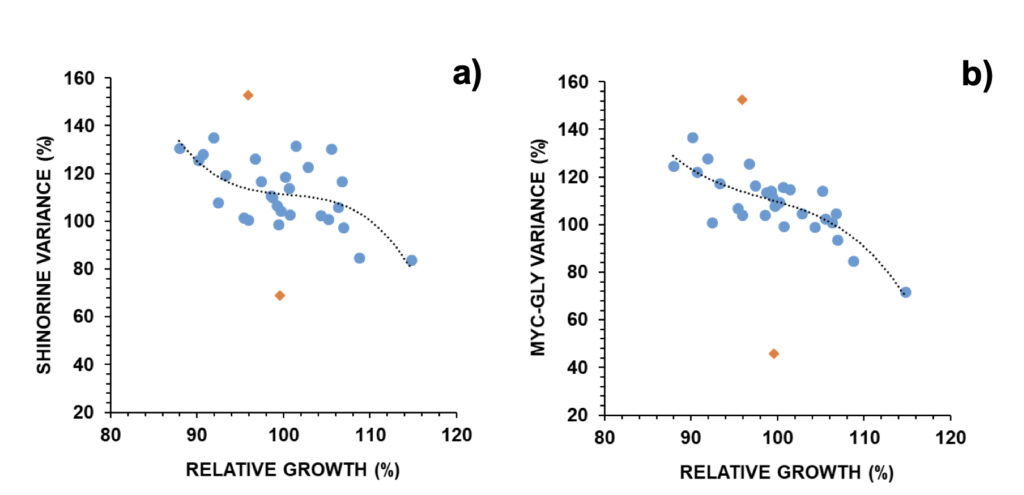
Figure 5. Relative changes in a) shinorine and b) mycosporine-glycine versus relative growth in A. pacificum (N=30). Outliers (orange diamonds) were removed for non-linear regression analysis: a) R=0.637; p < 0.01, b) R=0.828; p < 0.001.
Previous experiments had alerted that these kinds of experiments could suffer influence, in addition to SPW, from seismo-electromagnetic (SEM) phenomena from earthquakes and volcanic eruptions. Such phenomena might take place hours or days before earthquakes. In cases where seismic activity near the experimental location was close to or higher than M3 on the Richter scale, unexpected results were obtained in dinoflagellate cultures (Vale, 2017b). No similar explanation could be associated with other outliers. Overall, all MAAs increased in MTCM-exposed cells, with a median at circa 110% and the 25-75% probability intervals at circa 100-120% from control (Fig. 6a).
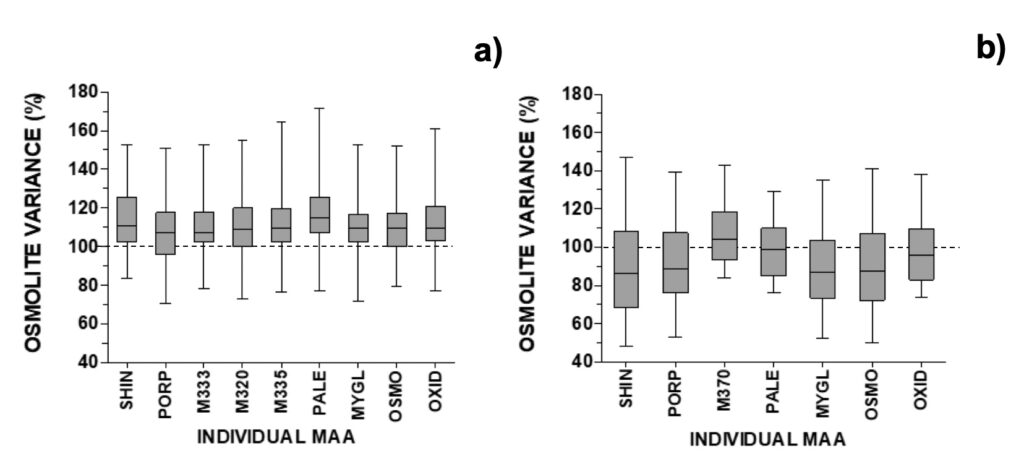
Figure 6. Relative changes in individual MAAs after removal of selected outliers for a) A. pacificum (N=29), b) G. catenatum (N=26). See section 2.4 for abbreviations and spectroscopic details.
Next, we compared the relative difference in cell concentration observed between control and MTCM-exposed G. catenatum cells regarding GMA and SA parameters. Cell concentrations were inferior to control when solar radio flux was below 77 F10.7 units (90.0%) or geomagnetic activity was above 10 nT or 18 nT (90.0 and 89.0%, respectively; Fig. 7). In contrast, cell concentrations were slightly above control when these parameters fell outside these windows: 103% and 104% for SA and GMA, respectively (Fig. 7). For GMA below 10 nT, the relative cell concentration was significantly different from all results obtained above 10 nT (data not shown; p < 0.05).
