Oxidative Stress Induced by Iodoacetamide (An Emerging Disinfection By-Product) on HepG-2 Cells
Junbiao Hua,1, Minjie Chenb1, Huan Wub, Lili Zhengb, Qinglan Zhangb, Xiaoling Zhoub*
a Jinhua People’s Hospital, Jinhua, China
b College of Geography and Environmental Science, Zhejiang Normal University, Jinhua, China
1 Equal contribution and co-first author
*Corresponding author: Tel: +86 (0579) 82282273;
E-mail address: zhouxiaoling@zjnu.cn
Keywords: Iodoacetamide; HepG-2 cell; oxidative stress; oxidative damage; gene expression
Submitted: June 12, 2022
Reviewed: October 9, 2022
Accepted: November 13, 2022
Published: January 28, 2023
Abstract
Iodoacetamide (IAcAm) is a type of emerging nitrogenous disinfection by-product (N-DBP) with high health risk. Up to now, several studies have been carried out on the toxicity of IAcAm, but the study on oxidative damage of IAcAm on human cells was not available. In this study, the oxidative stress and damage induced by IAcAm on HepG-2 cells were investigated. Results showed that superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity exhibited a decreasing trend; but nuclear factor erythroid 2-related factor 2 (NRF2) and glutamate cysteine ligase catalytic subunit (GCLC), both the mRNA and protein level, generally appeared an increasing trend with the increase of IAcAm concentration. These results suggested that IAcAm-exposed cells produced excessive reactive oxygen species (ROS) and initiated the compensation mechanism of NRF2 to deal with oxidative stress. Malonaldehyde (MDA), an index for oxidative damage, had no obvious change at 24 h (p>0.05) but significantly increased at 48 h (p<0.05). This result indicated that HepG-2 cells could protect themselves from ROS attack by consuming antioxidant enzyme (e.g., SOD and GSH-Px) and upregulating the genes related to antioxidation after 24 h exposure of IAcAm; yet, at 48 h, the antioxidant defense system could no longer prevent oxidative damage of ROS, causing severe damage of the lipid membrane.
Introduction
To prevent the occurrence of water-borne diseases and ensure the safety of drinking water, the use of disinfectants (such as chlorine, chloramines, and ozone) is indispensable in the water treatment process. However, disinfectants tend to react with organic matter in water to generate disinfection by-products (DBPs) (Krasner et al., 2006; Zhou et al., 2019; Xu et al., 2022), which have carcinogenic, teratogenic and mutagenic effects (WHO 2000; Richardson et al., 2007; Li and Mitch 2018). Up to now, more than 600 kinds of DBPs have been found in drinking water (Cortes and Marcos 2018). Among them, trihalomethanes and haloacetic acids are the most abundant, and have been regulated by many countries and regions (Richardson et al., 2007; Ding et al., 2013; Lin et al., 2018; Zheng et al., 2020; Weng et al., 2022). Nitrogenous and iodinated DBPs such as haloacetamide (HAcAms) are usually in low levels in drinking water and have not been regulated (Krasner et al., 2006; Ding et al., 2013; Dong et al., 2019), yet it has attracted much attention due to its extremely high cytotoxicity and genotoxicity (Richardson et al., 2007; Wagner and Plewa 2017; Hong et al., 2023). Many studies have been carried out on the toxicity of haloacetonitriles since the 1980s (Lin et al., 1986; Ahmed et al., 1989; Ahmed et al., 1991; Lipscomb et al., 2009; Komaki et al., 2014; Dong et al., 2018b), but the toxicology research on HAcAms, especially on iodinated haloacetamides (I-HAcAms), remained sparse. This may be because commercial standards for I-HAcAms have not been available until recently.
Among the I-HAcAms species, the earliest commercially available standard was for iodoacetamide (IAcAm), which was widely used as a typical thiol reagent in proteomic study in addition to its use as a DBP standard (Young 1980; Shau and Dawson 1985; Fuentes et al., 1994; Sarkany et al., 2000; Marchand et al., 2006; Schmidt and Dringen 2009). Therefore, IAcAm had been the subject of more toxicology studies as compared to other I-HAcAms species. Previous studies have shown that IAcAm causes extremely high cytotoxicity and genotoxicity to Chinese hamster ovary cells (Plewa et al., 2008), and apoptosis or necrosis in porcine kidney cells of LLC-PK1 (van De Water et al., 1999). Chen and Stevens (1991) reported that IAcAm caused lipid peroxidation to LLC-PK1 cells (as indicated by leakage of lactate dehydrogenase), but this damage can be reversed by addition of exogenous antioxidants. A study from Deng et al., (2014) showed that after mice were exposed to IAcAm for 30 days, the activity of antioxidant enzyme, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase were reduced; nevertheless the level of 8-hydroxy-2-deoxyguanosine, an indicator for DNA oxidative damage, increased. These studies provide important information about the toxicity of IAcAm, and indicate that oxidative stress may be an important mechanism driving IAcAm toxicity. But, these studies were mainly focused on animals or animal cells. Since toxicity and the related mechanism may vary by species (Kong 2012), it is necessary to investigate the toxicity and the mechanism of IAcAm using human cells, which may help to better understand its health risk to the people who may be exposed.
