 Infra-Red Spectroscopic Study of Structural Change of Liquid Water Induced by Sunlight Irradiation
Infra-Red Spectroscopic Study of Structural Change of Liquid Water Induced by Sunlight Irradiation
Yokono T1, Shimokawa S*2, Yokono M3, Hattori H4
1Techno-Science Laboratory, CRIS, Hokkaido University, Kita-ku, Kita-21, Nishi-10, Sapporo 001-0021, Japan; E-mail: tyokono@me.com
*2 Techno-Science Laboratory, CRIS, Hokkaido University , Kita-ku, Kita-21, Nishi-10, Sapporo 001-0021, Japan; E-mail: shimokawa@cast.hokudai.ac.jp
3Institute of Low Temperature Science , Hokkaido University, Kita-ku, Kita-19, Nishi-8, Sapporo 060-0819, Japan; E-mail: filia@mac.com
4Catalysis Research Center, Hokkaido University , Kita-ku, Kita-21, Nishi-10, Sapporo 001-0021, Japan; E-mail: hattori@cat.hokudai.ac.jp
Correspondence: shimokawa@cast.hokudai.ac.jp
Key Words: Liquid water; Sunlight irradiation; Clathrate-like structure; IR of O-H stretching.
Received 15 October 2008; revised 9 January 2009; accepted 11 March 2009. Published 1 July 2009. Available online: 1 July 2009.
Summary
Distinct changes in the infra-red (IR) spectrum of liquid water in the O-H stretching region were observed on irradiation of sunlight at room temperature. The spectrum shape in the range 2800 – 3800 cm -1 became trapezoidal on irradiation of the sunlight for more than 30 minutes under the sunlight strength of 3 MJ m-2. The spectrum gradually changed to restore the original spectrum in 180 minutes when sunlight irradiation was shut off. These results indicate that the formation and elimination of a clathrate-like structure of water occur reversibly at room temperature in response to irradiation and shutoff of the sunlight, respectively. The structural change caused by the sunlight irradiation occurs over a period of several minutes to hours.
Article Outline
- Introduction
- Experimental
- Results and Discussion
- Conclusions
- Acknowledgments
- References
- Discussion with Reviewers
Introduction
Liquid water forms a variety of structures arising from hydrogen bonding of water molecules. There are many examples of the formation of dodecahedral clathrates in aqueous solutions containing NH 4+, H3O+, Cs+, N2, O2, CH4, etc. (Sloan, 1998). The clathrates are stable under a high pressure. Clathrate-like structures are proposed as being part of the normal structure of water at atmospheric pressure, which becomes increasingly important as the water is supercooled (Kanno, 2001).
In our previous papers, we reported on the basis of XRD measurements that IR irradiation or sunlight irradiation induces structural change in the network of water molecules; a clathrate structure of water appears on irradiation of IR light (Shimokawa et al., 2004; Yokono et al., 2004) or sunlight (Shimokawa et al., 2007) even at room temperature. A water molecule itself functions as the guest molecule in the cage of the clathrate.
The structural change induced by irradiation of sunlight is slow as compared to the quantum mechanical transition with single photon excitation and to the hydrogen bond cleavage and formation occurring in pico second order. XRD measurements indicated that the clathrate structure induced by irradiation of sunlight was restored gradually to the original structure if the irradiation was stopped; it took more than 70 min to be restored to the original structure (Shimokawa et al., 2004). The clathrate is in a metastable state.
The clathrate structure is strongly associated with the hydrogen bondings among water molecules. Any structural changes should reflect in the O-H vibration modes, and, accordingly, the frequencies of the IR band positions for ν 1, ν2 and ν3 vibration modes should change with the structural change. For example, Miyazaki et al. (2004) reported changes in IR spectrum with cluster size of water by combined mass spectroscopy-IR spectroscopy. If structural change occurs on the sunlight irradiation, IR spectral changes will appear in the O-H vibration region.