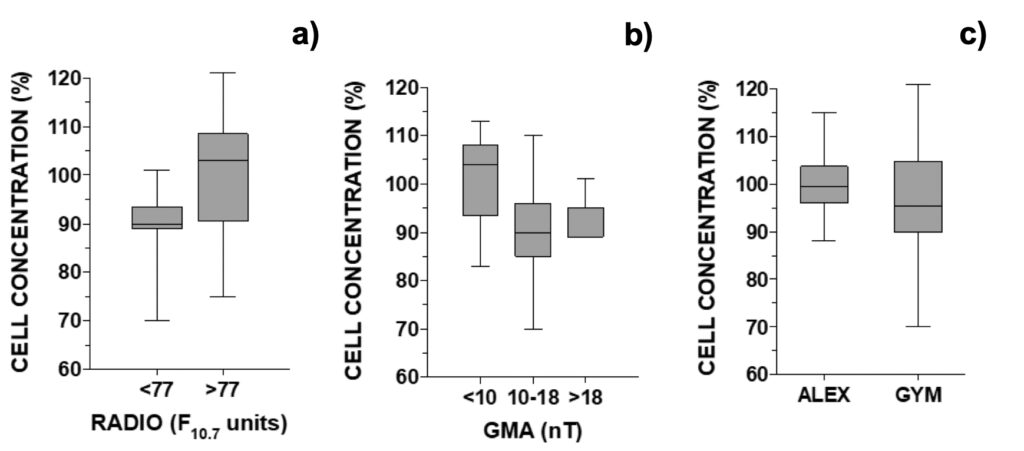
Figure 7. Relative growth of G. catenatum observed 24-hour after addition of culture media pre-treated with a magnetic field versus non-treated distributed accordingly with the daily a) RADIO flux (N=18, when GMA < 10 or >18 nT), b) GMA activity (N=23, when RADIO <81 F10.7 units). In c) global comparison for treatment effect between A. pacificum and G. catenatum (N=30).
These observations were the reverse of those observed for normal growth. Median growth in the control was higher below 77 F10.7 units or above 10 nT: 114% and 117%, respectively (Fig. S7). Outside these intervals, median growth was 104% and 111% for SA and GMA parameters, respectively (Fig. S7). The inverted-U shaped curve typical of hormesis dose-response was not discernible when observing cell counts. Comparing the global effect of treatment between both microalgae, in A. pacificum the concentration of treated cells was 99.5% from control while in G. catenatum, treated cells represented only 95.5% of control (Fig. 7c).
For analysis of UV-photoprotective pigments in G. catenatum, MAAs were grouped into those with an additional relevant role as osmolytes (shinorine, porphyra-334) or antioxidants (mycosporine-glycine, M-370, palythene). Distribution of MAA variance in the MTCM-exposed cells followed an inverted-U shaped curve for GMA, but not for solar activity (Fig. 8). For geomagnetic activity, both osmolytes and antioxidants increased above control in the middle 10-18 nT interval: 110% and 108%, respectively, decreasing outside this interval down to 74% and 84%, respectively (Figs. 8a-b). For SA, there was a non-significative but decreasing trend with increasing radio flux (Figs. 8c-d). When relating MAAs with another proxy for solar activity, the number of solar X-ray flares, MAAs generally decreased either at 0 or ≥ 1 solar flares (80-93%), except for antioxidants at F ≥ 1 (107%) (Fig. 4b).
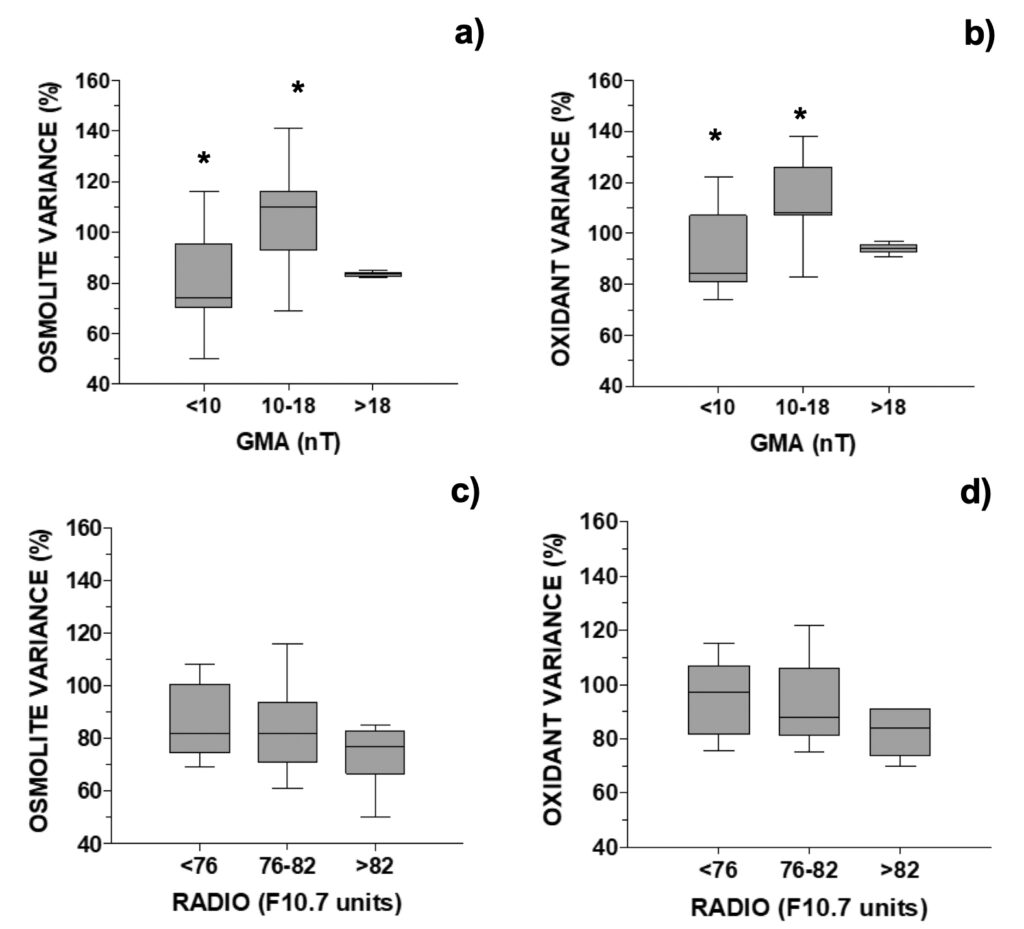
Figure 8. Changes in selected MAA’s concentration relative to control in G. catenatum distributed accordingly with the daily: a) and b) GMA activity (N=26, 4 outliers removed, see Fig. 8); c) and d) RADIO flux (N=19; data selected for days when GMA ≤10 or ≥18 nT, 4 outliers removed).