Though there are several studies available on the toxicity of IAcAm to human cells (e.g. HepG-2, CCD 841 CoN cell and MCF7), they are mainly focused on cell proliferation rates, apoptotic pathways and the reactivity of IAcAm with cellular proteome thiols (Sayess et al., 2017; Hong et al., 2018; Hall et al., 2020). Considering that oxidative stress has been considered the central mechanisms responsible for harmful effects caused by pollutants/toxicants including IAcAm (Deng et al., 2014; Sun et al., 2019; Zhang et al., 2021), and our previous study also detected significant reactive oxygen species (ROS) signal in IAcAm exposed HepG-2 cells, it can be inferred that oxidative damage may occur within human cells exposed to IAcAm (Hong et al., 2018). But, there is a data gap regarding the direct evaluation of oxidative stress of IAcAm on human cells.
Based on the above information, the HepG-2 cell line was used to investigate the oxidative stress on human cells induced by IAcAm. The parameters of SOD and GSH-Px, two important antioxidant enzymes, as well as malonaldehyde (MDA), an indicator for oxidative damage, were included. The expression of NRF2 (nuclear factor erythroid 2-related factor 2, a main regulatory master of cell redox homeostasis) and GCLC (catalytic subunit of glutamate cysteine ligase, a rate-limiting enzyme during GSH synthesis) were also evaluated. It is hoped that the present study will provide a better toxicological basis for the health risk assessment of IAcAm and the management of drinking water.
2. Materials and Methods
Reagents and Kits. IAcAm (≥ 98.0%) was purchased from CanSyn (German); Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, non-essential amino acids, penicillin-streptomycin, 0.25% trypsin, and phosphatic buffer solution (PBS) were obtained from Gibco USA; Dimethyl sulfoxide was purchased from American sigma company; Phenylmethanesulfonyl fluoride, Western and IP Lysate and BCA protein kit were provided by Shanghai Beyotime Biotechnology Company; SOD and MDA kits were bought from Nanjing Jiancheng Bioengineering Institute.
Cell culture and exposure. The HepG-2 cell line was provided by the Cell Bank of Type Culture Collection of Chinese Academy of Science (Shanghai, China). The reason to choose HepG-2 cells in this study is as follows: the liver is an important organ of detoxification in the human body, so it makes sense to use the liver cells to study the toxic effects of foreign chemicals, including IAcAm. However, the enzymatic activity of the normal human primary hepatocyte is unstable and will be weakened during cell passage/cell proliferation. HepG-2 cells, originating from human liver cancer tissue, contain biotransformed metabolic enzymes with homology to human normal cells, and the enzyme activity is stable and easy to measure, facilitating toxicology studies. HepG-2 cells have been widely used as an in vitro model for the study of human toxicity to pollutants (Leekumjorn et al., 2008; Sohn et al., 2013; Tsai et al., 2017; Wen et al., 2020).
HepG-2 cells were cultured in DMEM supplemented with 1% penicillin-streptomycin, 1% non-essential amino acids and 10% fetal bovine serum in a humidified atmosphere of 95% air and 5% CO2 at 37 oC. HepG-2 cells in log phase were inoculated into a 6-well plate with a density of 5×105/well. After the cells attached to the wall, IAcAm exposure (5 μM, 10 μM, 15 μM, 20 μM) was performed. Solvent treatment (dimethyl sulfoxide ≤ 1%) was used as the control. When the exposure time reached 24 h and 48 h, the cells were harvested for further analysis.
Preparation of protein extracts. Cells treated with IAcAm were washed softly with pre-cold PBS twice and then all the PBS was discarded. After that, 200 μL of cell lysis buffer (190 μL cell lysis buffer+10 μL Phenylmethanesulfonyl fluoride) was added. The whole cell lysis buffer was transferred to the tubes on ice and was treated by ultrasonic wave. Then the tubes were centrifuged at 4oC for 20 min at 12000 rpm. The supernatant was measured by the BCA protein kit.