In the present work, IR spectra of liquid water were measured following the sunlight irradiation. Marked changes were observed in the O-H stretching region, and the spectral changes persisted for 180 min to be restored to the original spectrum. The results confirm the conclusion obtained by XRD measurements (Shimokawa et al., 2007) that irradiation of the sunlight induces structural change of liquid water at room temperature; water becomes rich in clathrate structure.
Experimental
A water sample was purified with SA2000E1 ultrapure water production equipment (Eyela Co.), and its electrical conductivity was about 0.06 μS cm -1. The water was contained in a cylindrical polypropylene vessel of 2 cm3 capacity with a cap. Before exposure to sunlight, the water in the vessel was kept in a dark room at 293 K for 48 h. The vessel containing water was exposed to the sunlight for different periods ranging 15 min to 180 min. Strengths of sunlight on the days when the experiments were carried out were taken from the values measured by Japan Meteorological Agency. The water sample exposed to sunlight for a certain period was transferred to the dark room kept at 293 K or 288 K, and subjected to IR measurements in different periods of holding in the dark room.
For the measurement of IR spectrum, a 0.22 μL of water was withdrawn from the vessel with a micro syringe and dropped onto a CaF 2 window plate. Another CaF2 window plate was placed and pressed on the first CaF2 window plate to make the water droplet into a thin film. The light path length was adjusted by the pressure put on the plates to obtain the maximum absorbance in the range 0.5 – 1.0 for the peaks in the range 2 800 – 3 800 cm-1. Transmission IR spectra were measured with a Jasco FT-IR 460 spectrometer, a resolution being 4 cm-1.
Results and Discussion
Figure 1 shows the IR spectra of the water samples before and after being exposed to sunlight for different period of time. As the light path length of the cell varied a little for each measurement, the spectrum was normalized to a constant integral absorbance in the range 2800 – 3800 cm -1. This normalization is justified on the assumption that the integral molar absorption coefficient of each component of the band does not change with the band position.
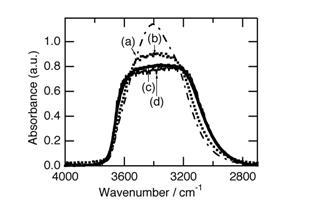
Figure 1: FT-IR spectral changes for liquid water induced by irradiation of sunlight. Before irradiation (a), and after irradiation of sunlight of 3 MJ m-2 h-1 for 15 min (b), 30 min (c), and irradiation of sunlight of 1 MJ m-2 h-1 for 180 min (d).
Before exposure to the sunlight, the water showed a broad peak centered at 3404 cm -1 (Fig. 1, spectrum (a)). This spectrum is exactly the same as those reported in literature (Venyaminov and Prendergast, 1997); Śmiechowski and Stangret (2008). The spectra for the samples exposed to sunlight were measured 2 min after the samples were transferred to the dark room. The peak shape drastically changed when the water was exposed to the sunlight. The center of the broad peak decreased and the shoulders at both higher and lower frequencies increased. As a result, the peak shape now approximates to trapezoidal.
The degree of the decrease in the absorbance at the center of the broad band caused by exposure of the water to the sunlight depended on the period of the exposure. The spectrum for the sample exposed to sunlight for 15 min under 3 MJ m -2 was not in an equilibrated state (Fig. 1, spectrum (b)). The spectrum became constant for the samples exposed to the sunlight for more than 30 min under the sunlight strength of 3 MJ m-2 (Fig. 1, spectrum (c)). The same spectrum was observed for the sample exposed to the sunlight of 1 MJ m-2 for 180 min (Fig. 1, spectrum (d)).
When the water was shielded from sunlight irradiation, the shape of the peak in the range 3600 – 3200 cm -1 gradually changed, and was restored in 180 min to the original shape for the water unexposed to the sunlight as shown in Fig. 2. The temperature of the dark room was kept at 288 K for the experiments. For each IR measurement, the sample was taken from the polypropylene vessel placed in the dark room and subjected to IR measurement. The spectrum (a) in Fig. 2 is the same as the spectrum (d) in Fig. 1. In 60 min, the peak became broader (Fig. 2, b), and was restored to the original peak in 180 min (Fig. 2, c).