These trends were opposite to those found when observing cell numbers (Fig. 7). The relative MAA concentration was then inversely related to relative growth. Examples were provided in Fig. 9: shinorine, one MAA with an additional role as an osmolyte, and mycosporine-glycine, one MAA with an additional role as an antioxidant. In the case of an MAA with an additional role as an antioxidant, its co-relation (p<0.001) with relative growth was stronger than in the case of an MAA with an additional role as an osmolyte (p<0.01) (Fig. 9).
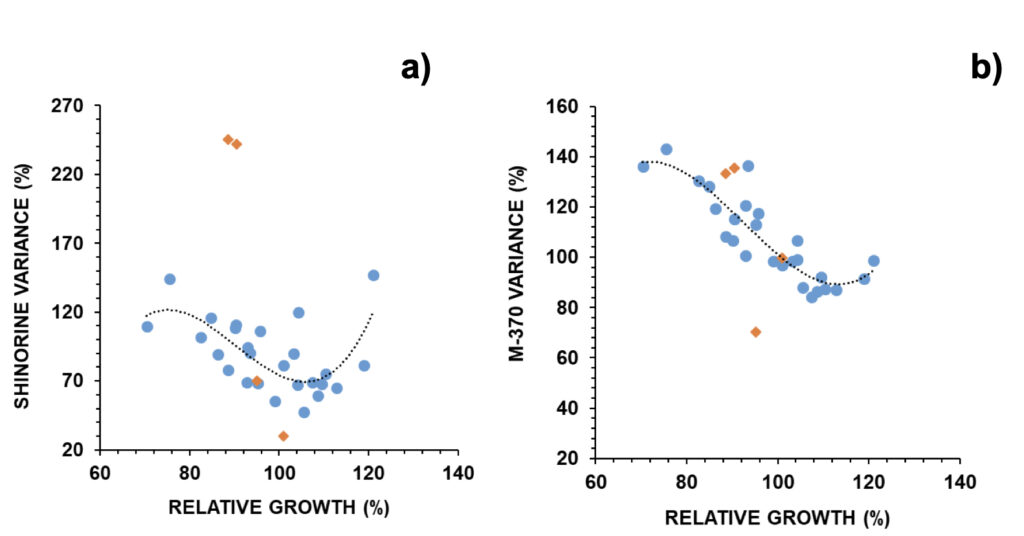
Figure 9. Relative changes in a) shinorine and b) M-370 versus relative growth in G. catenatum (N=30). Selected outliers (orange diamonds) were removed for regression analysis: (a) R=0.694; p < 0.01; b) R=0.883; p < 0.001.
Selected outliers were removed from regression analysis (Fig. S5). Two outliers were associated with earthquakes: outlier 1 was related to an M3.4 event that took place 3 days after the experiment onset with an epicentre at 20 km northeast (on March 28, 2021), and outlier 2 was related to a stronger M3.7 event that took place 12 hours after the experiment onset with an epicenter at 300 km north (on May 20, 2021) (IPMA, 2022b). In outlier 1, both osmolytes and antioxidants reduced to 70% from control; in outlier 2 these reduced to 38% (30% for shinorine) and 68% (36% for mycosporine-glycine), respectively (Fig. 9). Outliers 3 and 4 refer to experiments carried out on two consecutive days, when mucilage was prominently observed (Fig. S5). In these cases, MAAs with an osmolyte role were mainly altered, with shinorine surpassing 240% from control, while those with an antioxidant role not deviating from expected (Fig. 9). Overall, most MAAs decreased to 86-89% from control with the 25-75% probability intervals circa 70-105% from control. The exceptions were M370 and palythene (104% and 99%, respectively) (Fig. 6b). The pigment M-370, so far has only been described in G. catenatum, and its broad absorption covers the entire UVA up to the violet range (Vale, 2015).