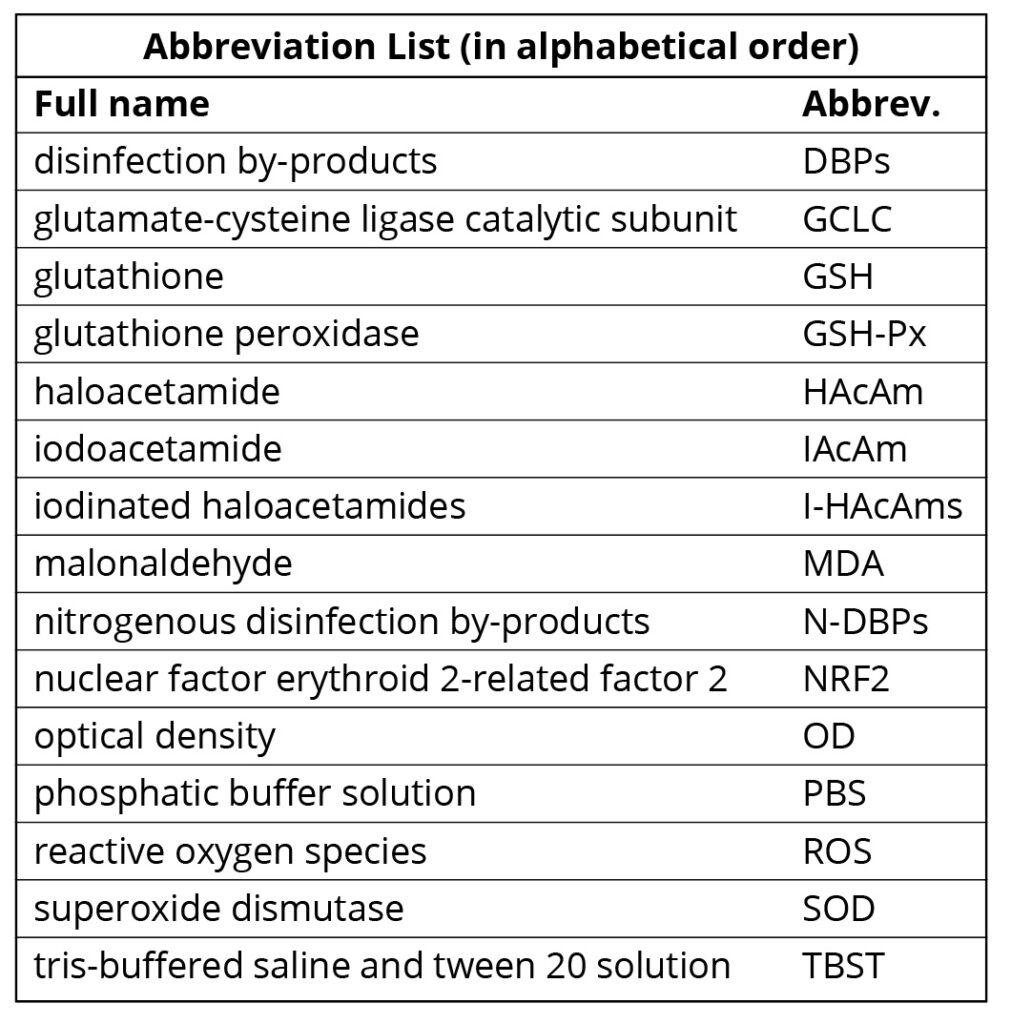
Detection of MDA and SOD. SOD was measured using xanthine oxidase assay in this study. Xanthine oxidase catalyzes xanthine to produce superoxide anion radical (O2–), and O2– oxidizes hydroxylamine to form nitroso salt. The nitroso salt can react with chromogenic agent (P-aminophenol sulfonic acid and methamamine) to form purple red color, which can be quantified by UV-visible spectrophotometer. When the tested sample contains SOD, the nitrite formation will reduce, the measured absorbance value is lower than the control tube, and the SOD activity of the tested sample can be obtained through formula calculation. The reagents and the specific procedures followed the kit instructions.
The formula for calculation is listed below as Equation 1. In Equation 1, OD means optical density at 550 nm. ODc means the OD value of the control sample; ODt means the OD value of the test sample; Vreaction means the volume of the reaction solution; Vsample means the volume of the test sample; and Conprot means protein concentration.
Equation (1)
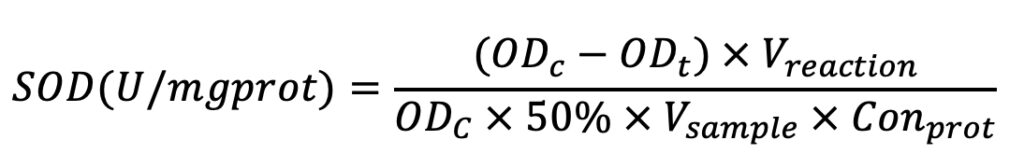
MDA was measured using thiobarbituric acid (TBA) assay. The condensation reaction between TBA and MDA results in the formation of red products with a maximum absorption peak at 532 nm. The absorbance at 532 nm was measured with an UV-visible spectrophotometer, and then the MDA content was calculated by the formula. The reagents and the specific procedures followed the kit instructions.
The formula used for calculation is listed below as Equation 2. In Equation 2, OD means optical density at 532 nm. ODt means the OD value of the test sample; ODt-bk means the OD value of the test blank sample; ODst means the OD value of the standard sample; ODst-bk means OD of the standard blank sample; Conprot means protein concentration.
Equation (2)
RT-PCR assay. Cells were cultured in a series of 6-well plates at an initial density of 3×105/well. After exposure to IAcAm for 4 h, total RNA was isolated with the RNA prep pure Cell/Bacteria Kit. The concentration, as well as the purity of RNA, was measured with Nano Drop. Reverse transcription was performed following the steps of kit instructions. The obtained cDNA was stored at -80oC. A two-step method was used to determine the gene expression. The primer sequence of NRF2, GCLC is presented in Table 1. The sequence.18S, was used as the internal control. PCR procedures were carried out following the steps below: set temperature at 95oC to make the cDNA denaturate, then amplification through 39 cycles (95oC for 5 seconds, annealing at 60oC for 30 seconds, extension at 72oC).