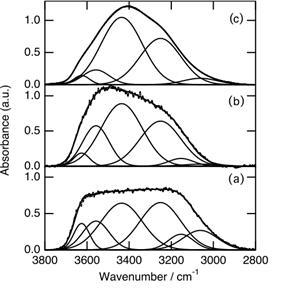
Figure 2: FT-IR spectral changes for liquid water after sunlight was shut off. (a): Water irradiated by sunlight of 1 MJ m-2 h-1 for 180 min (the same as spectrum (d) in Fig. 1). Sample of (a) was stored in the dark room at 288 K for 60 min (b), and 180 min (c). Narrow lines are deconvoluted curves.
The broad peak in the range 2800 – 3800 cm -1 is ascribed to the O-H stretching vibrations, and has several components. Assignments of each component and interpretations were reported in literature in different ways (Freda et al., 2005; Schmidt and Miki, 2007; Venyaminov and Prendergast, 1997; Ohno et al., 2005). In general, an O-H group with a high degree of hydrogen bond order shows a band at a lower wave number. Schmidt and Miki (2007) reported that the ν(OH) band appearing in the range 2800 – 3800 cm-1 has six components that are dominated by differences in their O-H bond lengths. The classification of O-H groups was based on the hydrogen bond ordering parameters (MOH values) proposed by Ohno et al. (2005).
We deconvoluted the O-H vibration band shown in Fig. 2 into six components. The peak positions of the six components (3626, 3557, 3436, 3251, 3154, and 3062 cm -1) were slightly different from those (3629, 3535, 3390, 3246, 3124, and 3045 cm-1) reported by Schmidt and Miki (2007). Deconvolution by use of six components reported by Schmidt and Miki could not reproduce all the spectra obtained in the present study. The percentages of each component for spectra (a) – (c) are summarized in Table 1.
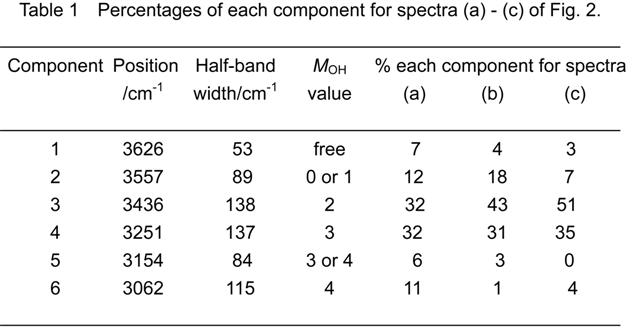
On sunlight irradiation, OH groups of both high hydrogen bond order ( MOH = 4) and less hydrogen bond order (MOH = 0 or 1) increased, and the OH groups of intermediate hydrogen bond order (MOH = 2) decreased (Table 1, spectrum (a), and Fig. 1, spectrum (d)).
In our preceding paper, we reported the formation of clathrate-like structure on irradiation of sunlight or IR light based on the appearance of XRD pattern in a low 2θ range (Shimokawa et al., 2004; Yokono et al., 2004; Shimokawa et al., 2007). It was suggested that the clathrate-like structure includes the clathrate structure StI consisting of two 12-hedra and six 14-hedra, the clathrate structure StII consisting of six 12-hedra and eight 16-hedra, and their fragments (Ripmeester et al., 1987). Each of 14-hedra, 12-hedra, and 16-hedra has two three-coordinated water molecules which correspond to MOH = 0 or 1.