Another metabolite, the PSTs, were next evaluated. A limited number of A. pacificum samples were processed (N=10), while in G. catenatum, PSTs were searched in most samples (N=26), as the screen of the PSTs was not initially in the goals of the present research. For MTCM-exposed cells, an increase in PSTs was observed for both dinoflagellates, mainly with an increase of C1+2 up to 107% and 109%, respectively (Figs. 10a-b). C1+2 are commonly dominant over B1 in both microalgae, with a ratio that often approaches 10:1 (Vale, 2022). The cellular concentration of PSTs was then inversely related to growth in G. catenatum (Fig. 10c), but not in A. pacificum (not shown).
Figure 10. Changes in individual PSTs observed 24-hour after addition of culture media pre-treated with a magnetic field in relation to control cells in: a) A. pacificum (N=10), b) G. catenatum (N=25). In c) variation of toxins (C+B toxins) in G. catenatum versus relative growth (N= 26; R=0.839; p < 0.001).
4. Discussion
The present research explored the so-called “water memory” effect for transferring magnetic information into microalgae cultures. The minimal time required for imprinting or the duration of this imprinted information was not the subject of this research. In the absence of this information, a long exposure period was chosen: 24 hours. Other authors have transferred information electronically in a much shorter time, such as 15-min (Jerman et al., 2005). Previous experiments with the transfer of molecular information showed the stored information can work in a similar way as the donor substance (Davenas et al., 1987; Endler et al., 1994; Rey, 2003; Thomas et al., 2000; Jerman, I., 2005). The present research also highlighted that imprinted information can work in a similar way as the original information source, which in this case is an extremely weak low-frequency magnetic field (Vale, 2020a). Addition of distilled water (hypo-osmotic shock) following a 7-hour exposure to an ELFMF reduced cell concentration in relation to the control, in particular when GMA was around 7-12 nT (Vale, 2020a). The transfer of the electromagnetic imprint in the culture medium, although not producing the hypo-osmotic shock, also originated “shock” and reduced cell concentration in relation to the control, in particular when GMA was around 9-18 nT, or radio flux was 76-83 F10.7 units for A. pacificum or below 77 F10.7 units for G. catenatum.
The frequency used here was chosen not only to mimic geomagnetic storms, but also because several biological effects have already been observed in vitro combining weak static and alternating magnetic fields, with an alternating component of tens to hundreds nT and frequencies around 5 Hz or lower with a collinear static field of 42 µT, which is equivalent to the geomagnetic field (Novikov et al., 2010). The same authors also concluded that the presence of accompanying technogenic fields does not cause a noticeable influence on the effects of weak MF with a very small alternating component, at least in those cases when its frequency significantly differs from the industrial frequency (Novikov et al., 2010).
In addition, a clear distinction in susceptibility to MTCM was observed between the two species, with G. catenatum more susceptible than A. pacificum regarding reduction in growth and in the cellular pool of UV-photoprotectors/antioxidants. PSTs increased by direct exposure to an ELFMF in a previous study (Vale, 2022), and here increased also by exposure to MTCM. The small increase in the synthesis of PSTs might simply result from the increase in oxidative stress resulting from magnetic fields (Vale, 2022). PSTs might have no role in antioxidant defense, but their synthesis is rather triggered by exposure to predators (Selander et al., 2015). So far, among marine PST-producers worldwide, such a remarkable natural susceptibility to high solar activity (comprising geomagnetic activity among other solar cycle derived variables) was found only for Iberian G. catenatum strains (Vale, 2013; Vale, 2020b).
The present set of experiments also provided additional evidence on how these microalgae promptly respond to subtle daily variations in space weather. SPW effects have been mainly studied in humans inhabiting high latitudes but are not widely recognized in the biology field (Chernouss et al., 2001; Krylov et al., 2014; Vale, 2020a). During periods of low solar activity, these kinds of experiments might suffer interference from other types of electromagnetic signals, such as those derived from SEM phenomena. Such phenomena, which might take place hours or days before earthquakes, include the changes in the following observations: the earth’s resistivity, the geoelectric field, the geomagnetic field, electromagnetic emissions at various frequencies, and secondary electromagnetic phenomena (e.g., the disturbance of the ionosphere) (Huang and Liu 2006). Observation of SEM effects in wildlife has been mainly restricted to animal behavior and could explain how terrestrial or aquatic animals apparently can sense impending earthquakes in advance (Bhargava et al. 2009; Freund and Stolc, 2013). During nearby earthquakes, cell loss was often observed in both species (Figs. S4-5). The relevance of SEM precursors was best related to alterations in the cellular pool of MAAs, which can function also as antioxidant molecules. Other instances of cell losses were unrelated to M3 earthquakes and occurred mainly in G. catenatum. These might simply reflect the fragility of this microalga in comparison to the Alexandrium strain.
Information imprinting and erasing into water is still a restricted and poorly understood scientific field. Water imprinting, such as in homeopathic preparations, can be erased by a microwave oven or mobile phone energy, while airport X-ray and red- light barcode scanning do not diminish the effect of homeopathic solutions (Weber et al., 2008). In addition, homeopathic medicines lose their properties at around 70-80ºC and in strong magnetic fields (Thomas, 2007). Successful microalgae cultures have been performed by the author since 2014 without autoclaving at 120ºC (Vale, 2015). Heating seawater culture media above 70ºC (80ºC for 1 h) was enough as a sterilization procedure. But, in addition to killing living microorganisms by heat, this procedure was also enough to “erase” previous seawater “memory.” It has been routinely used for cultures and was used here for the transfer of magnetic information.