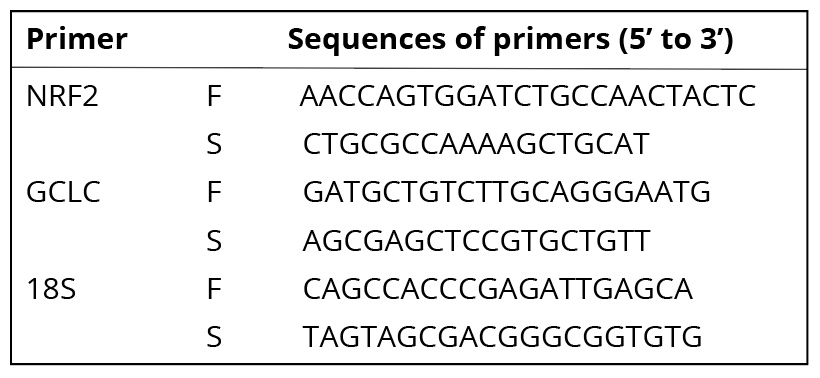
Table 1. Sequences of primers used in quantitative RT-PCR
Western blotting. Western blotting was carried out after HepG-2 cells were exposed to IAcAm for 24 h. The protein was quantified with the BCA Protein Assay Kit. After adjusting the protein of each treatment to the same level, they were separated by electrophoresis on SDS-PAGE gel. The protein was then transferred to nitrocellulose membrane and blocked with 5% BSA at room temperature for 1 h. Antibodies of NRF2 (1:1000), GCLC (1:1000) and β-actin (internal control,1:1000) were incubated overnight at 4oC. Mouse and anti-rabbit IgG (1:5000) were washed with tris-buffered saline and tween 20 solution (TBST) extensively, and then used as the secondary antibodies and incubated for 1 h, and washed with TBST again (3 times,10 min/time). The images were obtained through Kodak 4000 MM (Kodak, Japan).
3. Results
3.1 Effect of IAcAm on antioxidant enzymes
SOD activity in HepG-2 cells (Fig. 1a) showed no significant change when treated with low concentration of IAcAm (5 μM) (p>0.05). But when the dose of IAcAm increased to 10~20 μM, the activity of SOD decreased significantly (p<0.05) as compared with solvent control (Fig. 1a). Moreover, from 5 to 20 µM IAcAm, SOD activity decreased in a concentration-dependent manner (Fig. 1a). The trend of GSH-Px activity (Fig. 1b) also showed a similar pattern to that of SOD (decreasing with IAcAm concentration),yet appeared less sensitive as compared to SOD. The significant reduction of GSH-Px activity was observed at 15 (48 h) or 20 μM (24 h and 48 h).
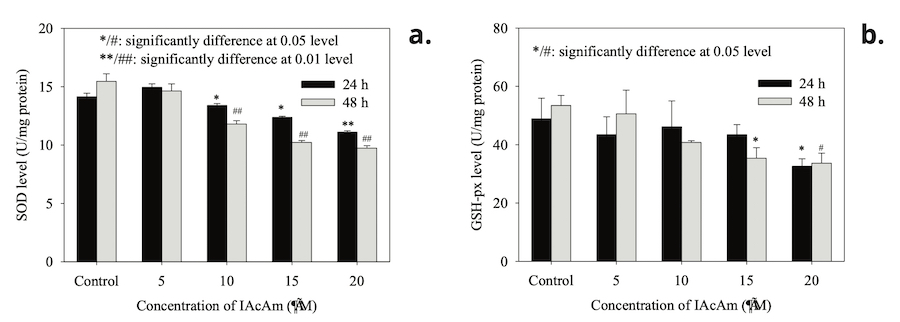
Figure 1. SOD and GSH-px activity after HepG-2 cells exposed to IAcAm (Data were expressed as average ± standard deviation from three replicates; “*” and ”#” were used to mark significant difference as compared to the control for 24 h and 48 h treatment, respectively.
3.2 Effect of IAcAm on MDA level
The MDA level showed no significant change in HepG-2 cells at 24 h treatment as compared with the control (Fig. 2). However, as the exposure time prolonged to 48 h, the MDA levels increased significantly (p< 0.05) in all IAcAm treatment groups (5-20 μM), and that increase occurred in a dose-dependent pattern (Fig. 2). MDA levels increased by 53%, 59%, 76% and 76% at 5, 10, 15 and 20 μM concentrations, respectively.
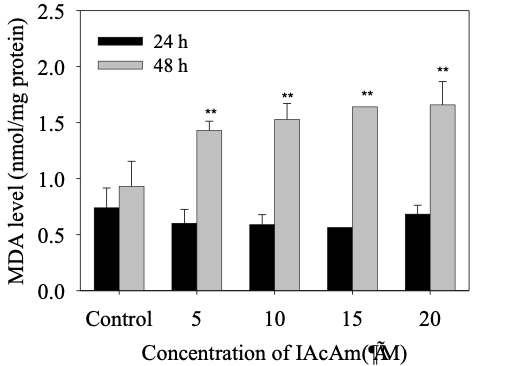
Figure 2. MDA level in HepG-2 cells induced by IAcAm (Data were expressed as average ± standard deviation from three replicates; “**” means significant difference compared to the control at 0.01 level.)
3.3 Effect of IAcAm on NRF2 and GCLC gene expression
IAcAm exposure significantly influenced the NRF2 transcriptional levels (Fig. 3). The expression of NRF2 was firstly inhibited at 5 µM IAcAm (66% of the control), and then slowly recovered at 10 µM (80% of the control) and 15µM IAcAm (103% of the control). At 20µM, the mRNA expression of NRF2 increased markedly (231% of the control). The NRF2 protein level expressed in the IAcAm treatment group also showed a similar pattern with mRNA expression (Fig.4).