The O-H stretching vibration for MOH = 0 or 1 shows a peak at 3 557 cm-1. Increase in the intensity of the band at 3 557 cm-1 on the sunlight irradiation indicates the formation of 12-, 14-, or 16-hedral structure, which is a unit of clathrate-like structure. Increase in the intensity of the band 3 062 cm-1 indicates the formation of high hydrogen bond cooperativity. The increases in the IR bands of less and high hydrogen bond order components, combined with the XRD results, strongly suggest that clathrate-like structures form on the sunlight irradiation.
On stopping irradiation, restructuring of water occurs; hydrogen bonds repeat cleavage and formation, and gradually approach an equilibrated state at 288 K in the dark. Although the causes of the structural change of water by sunlight irradiation are not certain, the formation of clathrate-like structure on sunlight irradiation has become more plausible by the IR measurements.
Ohno et al. (2005) reported structural change of the water cluster on UV irradiation at 12 K. By UV irradiation, aggregation occurred; the cyclic pentamer was predominantly formed from the monomer and dimer. The formation of hydrogen bonding by irradiation is common for UV and for sunlight. However, the temperature of irradiation was very different. It is noted that structural change induced by sunlight irradiation occurs at room temperature.
Zheng et al. (2006) disclosed the idea that ordering of molecular water accelerates on sunlight irradiation to form the exclusion zone near the surface of liquid water contacting with air (Web ref.1). The process of the formation of the exclusion zone occurs slowly; the width of the zone changes over minutes to hours. It is plausible that the formation of the clathrate-like structure proposed in the present paper is closely related to the exclusion zone.
Conclusions
A clathrate-like structure of liquid water forms on irradiation of sunlight at room temperature, which is reflected on a drastic change in the IR spectrum in the O-H stretching region.
Acknowledgment
We are grateful to Dr. T. Fukuhara and Prof. S. Hara of Hokkaido University for providing us with their experimental facilities, and to Prof. T. Araiso for his encouragement.
References
Freda M, Piluso A, Santucci A, Sassi P (2005). Transmittance Fourier transform infrared spectra of liquid water in the whole mid-infrared region: temperature dependence and structural analysis. Appl Spectros 59: 1155-1159.
Kanno H, Yokoyama H, Yoshimura Y (2001). A new interpretation of anomalous properties of water based on Stillinger’s postulate. J Phys Chem B 105: 2019-2026.
Miyazaki M, Fujii A, Ebata T, Mikami N (2004). Infrared spectroscopic evidence for protonated water clusters forming nanoscale cages. Science 304: 1134-1137.
Ohno K, Okimura M, Akai N, Katsumoto Y (2005). The effect of cooperative hydrogen bonding on the OH stretching-band shift for water clusters studied by matrix-isolation infrared spectroscopy and density functional theory. Phys Chem Chem Phys 7: 3005-3014.
Ripmeester JA, Tse JS, Ratcliffe CI, Powell BM (1987). A new clathrate hydrate structure. Nature 325:135-136.
Schmidt DA, Miki K (2007). Structural correlations in liquid water: A new interpretation of IR spectroscopy. J Phys Chem A 111:10119-10122.
Shimokawa S, Yokono T, Mizuno T, Tamura H, Erata T, Araiso T (2004). Effect of far-infrared light irradiation on water as observed by X-ray diffraction measurements. Jpn J Appl Phys 43: L545-L547.
Shimokawa S, Yokono T, Yokono M, Yokokawa T, Araiso T (2007). Effect of sunlight on liquid structure of water. Jpn J Appl Phys 46: 333-335.
Sloan ED (1998). Clathrate Hydrates of Natural Gases, 2nd edition, Marcel Dekker, New York, 286-386.
Śmiechowski M , Stangret J (2008). ATR FT-IR H 2O spectra of acidic aqueous solutions. Insights about proton hydration. J Mol Struct 878: 104-115.
Venyaminov SY , Prendergast FG (1997). Water (H 2O and D2O) molar absorptivity in the 1000-4000 cm-1 range and quantitative infrared spectroscopy of aqueous solutions. Anal Biochem 248: 234-245.