Besides the classical feature of bonding in liquid water, the hydrogen bonds, a wide range of van der Waals bonds, are present between and among the various oligomeric (cluster) structural units. These weak bonds could account for the remarkable ease of changing the structure of water. Such weak bonds would also allow for the changes in structure caused by electric and magnetic fields and by radiation of all kinds (reviewed by Roy et al., 2005). Only a few biologists have plunged into exploring the complexity behind the physics theory paradigm of quantum fields explaining water-water and water-molecule interactions (Alfinito and Vitiello, 2002; Montagnier et al., 2017).
In the case of marine cultures, not only water contributes to the storing media. Seawater contains circa 35 g per litre of dissolved salts. These are mostly Cl–, Na+, Mg2+, SO2-4, Ca2+ and K+. Magnetic water treatment can influence limescale formation, favoring the precipitation of aragonite (Knez and Pohar, 2005). Coey (2012) hypothesized how a non-classical nucleation mechanism for calcium carbonate via prenucleation clusters, which exist in equilibrium under ambient conditions, could originate a long-lived chemical memory imprinted on these clusters.
Water imprints from biological molecules have also been discovered. The subtle field of biological origin in coral calcium can influence water status if transferred at a distance, i.e., in a field-like manner (Jerman, 2021). Some bacterial and viral DNA sequences have been found to induce low-frequency electromagnetic waves in high aqueous dilutions (Montagnier et al., 2011). In turn, Montagnier et al. (2015, 2017) showed that recorded electromagnetic signals and nanostructures induced in water carry the DNA information (sequence) by retrieving that same DNA by classical PCR amplification using the TAQ polymerase, including both primers and nucleotides.
To understand the effect of the magnetically treated aqueous culture media, results had to be interpreted in light of the hormesis dose-response curve regarding natural fluctuations in space weather. These biphasic dose-responses have been observed with animals, plants and bacteria (Calabrese and Blain, 2009). Hormetic responses occur at low doses of gamma radiation, toxic metals, synthetic drugs and natural molecules (reviewed in Calabrese and Blain, 2011). Plants also present hormetic responses to a variety of environmental factors resulting from physiological or biological stress (temperature, ultraviolet radiation, nutrients, ozone, CO2, O2) (reviewed in Agathokleous, 2018; Agathokleous et al., 2018). Hormesis is also fundamental to understanding response to homeopathic preparations (Calabrese and Jonas, 2010).
Hormesis responses were already observed in these two microalgae in several instances (reviewed in Vale, 2020b). A “hormesis optimum” regarding geomagnetic activity was observed several times in a wide interval of 7-20 nT or a more restricted interval of around 8-12 nT. The MTCM reduced growth at the hormesis optimum for selected SPW parameters. In these cells with reduced growth, the metabolite MAAs probably increased due to the cell arrest not allowing their dilution by daughter cells. However, the overall trend for paralytic shellfish poisoning toxins was an increase in concentration (Fig. 10), while for mycosporine-like amino acids there was a net increase in A. pacificum and a net decrease in G. catenatum (Fig. 6).
These indirect exposures to an ELFMF reinforce the existence of a magnetic window for optimal growth, which may derive from the combination between the local (Earth’s) static field and the oscillating (GMA) field (Novikov et al., 2010), meaning that values of geomagnetic activity close to zero are not optimal. The existence of “frequency windows” and “amplitude windows” is now widely recognized in magnetobiology (Markov, 2005). For example, reducing background (ambient) extremely high-frequency radiation (such as X-rays, Gamma-rays) can impact negatively in bacteria, Paramecium, hamster and mouse cells, the so-called radiation hormesis effect (Luckey and Lawrence, 2006; Kawanishi et al., 2012; Fratini et al., 2015; Castillo and Smith, 2015).
Exploring the memory of water was used here to understand if, indeed, water can be important as a primary receptor for electromagnetic fields, as postulated some time ago by Fesenko and collaborators (1995). From these and previous experiments by the author, an important issue arises for biology: cell division does not occur at a constant pace even under optimal culture conditions (sufficient nutrients, light, absence of predation, etc), and can fluctuate on a daily basis due to the pervasive exposure to SPW. Even inside a building, time-fluctuating magnetic fields penetrate quite easily at all times. And these extremely low frequencies are quite relevant in biological processes. Here, two organisms closely related behaved slightly differently regarding these external informational fields.
Acknowledgments
Great appreciation is due to Margarita Tejedor (IRTA, Cataluna, Spain) and Luz Mamán Menéndez from Laboratorio de Control de Calidad de los Recursos Pesqueros (LCCRRPP, Andalucía, Spain), who provided the A. pacificum strain. Great appreciation is due to Lia Godinho and Laboratorio de Fitoplancton staff (IPMA, Portugal), who provided the G. catenatum strain. M. Manuel Angélico provided access to the stereo microscope at IPMA-DMRM. The results presented in this paper rely on geomagnetic indices calculated and made available by ISGI Collaborating Institutes from data collected at magnetic observatories. We thank the involved national institutes, the INTERMAGNET network and ISGI, and the British Geomagnetic Survey. Appreciation is due to NOAA’s Space Weather Prediction Center, USA, for usage of solar data. Funding for IPMA-DMRM was obtained from ‘SNMB-MONITOR II’ and ‘SNMB-INOV’ (FEAMP – 2020) projects. This research did not receive other specific grants from funding agencies in the public, commercial, or not-for-profit sectors. Appreciation is also due to the external reviewers, whose helpful comments aided in improving the manuscript.