Gene expression of GCLC was inhibited (decreased by 56%) significantly (p<0.05) at 5 µM, but increased by 31%, 117%, 587% when IAcAm concentration increased to 10, 15 and 20µM, respectively (Fig. 3). Western-blot results for protein expression also showed a similar trend (Fig. 4), demonstrating the validation of gene expression. Overall, the trend of GCLC expression was consistent with NRF2, i.e., inhibited at low IAcAm concentrations, recovered/activated at high IAcAm concentrations.
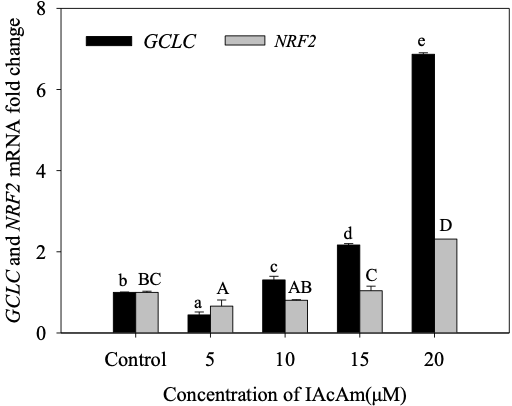
Figure 3. The mRNA expression of NRF2 and GCLC in HepG-2 cells after exposure to IAcAm. (Data were expressed as average ±standard deviation from three replicates; same letter means no significant difference (p<0.05) according to Duncan test; lowercase letter for GCLC and uppercase letter for NRF2).

Figure 4. The protein expression of NRF2 and GCLC in HepG-2 cells after exposure to IAcAm.
4. Discussion
SOD and GSH-Px, two important antioxidant enzymes in biological systems, are responsible for removing dangerous ROS (e.g. superoxide anion and peroxide) and protecting cells from oxidative damage (Kong 2012; Mukherjee et al., 2015; Dong et al., 2018a; Wang et al., 2020). Therefore, activity of SOD and GSH-Px can reflect the body’s ability to scavenging ROS and are often used to evaluate oxidative stress (Ma et al., 2008; Nong et al., 2016; Ma et al., 2021). In this study, both the SOD and GSH-Px activity decreased significantly at high IAcAm concentrations (Fig. 1a), suggesting that high levels of IAcAm exposure caused oxidative stress, and it increased with IAcAm concentrations. This result was consistent with the previous study on IAcAm, where the ROS level (a direct index for oxidative stress) increased to 1.0, 1.3, 1.4 and 2.0 times of control at 5, 10, 15 and 20 μM of IAcAm, respectively (Hong et al., 2018). We theorize that, to deal with the high level of ROS, SOD and GSH-Px were gradually exhausted, and excessive ROS could, in turn, inactivate the activities of SOD and GSH-Px. Furthermore, the variation trends of SOD and GSH-Px activity were in agreement with the cytotoxicity of IAcAm on HepG-2 cells observed in a previous study (Hong et al., 2018), where the cell viability reduced in a dose-dependent manner as the IAcAm level increased from 0 to 20 μM, further indicating that oxidative stress may be the cause for the cytotoxicity of IAcAm. The significant reduction of SOD and GSH-Px activity in the IAcAm-treated group suggested that the cells generated ROS, outweighing the self-scavenging capacity, and may cause oxidative damage, which can be seen from MDA levels occurring within the HepG-2 cells.
MDA is the final product of membrane lipid peroxidation and a sensitive diagnostic parameter for oxidative damage of cells (Kong 2012; Sun et al., 2020; Gawron-Skarbek et al., 2021). In this study, the MDA level in HepG-2 cells showed no significant change at 24 h but enhanced greatly at 48 h (Fig. 2). These results suggested that during the first 24 h of exposure, the cells can protect themselves from ROS attack through initiating an antioxidant system (such as sacrificing SOD, GSH-Px). Yet, at 48 h, the ROS could not be scavenged completely by the antioxidant system and this caused oxidative damage on the cell membrane. Moreover lipid peroxidation products represented by MDA can further polymerize or cross-link with proteins/enzymes/nucleic acid within the cell, aggravating membrane damage and cell toxicity (Kong 2012).
Combining the results of SOD, GSH-Px, and MDA, we conclude that short term IAcAm exposure is harmful to HepG-2 cells, which produce excessive ROS, not only causing the significant reduction of SOD and GSH-PX, but also damaging the cell membrane and leading to a significant accumulation of MDA. Since the exogenous antioxidant can also be a good quencher for ROS occurring in the organism/cell (Chen and Stevens 1991; Kong 2012), the addition of dietary antioxidants (e.g. N-acetylcysteine, vitamin C) may block the toxicity of IAcAm on human cells.