Yokono T, Shimokawa S, Mizuno T, Yokono M, Yokokawa T (2004). Clathrate-like ordering in liquid water induced by infrared irradiation. Jpn J Appl Phys 43: L1436-L1438.
Zheng JM, Chin WC, Khijinak E, Khijinak Jr E, Pollack GH (2006). Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv Colloid Interface Sci 127: 19-27.
Web References
1. http://uwtv.org/programs/displayevent.aspx?rID=22222 [01-30-2008]
Discussion with Reviewers
Reviewer: This manuscript is a confirmatory contribution to such studies as are already published. But I have a question about a word in the Abstract. Isn’t the word “drastic” too strong a word in this context? Perhaps the word “measurable” would be more appropriate.
Shigezo Shimokawa: The word “drastic” has been replaced by the word “distinct.” Any small change could be measurable with a sophisticated machine. The observed changes are better expressed by the word “distinct.”
Reviewer: There is another tentative suggestion. Although these studies were confined to the laboratory situation, there may be considerations outside of it. There is the influence of sunlight on the waters of the oceans, the seas, and lakes. But movement of the waters and the day and night cycles exclude such permanent changes as indicated in this study. However, the human brain is another matter because here conditions are close to the laboratory experiments described above. Exposure of the head to sunlight causes headaches. These can be attributed to small changes in the structure of the water of the brain as brought about by changes in temperature. Could sunlight also change ‘brain water’ as suggested above?
Shimokawa: Microscopic changes in the water structure exert influences on both macroscopic changes in physical property of water and microscopic changes in the coordination field including local electrostatic field around the water clathrates.
The former influences appear in viscosity, density, and thermodynamic properties such as heat of evaporation and vapor pressure. These changes in the physical properties should affect water movement occurring in nature to some extent. Change in evaporation rate, for instance, could change in the rate of global circulation of water. Change in viscosity could change in fluid dynamics. Increase in viscosity of the blood flowing in brain capillaries may cause headaches.
The latter influences could appear in the activities of brain as the referee suggested. In recent reports, it has been revealed that the state of the water in the extra-cellular space in brain is important in the activities of nerve cell in brain. The water molecules coordinate with the ion-channel membrane, and the coordination controls the function of the brain. Accordingly, the change in the structure of water caused by sunlight irradiation possibly affects the threshold of neuronal firing , which is closely associated with the memory.
Wei-Chun Chin1: Could you speculate the implications of your experimental results on photosynthesis?
Shimokawa: Photosynthesis is a photocatalytic reaction of water with carbon dioxide. It is assumed that the reactivity of water varies with the structure of water; the water of clathrate-like structure has reactivity different from normal water. Accordingly, structural change caused by sunlight irradiation affects the rate of photosynthesis. Irradiation of sunlight on water, however, causes not only structural change but also other changes such as electrostatic properties of water which also affects photosynthesis.
Chin: Do you expect the water structural changes that you observed affect aquatic ecology, especially the microbial community near the water surface layer?
Shimokawa: In microbial structuring of marine ecosystem (F. Azam, F. Malfatti, Nature Rev. Microbiology, Vol. 5, 2007, 782; www.nature.com/reviews/micro), photosynthesis is limited to the near surface layer. Photosynthesis is active when the mixed layer is shallower than the euphotic depth. If the change in water structure induces change in water density, the vertical position of the mixed layer will change. Change in the depth of the mixed layer implies change in the nutrient supply from deep layer to surface, thus inducing change in photosynthetic activities.
Near the surface layer, the aquatic ecosystem has two primary producers; e.g., phytoplankton and heterotrophic bacteria. Phytoplankton produces particulate organic matter from inorganic matter and bacteria produces particulate organic matter from dissolved organic matter. Changes in the water structure may affect solubility of both inorganic and organic matter, which also affects on the rates of primary production. Since the primary production is greatest in near the surface layer, the change in the amount of primary production induces the change in the structure of the aquatic ecosystem.
1 Assistant Professor, School of Engineering, University of California, Merced.