Disclosure of Potential Conflicts of Interests
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Materials
Additional Supporting Information may be found here.
Fig. S1. The LED illumination wood structures
Fig. S2. Daily variation of geomagnetic activity during the experimental period
Fig. S3. Description of the extremely low frequency magnetic field apparatus
Fig. S4. Raw data for Gymnodinium catenatum experiments
Fig. S5. Raw data for Alexandium pacificum experiments
Fig. S6. Daily growth for A. pacificum observed in the control versus SA and GMA
Fig. S7. Daily growth for G. catenatum observed in the control versus SA and GMA
References
Kawanishi M, Okuyama K, Shiraishi K, Matsuda Y, Taniguchi R, Shiomi N, Yonezawa M, Yagi T (2012). Growth retardation of Paramecium and mouse cells by shielding them from background radiation. J.Radiat Res 53: 404–410.
Knez S, Pohar C (2005). The magnetic field influence on the polymorph composition of CaCO3 precipitated from carbonized aqueous solutions. J Colloid Interface Sci 281: 377.
Krylov VV, Zotov OD, Klain BI, Ushakova NV Kantserova NP, Znobisheva AV, Izyumov YG, Kuz’mina VV, Morozov AA, Lysenko LA, Nemova MN, Osipova EA (2014). An experimental study of the biological effects of geomagnetic disturbances, The impact of a typical geomagnetic storm and its constituents on plants and animals. J Atmospheric Sol-Terr Phys 110-111: 28-36.
Lednev VV, Belova NA, Ermakov AM, Akimov EB, Tonevitsky AG (2008). Modulation of cardiac rhythm in the humans exposed to extremely weak alternating magnetic fields. Biofizika 53: 1129–1137.
Luckey TD, Lawrence KS (2006). Radiation hormesis: the good, the bad, and the ugly. Dose-Response 4(3):169–190, 2006.
Markov MS (2005). “Biological Windows”: A Tribute to W. Ross Adey. The Environmentalist, 25: 67–74.
McKay BE, Koren SA, Persinger MA (2003). Behavioural effects of combined perinatal L-NAME and 0.5 Hz magnetic field treatments. Int J Neurosci 113: 119–139.
Moita MT, Palma S, Oliveira PB, Vidal T, Silva A., Vilarinho MG (2008). The return of Gymnodinium catenatum after 10 years, Bloom initiation and transport off the Portuguese coast. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, Intergovernmental Oceanographic Commission (IOC) of UNESCO, Copenhagen, Denmark, p. 242.
Montagnier L, Aïssa J, Capolupo A, Craddock TJA, Kurian P, Lavallee C, Polcari A, Romano P, Tedeschi A, Vitiello, G (2017). Water bridging dynamics of polymerase chain reaction in the gauge theory paradigm of quantum fields. Water 9: 339.
Montagnier L, Aïssa J, Del Giudice E, Lavallee C, Tedeschi A, Vitiello G (2011). DNA waves and water. J Phys Conf Ser 306: 012007.
Montagnier L, Del Giudice E, Aïssa J, Lavallee C, Motschwiller S, Capolupo A, Polcari A, Romano P, Tedeschi A, Vitiello G (2015). Transduction of DNA information through water and electromagnetic waves. Electromagn Biol Med 34 (2): 106-112.
NOAA (2022a). ftp//ftp.swpc.noaa.gov/pub/indices/old_indices/ (accessed March-10 2022).
NOAA (2022b). https://www.ngdc.noaa.gov/geomag/calculators/magcalc.shtml#igrfwmm (accessed March-10 2022).
Novikov VV, Ponomarev VO, Novikov GV, Kuvichkin VV, Yablokova EV, Fesenko EE (2010). Effects and molecular mechanisms of the biological action of weak and extremely weak magnetic fields. Biophysics 55(4): 565–572.
Rey L (2003). Thermoluminescence of ultra-high dilutions of lithium chloride and sodium chloride. Physica A 323: 67–74.
Roy R, Tiller WA, Bell I, Hoover MR (2005). The structure of liquid water, novel insights from materials research, potential relevance to homeopathy. Mater Res Innov 9(4): 98-103.
Selander E, Kubanek J, Hamberg M, Andersson MX, Cervin G, Pavia H (2015). Predator lipids induce paralytic shellfish toxins in bloom-forming algae. PNAS 112(20): 6395–6400.
Thomas Y (2007). The history of the Memory of Water. Homeopathy 96: 151–157.
Thomas Y, Schiff M, Belkadi L, Jurgens P, Kahhak L, Benveniste J (2000). Activation of human neutrophils by electronically transmitted phorbolmyristate acetate. Medical Hypotheses 54: 33-39.
UTEX (2022). https,//utex.org/products/f-2-medium?variant=31722216915034#recipe (accessed March-10 2022).