In response to oxidative stress, the cell will tweak at the gene expression level spontaneously (Kalayarasan et al., 2009; Liu et al., 2017; Li et al., 2021). NRF2 is a vital protein in regulating the process of the antioxidant reaction (Copple et al., 2008; Soetikno et al., 2013; Choudhury et al., 2021). Under normal physiological conditions, NRF2 mainly binds to its inhibitor Keap1 and exists in the cytoplasm in an inactive state. When cells are subjected to oxidative stress, NRF2 will dissociate from Keap1, translocate to the nucleus, and bind to the ARE (anti-oxidation response element) sequence, and activate the expression of a variety of antioxidant enzymes (e.g. SOD, GSH-Px, etc) (Antelmann and Helmann 2011; Yamamoto et al., 2018; Patinen et al., 2019; Wang et al., 2020). In this study, the expression of NRF2 (both the mRNA and protein level) was firstly inhibited and then slowly recovered, and finally increased markedly. Combining the results of antioxidant enzymes, the trend of NRF2 expression at 5-20µM IAcAm was opposite to the trend of SOD and GSH-Px activity. This can be attributed to the compensation mechanism of NRF2, which can upregulate its transcriptional level to deal with oxidative stress via adjusting antioxidant enzymes (e.g., SOD, GSH-Px, etc). But because of the excessive ROS, the increased SOD/GSH-Px level stimulated by NRF2 was still not enough to scavenge free radicals, and the SOD/GSH-Px activity still reduced significantly as compared to the control (Fig.1).
GCLC is one of the downstream target genes of NRF2; it is the catalytic subunit of glutamate cysteine ligase, a rate-limiting enzyme during synthesis of GSH (Guan et al., 2015; Zhang et al., 2019), which can remove hydroxyl radicals and provide reducing power to other antioxidant enzymes (Atkuri et al., 2007; Kong 2012). In the present study, the trend of GCLC gene expression (both mRNA and protein level) was consistent with NRF2, i.e., inhibited at a low exposure level, but recovered/stimulated at a high exposure level, indicating it was strictly regulated by NRF2. The significant increase of GCLC expression in a high IAcAm concentration reflected the great demand for GSH production, which may be used to 1) resist the excessive ROS induced by IAcAm (Hong et al., 2018); and 2) directly react with IAcAm to protect the thiol-containing proteins and enzymes in the cell as IAcAm was a well-known thiol-alkylating reagent (Schmidt and Dringen 2009; Pals et al., 2017; Hall et al., 2020).
4. Conclusions
At 24 h, HepG-2 cells can marginally resist the ROS caused by IAcAm through consumption of antioxidant enzymes (SOD, GSH-Px) and upregulating the genes related to oxidative stress (NRF2, GCLC). However, when the exposure time was prolonged to 48 h, the antioxidant defense system could not resist ROS induced by IAcAm anymore, leading to severe oxidative damage and significant accumulation of MDA. The results in this study demonstrated that oxidative stress is an important mechanism for the toxicity of IAcAm on human cells, which suggested that dietary antioxidants may be a good way to prevent the health risk from IAcAm in drinking water.
Acknowledgments
This study was financially supported by Basic Public Welfare Research Project in Zhejiang Province (LGF21B070004).
References
Ahmed AE, Jacob S and Loh JP (1991). Studies on the mechanism of haloacetonitriles toxicity: quantitative whole body autoradiographic distribution of [2-14C]chloroacetonitrile in rats. Toxicology 67(3): 279-302.
Ahmed AE, Soliman SA, Loh JP and Hussein GI (1989). Studies on the mechanism of haloacetonitriles toxicity: inhibition of rat hepatic glutathione S-transferases in vitro. Toxicol Appl Pharmacol 100(2): 271-279.
Antelmann H and Helmann J (2011). Thiol-based redox switches and gene regulation. Antioxid Redox Sign 14(6): 1049-1063.
Atkuri K, Mantovani J, Herzenberg L and Herzenberg L (2007). N-Acetylcysteine–a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7(4): 355-359.
Chen Q and Stevens JL (1991). Inhibition of iodoacetamide and t-butylhydroperoxide toxicity in LLC-PK1 cells by antioxidants: a role for lipid peroxidation in alkylation induced cytotoxicity. Arch Biochem Biophys 284(2): 422-430.
Choudhury C, Mazumder R, Kumar R, Dhar B and Sengupta M (2021). Cadmium induced oxystress alters Nrf2-Keap1 signaling and triggers apoptosis in piscine head kidney macrophages. Aquat Toxicol 231: 105739.
Copple IM, Goldring CE, Jenkins RE, Chia AJL, Randle LE, Hayes JD, Kitteringham NR and Park BK (2008). The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology 48(4): 1292-1301.
Cortes C and Marcos R (2018). Genotoxicity of disinfection byproducts and disinfected waters: A review of recent literature. Mutat Res-GenTox En 831: 1-12.
Deng Y, Zhang Y, Zhang R, Wu B, Ding L, Xu K and Ren H (2014). Mice In vivo toxicity studies for monohaloacetamides emerging disinfection byproducts based on metabolomic methods. Environ Sci Technol 48(14): 8212-8218.
Ding H, Meng L, Zhang H, Yu J, An W, Hu J and Yang M (2013). Occurrence, profiling and prioritization of halogenated disinfection by-products in drinking water of China. Environ Sci-Proc Imp 15(7): 1424.
Dong H, Qjang Z and Richardson SD (2019). Formation of iodinated disinfection byproducts (I-DBPs) in drinking water: Emerging concerns and current issues. Accounts Chem Res 52(4): 896-905.
Dong WQ, Sun HJ, Zhang Y, Lin HJ, Chen JR and Hong HC (2018a). Impact on growth, oxidative stress, and apoptosis-related gene transcription of zebrafish after exposure to low concentration of arsenite. Chemosphere 211(NOV.): 648-652.
Dong Y, Li F, Shen H, Lu R, Yin S, Yang Q, Li Z and Wang S (2018b). Evaluation of the water disinfection by-product dichloroacetonitrile-induced biochemical, oxidative, histopathological, and mitochondrial functional alterations: Subacute oral toxicity in rats. Toxicol Ind Health 34(3): 158-168.
Fuentes JM, Campo ML and Soler G (1994). Kinetics of manganese reconstitution and thiol group exposition in dialyzed rat mammary gland arginase. Int J Biochem 26(5): 653-659.
Gawron-Skarbek A, Chrzczanowicz J, Nowak D, Gawor R and Kostka T (2021). Effects of two different types of single exercise modes on salivary C-reactive protein concentration, oxidative stress and antioxidant capacity in post-myocardial infarction patients. Redox Rep 26(1): 29-34.
Guan D, Su Y, Li Y, Wu C, Meng Y, Peng X and Cui Y (2015). Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity through enhancement of Nrf2/GCLC/GSH and suppression of HIF1 alpha/NOX2/ROS pathways. J Neurochem 134(3): 551-565.
Hall DR, Yeung K and Peng H (2020). Monohaloacetic acids and monohaloacetamides attack distinct cellular proteome thiols. Environ Sci Technol 54(23): 15191-15201.
Hong H, Lu Y, Zhu X, Wu Q, Jin L, Jin Z, Wei X, Ma G and Yu H (2023). Cytotoxicity of nitrogenous disinfection byproducts: A combined experimental and computational study. Sci Total Environ 856: 159273.
Hong H, Wu H, Chen J, Wu B, Yu H, Yan B and Liang Y (2018). Cytotoxicity induced by iodinated haloacetamides via ROS accumulation and apoptosis in HepG-2 cells. Environ Pollut 242: 191-197.
Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M and Sudhandiran G (2009). Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol 606(1-3): 162-171.
Komaki Y, Marinas BJ and Plewa MJ (2014). Toxicity of drinking water disinfection byproducts: cell cycle alterations induced by the monohaloacetonitriles. Environ Sci Technol 48(19): 11662-11669.
Kong Z (2012). Environmental Toxicology, Nanjing University Press, Nanjing (in Chinese).
Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad GD and Thruston AD, Jr. (2006). Occurrence of a new generation of disinfection byproducts. Environ Sci Technol 40(23): 7175-7185.
Leekumjorn S, Wu Y, Sum AK and Chan C (2008). Experimental and computational studies investigating trehalose protection of HepG2 cells from palmitate-induced toxicity. Biophys J 94(7): 2869-2883.
Li R, Yu L, Qin Y, Zhou Y, Liu W, Li Y, Chen Y and Xu Y (2021). Protective effects of rare earth lanthanum on acute ethanol-induced oxidative stress in mice via Keap 1/Nrf2/p62 activation. Sci Total Environ 758: 143626.
Li XF and Mitch WA (2018). Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52(4): 1681-1689.
Lin EL, Daniel FB, Herren-Freund SL and Pereira MA (1986). Haloacetonitriles: metabolism, genotoxicity, and tumor-initiating activity. Environ Health Persp 69: 67-71.
Lin J, Chen X, Zhu A, Hong H, Liang Y, Sun H, Lin H and Chen J (2018). Regression models evaluating THMs, HAAs and HANs formation upon chloramination of source water collected from Yangtze River Delta Region, China. Ecotox Environ Safe 160: 249-256.
Lipscomb JC, El-Demerdash E and Ahmed AE (2009). Haloacetonitriles: metabolism and toxicity. Rev Environ Contam Toxicol 198: 169-200.
Liu X, Liu H, Zhai Y, Li Y, Zhu X and Zhang W (2017). Laminarin protects against hydrogen peroxide-induced oxidative damage in MRC-5 cells possibly via regulating NRF2. PeerJ 5: e3642.
Ma A, Yang XZ, Wang ZX, Shi DZ and Chen YX (2008). Adult exposure to diethylstilbestrol induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male hamster. Reprod Toxicol 25(3): 367-373.
Ma Z, Zhang W, Wu Y, Zhang M, Wang L, Wang Y, Wang Y and Liu W (2021). Cyclophilin A inhibits A549 cell oxidative stress and apoptosis by modulating the PI3K/Akt/mTOR signaling pathway. Biosci Rep 41(1): BSR20203219.
Marchand C, Le Marechal P, Meyer Y and Decottignies P (2006). Comparative proteomic approaches for the isolation of proteins interacting with thioredoxin. Proteomics 6(24): 6528-6537.
Mukherjee A, Haldar C and Vishwas DK (2015). Melatonin prevents dexamethasone-induced testicular oxidative stress and germ cell apoptosis in golden hamster, Mesocricetus auratus. Andrologia 47(8): 920-931.
Nong KT, Wang WW, Niu X, Hu B, Ma CC, Bai YQ, Wu B, Wang Y and Ai KX (2016). Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 18(12): 1548-1559.
Pals JA, Wagner ED, Plewa MJ, Xia M and Attene-Ramos MS (2017). Monohalogenated acetamide-induced cellular stress and genotoxicity are related to electrophilic softness and thiol/thiolate reactivity. J Environ Sci 58: 224-230.