Vale P (2013). Can solar/geomagnetic activity restrict the occurrence of some shellfish poisoning outbreaks? The example of PSP caused by Gymnodinium catenatum at the Atlantic Portuguese coast. Biophysics 58 (4): 554–567.
Vale P (2016). Can mycosporine-like amino acids act as multifunctional compounds in Gymnodinium catenatum (Dinophyceae)? Photochem Photobio 92 (2): 264-275.
Vale P (2017a). Influence of solar and geomagnetic activity in Gymnodinium catenatum (Dinophyceae) cultures. Gen Physiol Biophys 36(1): 7-21.
Vale P (2017b). Influence of static magnetic fields in phototaxis and osmotic stress in Gymnodinium catenatum (Dinophyceae). Gen Physiol Biophys 36(3): 235-245.
Vale P (2018a). Resistance to hydrogen peroxide highlights Gymnodinium catenatum (Dinophyceae) sensitivity to geomagnetic activity. Photochem and Photobio 94: 95-104.
Vale P (2018b). Impact of light quality and space weather in Alexandrium catenella (Dinophyceae) cultures. Life Sci Space Res 19: 1-12.
Vale P (2019). Intoxicação paralisante por marisco (PSP), uma síndroma rara com recorrência decadal? Medicina Interna 26(4): 326-334.
Vale P (2020a). Extremely-low frequency magnetic field exposure for simulating geomagnetic pulsations in Alexandrium pacificum and Gymnodinium catenatum cultures. Life Sci Space Res 26: 85-96.
Vale P (2020b). The role of hormesis in interpreting the biological effects of space weather. In, Veress, B. and Szigethy, J. (Eds.), Horizons in Earth Science Research. Volume 20, Chapter 6. Nova Science Publishers, Inc., Hauppauge, NY, USA, pp. 153-182.
Vale, P (2022). Physical factors influencing the production of saxitoxin analogues in Gymnodinium catenatum and Alexandrium pacificum cultures. In: Band-Schmidt, C.J. and Rodríguez-Gómez, C.F. (Eds.), Proceedings of the 19th International Conference on Harmful Algae, La Paz, B.C.S., Mexico. International Society for the Study of Harmful Algal Blooms, pp O35-O39.
Vale P, Botelho MJ, Rodrigues SM, Gomes SS, Sampayo MAM (2008). Two decades of marine biotoxin monitoring in bivalves from Portugal (1986-2006), a review of exposure assessment. Harmful Algae 7 (1): 11-25.
Vale P, Rodrigues SM, Ribeiro I (2021). Workflow of the pre-chromatographic ‘Lawrence’ method for bivalves contaminated with Gymnodinium catenatum’s paralytic shellfish poisoning toxins. Food Control 126 (8): 108081.
Weber S, Endler PC, Welles SU, Suanjak-Traidl E, Scherer-Pongratz W, Frass M, Spranger H, Peithner G, Lothaller H (2008). The effect of homeopathically prepared thyroxine on highland frogs, influence of electromagnetic fields. Homeopathy 97: 3–9.
XiaoFeng P, Bo D (2008). Investigation of changes in properties of water under the action of a magnetic field. Science in China Series G: Physics, Mechanics & Astronomy, 51(11): 1621-1632.
Zhadin MN (2001). Review of Russian literature on biological action of DC and low-frequency AC magnetic fields. Bioelectromagnetics 22: 27–45.
Discussion with Reviewers
Reviewer #3: How long were samples cultivated before being tested?
Author: The algae were in culture for at least a couple of years before the experiments. Before each experiment, culture media was added, and each of the inocula were allowed to grow for 5-7 days, as already described.
Reviewer #3: Wouldn’t it be useful to run one sample on a (solar) maxima and compare?
Author: Choosing to run experiments in solar maxima or solar minima is a very limiting choice; if we are in solar minima conditions, we need to wait around 5 years to be in solar maxima conditions again. If we want to repeat again a set of experiments in solar maxima, we need to wait another 10 years or so!
Reviewer #3: Why not consider other pervasive forces? Mostly these are always active, with long coherence times, such as gravity tides.
Author: This ongoing research line has been devoted to electromagnetic signals. Gravity surely affects living organisms, but experiments were always carried out at the same place = same gravity (sea-level altitude). Gravity is circa 978 Gal at the equator. It varies more due to latitude (up to 5000 mGal) or altitude (up to 2000 mGal) than to tides (0.3 mGal) (https://gpg.geosci.xyz/content/gravity/gravity_basics.html).
For a constant magnetic field of circa 43000 nT (Lisbon), there were geomagnetic variations during the experiments of circa 40 nT or higher, which represents a variation of 0.09%. Tidal gravity variation is just 0.00003%. It seems variations in electromagnetic force are more “pervasive” than variations in gravitational force.
Reviewer #2: It is unclear to me whether the shape of the EM signal coming from an electronic clock would be sinusoidal as I would expect for natural phenomena; I would expect such a signal to be “square,” so quite different from a “natural” signal.
Author: Indeed, the signal is more square-like. But natural oscillations are not perfectly sinusoidal and they look like random “noise” (see Fig. S2b, exemplifying variation during an episode of natural geomagnetic unrest).