Patinen T, Adinolfi S, Cortés C, Härkönen J, Jawahar Deen A and Levonen A (2019). Regulation of stress signaling pathways by protein lipoxidation. Redox Biol 23: 101114.
Plewa MJ, Muellner MG, Richardson SD, Fasanot F, Buettner KM, Woo Y-T, McKague AB and Wagner ED (2008). Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection byproducts. Environ Sci Technol 42(3): 955-961.
Richardson SD, Plewa MJ, Wagner ED, Schoeny R and Demarini DM (2007). Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(1-3): 178-242.
Sarkany Z, Skern T and Polgar L (2000). Characterization of the active site thiol group of rhinovirus 2A proteinase. FEBS Lett 481(3): 289-292.
Sayess R, Khalil A, Shah M, Reckhow DA and Pollitt KJG (2017). Comparative cytotoxicity of six iodinated disinfection byproducts on nontransformed epithelial human colon cells. Environ Sci Technol Lett 4(4): 143-148.
Schmidt MM and Dringen R (2009). Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front. Neuroenergetics 1: 1.
Shau H and Dawson JR (1985). Regulation of human natural killing by lysosomotropic and thiol-reactive agents. Immunology 55(4): 647-654.
Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, Kawachi H and Watanabe K (2013). Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res 57(9): 1649-1659.
Sohn E-H, Koo HJ, Do Thi Thu H, Jang S-A, Namkoong S, Lim JD and Kang SC (2013). Protective effects of ellagic acid on ethanol-induced toxicity in hepatic HepG2 cells. Mol Cell Toxicol 9(3): 249-256.
Sun H-J, Zhang Y, Zhang J-Y, Lin H, Chen J and Hong H (2019). The toxicity of 2,6-dichlorobenzoquinone on the early life stage of zebrafish: A survey on the endpoints at developmental toxicity, oxidative stress, genotoxicity and cytotoxicity. Environ Pollut 245: 719-724.
Sun HJ, Zhao WJ, Teng XQ, Shu SP, Li SW, Hong HC and Guan DX (2020). Antioxidant responses and pathological changes in the gill of zebrafish (Danio rerio) after chronic exposure to arsenite at its reference dose. Ecotox Environ Safe 200: 110743.
Tsai T-H, Yu C-H, Chang Y-P, Lin Y-T, Huang C-J, Kuo Y-H and Tsai P-J (2017). Protective effect of caffeic acid derivatives on tert-butyl hydroperoxide-induced oxidative hepato-toxicity and mitochondrial dysfunction in HepG2 cells. Molecules 22(5): 702.
van De Water B, Wang Y, Asmellash S, Liu H, Zhan Y, Miller E and Stevens JL (1999). Distinct endoplasmic reticulum signaling pathways regulate apoptotic and necrotic cell death following iodoacetamide treatment. Chem Res Toxicol 12(10): 943-951.
Wagner ED and Plewa MJ (2017). CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review. J Environ Sci 58: 64-76.
Wang Y, Wang C, Bao S and Nie X (2020). Responses of the Nrf2/Keap1 signaling pathway in Mugilogobius abei (M. abei) exposed to environmentally relevant concentration aspirin. Environ Sci Pollut Res 27(13): 15663-15673.
Wen Y, Mirji N and Irudayaraj J (2020). Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol In Vitro 65: 104797.
Weng H, Wang C, Ye T, Xu Z, Sun H, Lin H, Deng W-J, Wu F and Hong H (2022). Precursor characteristics of mono-HAAs during chlorination and cytotoxicity of mono-HAAs on HEK-293T cells. Chemosphere 301: 134689.
WHO (2000). Environmental Health Criteria 216: Disinfectants and disinfectant by-products. World Health Organization, Geneva. .
Xu Z, Shen J, Qu Y, Chen H and Wu F (2022). Using simple and easy water quality parameters to predict trihalomethane occurrence in tap water. Chemosphere 286(12): 131586.
Yamamoto M, Kensler T and Motohashi H (2018). The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98(3): 1169-1203.
Young JD (1980). Effects of thiol-reactive agents on amino acid transport by sheep erythrocytes. BBA 602(3): 661-672.
Zhang C, Yang Y, Liang W, Wang T, Wang S, Wang X, Wang Y, Jiang H and Feng H (2019). Neuroprotection by urate on the mutant hSOD1-related cellular and Drosophila models of amyotrophic lateral sclerosis: Implication for GSH synthesis via activating Akt/GSK3 beta/Nrf2/GCLC pathways. Brain Res Bull 146: 287-301.
Zhang Z, Pan T, Liu C, Shan X, Xu Z, Hong H, Lin H, Chen J and Sun H (2021). Cyclophosphamide induced physiological and biochemical changes in mice with an emphasis on sensitivity analysis. Ecotox Environ Safe 211: 111889.
Zheng L, Sun H, Wu C, Wang Y, Zhang Y, Ma G, Hongjun Lin, Chen J and Hong H (2020). Precursors for brominated haloacetic acids during chlorination and a new useful indicator for bromine substitution factor. Sci Total Environ 698: 134250.
Zhou X, Zheng L, Chen S, Du H, Raphael BMG, Song Q, Wu F, Chen J, Lin H and Hong H (2019). Factors influencing DBPs occurrence in tap water of Jinhua Region in Zhejiang Province, China. Ecotox Environ Safe 171: 813-822.