Reviewer #2: No shielding was used for the exposure phase; this lack of shielding makes it hard to know what signal the sample actually received. It seems, at least, to overpower natural EM fields at that frequency (unclear what the natural intensity is at 0.5Hz).
Author: This is a shortcoming of these experiments. The requirements for biophysical experiments are quite complex. Unfortunately, at a fisheries department like ours, we are not devoted to such deep biophysical experimentation. Also, the relevance of this solar-terrestrial interaction is not easily accepted by my personal peers as well and by the general scientific community working on toxic microalgae. Recently I found another positive (stimulation) correlation between geomagnetic activity and the production of the amnesic toxin ASP. It presents both decadal-like periodicity and seasonal periodicity due to the stronger geomagnetic disturbance taking place annually near the equinoxes. It was first reported here: https://link.springer.com/article/10.1007/s10113-022-01972-6
Reviewer #2: I suggest keeping the topic of hormesis for the intro and discussion and removing it from the title and abstract as it distracts from the main topic for this paper. Your previous papers have already dealt with the hormesis aspect of the response curve, which is very interesting in its own right, but (again my suggestion) not the main topic for this paper.
Author: Hormesis has been removed from the title only. Although not the main topic here, I was obliged to incorporate this concept to interpret daily fluctuations in growth. Actually, since the topic of the journal is “WATER Journal,” water is seen here as a receptor of electromagnetic fields. Exploring water memory was used here to understand if indeed water can be important as a primary receptor of EMF as postulated long ago by Fesenko and collaborators. This was emphasized in the discussion section. From these and previous experiments, an important issue arises for biology: cell division might not be constant even under optimal conditions (sufficient nutrients, light, absence of predation, etc), and can fluctuate on a daily basis due to the pervasive exposure to space weather. Even inside a building, time-fluctuating magnetic fields penetrate quite easily at all times. And these extremely low frequencies are quite relevant in biological processes (text placed at the end of discussion).
Reviewer #1: The author points out that EMF was generated by the solenoid of an electronic clock with a frequency of 0.5 Hz (Materials and Methods section). Figure S1 clearly shows that the generated EMF is extremely inhomogeneous with a high intensity gradient. The leading edge of the liquid in the tube was irradiated with an intensity of approximately 60 μT, and the opposite front – about 20 μT. Accordingly, the different volumes of aqueous solution received different electromagnetic influence. So, the intensity 30 μT, pointed out in the manuscript, is the very approximate and average value…. At the same time, Figure S1 also shows that the electromagnetic signal generated by the clock solenoid is not monochromatic.
Author: These are the constraints of a low- or non-existent budget for biophysical experimentation. See also the answer above on the same subject. In bioelectromagnetic experiments, it has often been found that higher intensities do not produce necessarily a more detrimental effect in living objects.
The information (in this case the frequency) is more relevant than the intensity. «The presence of accompanying technogenic fields does not cause a noticeable influence on the effects of weak MF with a very small alternating component, at least in those cases when its frequency significantly differs from the industrial frequency; in realization of the effects of weak MF of essential importance are both MF components (static and alternating) (Novikov et al., 2010). Citation added to text.
Reviewer #1: The methodological weakness of the work is also the lack of control over the objective parameters of the aquatic media (water solution) upon the influence of the EMF, such as, for example, the refraction index or scattering of light (or scattering spectrum)
Author: This was an interesting addition to the experimental work. No equipment available for that! Citation added on changes of water parameters (XiaoFeng and Bo, 2008).
Reviewer #1: It is not clear what the author means by “The coefficient of variation between triplicate tubes was kept below 10% for A. pacificum and 15% for G. catenatum.” Is the reference to triplicate tubes a statistical array of three tubes with identical samples in each experiment? Is it something else? Why is the variability with one biological object 10% and with another 15%? For what index was the coefficient of variation calculated. Is it the variability of the number of cells per unit volume?
Author: Each day experiments were carried out in a 220-mL aliquot of culture. Sub-aliquots were removed in triplicate for analysis (cell counting and metabolite analysis). Accepted variability was higher in G. catenatum due to its long-chain forming habit, increasing experimental error.
Reviewer #1: Why are such ranges of space weather indices taken? Why were only three ranges, very different in width, used? On what principle were these ranges chosen? And what are the minimum and maximum values of space weather indices for the time period when these experiments were carried out?
Author: Although the concept of hormesis is controversial, nevertheless it cannot be ignored in the field of ionizing radiation: «Radiation hormesis proposes that radiation exposure comparable to and just above the natural background level of radiation is not harmful but beneficial…» (https://en.wikipedia.org/wiki/Radiation_hormesis)
Novel citation added: Luckey and Lawrence (2006) on the topic of radiation hormesis.
I found a similar need to use the hormesis interpretation for geomagnetic activity and solar activity (non-ionizing natural radiation).
Reviewer #1: On Fig.12c, the data are given only for the case of G. catenatum for toxin C1 + 2, but for B1, the data are absent. Why is this so? Why are data absent for A. pacificum?
Author: C1-2 is the dominant toxin (see Vale, 2022 cited in this manuscript). At the time only a limited number of analyses were carried out for A. pacificum and were not enough to analyze their correlation with growth. Later on it was decided to analyze all the individual experiments with G. catenatum.
