 Photochemical Removal of Pollutants from Air or Automobile Exhaust by Minimal Catalyst Water
Photochemical Removal of Pollutants from Air or Automobile Exhaust by Minimal Catalyst Water
Sugihara S1 and Hatanaka K2
1Department of Materials Science and Technology, Shonan Institute of Technology, Fujisawa, Japan
2MCM Institute of Technology, Osaka, Japan
Correspondence: sugihara@mate.shonan-it.ac.jp
Key Words: electromagnetic wave, exhaust gas reduction, automobiles, air pollution control, minimal catalyst
Received: 19 June 2009; revised 28 July; accepted 7 August; published 1 December 2009; available online 1 December 2009.
Summary
Minimal catalyst (MICA) water, produced by high-pressure treatment, activates liquids, solids, and gases (including nitrogen in air). By using MICA-treated devices, we have achieved a remarkable reduction in CO2 levels (27%) and the elimination of NOx (40%) from automobile exhaust, possibly as a result of better combustion of gasoline. A typical MICA-treated device consists of an activated copper-plated plastic appliance. Our simple low-cost purification systems operate on the inlet gases, and can be fitted at the front of the engine bay of an automobile. MICA water energy at near-infrared to terahertz wavelengths, the mechanism underlying the reduction in automobile exhaust gases and the deodorization effect, is discussed.
Article Outline
- Introduction
- Methods
- Results and Discussion
- Reduction of Automobile Exhaust Gases
- Conclusions
- Acknowledgements
- References
- Discussion with Reviewers
Introduction
Our system for purifying liquids, solids, and gases utilizes minimal catalyst (MICA) water (Hatanaka, 1991), which is obtained by modifying ordinary water through a special process involving high-pressure treatment. Materials activated by MICA water can behave as catalysts. In MICA water, hydrogen bonds between the molecules are broken, and the substance contains free electrons that are considered to be in a plasma-like state. These can activate other substances, generate electromagnetic waves, and they can transfer energy to other substances through their electrons. The crystal structure, density, and structure of water at various pressures have been discussed by Mishima et al. (1998) and Elington et al. (2001); they reported that water becomes more disordered when compressed, and that directional attractions (hydrogen bonds) combine with short-range repulsions to determine the relative orientation of neighboring molecules. H3O2-and H3O+ are involved in a proton-transfer mechanism for which the free-energy values are 0.23 × 10-23 to 0.48 × 10-23 kJ in the THz region (Novoa et al., 1997). However, these previous discussions do not deal with interactions of the modified water with other substances, as is the case with MICA energy. In our system, the subject is originally water, but devices activated by water with broken hydrogen bonds effect reductions in exhaust gases, deodorization, retention of the freshness of meat and vegetables, and purification of water. In this paper, we focus on the reduction of exhaust gases from automobile and on deodorization effects. The energy stored in MICA water acts a “catalyst” and, as a result, we do not require actual catalyst materials, as the air and/or gasoline is altered by electrons and electromagnetic waves in the near-infrared through THz region that are emitted as MICA energy from an activated device. We propose a concept involving electromagnetic waves that is new to the chemistry and physics of water, and we describe several devices that we have developed as applications of this basic concept.
Methods
Computations for Water Molecules
First, a calculation based on first principles is performed using the molecular-orbital method. A model cluster consisting of two molecules of water is shown in Figs. 1; this shows two electrons in two H2O molecules. Hydrogen bonds are shown by the dotted lines. Fig. 1(a) shows a plot of the bond-overlap population (BOP) versus the change in distance between the molecules Δx (Å) in the ground state. Fig. 1(b) figure shows a plot of the BOP versus the number of excited electrons in hydrogen bonds. The degree of covalency of the hydrogen bonds is less than that of other O-H bonds. Furthermore, it is interesting to note that on increasing the number of excited electrons, the Coulombic repulsion becomes smaller, and the molecular structure of water results in a lower energy state. According to Muro-oka’s thesis (graduation thesis, Hyogo University of Teacher Education, 1990; unpublished), charges on oxygen in an O-H hydrogen bond are attracted by hydrogen. From the results of these calculations, we propose the existence of free electrons in a plasma-like state in MICA water.
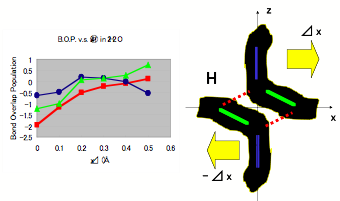
Figure 1a: Bond Overlap Population in Ground State
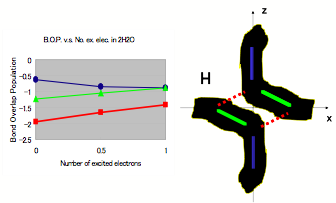
Figure 1b: Bond Overlap Population in Excited State
These plasma-like electrons contain energy that can be transferred by radiation, and when MICA water approaches the surface of a solid or liquid, atoms of the substance absorb energy from the MICA water through radiation or, if contact occurs, by conduction. The electromagnetic energy is the range from the near infrared through to the THz region. Essentially MICA energy is soft, as we show later. Perturbation of the electromagnetic wave of MICA electrons may initiate the transition. The energy quantum for transition from a level m to n can be formulated as follows:
[1] h ωn = Em– En
Therefore,transition to a higher level accompanies the absorption of a quantum. Thus, a substance can be energized by MICA electron through radiation (electromagnetic waves) or by conduction.
Spectroscopic Analysis of Water, and Measurement of Automobile Exhaust Gases and Volatile Organic Compounds
MICA water can be produced from tap water or any other type of water by high-pressure treatment (0.5 to 3 MPa). During this process, hydrogen bonds between the water molecules gradually break. MICA water and MICA-activated substances were analyzed by Fourier-transform IR (FTIR), and the dielectric parameters were measured by terahertz time-domain spectroscopy (TDS).
An automobile (Nissan Cube, UA-BZ11) fitted with the device and another identical model lacking the device were both subjected to idling tests for one hour in vinyl tents. Exhaust gases were analyzed with a FERRER F16P 5 Gas Analyzer (GXT International, NY, USA) in real time. The humidity in the tents was measured by using a standard hygrometer. One end of a vinyl hose was connected to the exhaust pipe of an idling automobile and the other end was immersed in water in a pail; the water in the pail was subsequently analyzed by a standard method (JIS-K0102 45.2). Volatile organic compounds (VOCs) were also measured by using a flame-ionization detector (Horiba FV-250).
The effects of MICA-activated devices on the fuel consumption of vehicles were assessed by driving a car in a city for a certain period, and monitoring the fuel consumption day by day. The deodorization effects of MICA-activated devices were also examined.
Results and Discussion
The frequency of MICA electrons in the plasma-like state is calculated by analogy with a plasmon in a metal surface. The plasma frequency in our system is given by the expression ωp2 ≈ 4π n e2/(m* ε∞), and this calculation shows it to be equal to be approximately 1 THz (ε∞ is 4 according to Figure 2; other symbols are used conventionally: m* = m0 of a free electron, n = number of electrons).
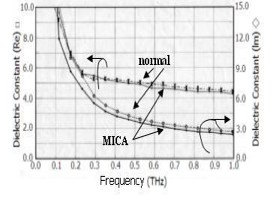
Figure 2: Complex dielectric constants of MICA water and normal mineral water; the upper two curves show the dielectric constant (Re: real part) on the left-hand axis, and lower two curves show the dielectric constant (Im: imaginary part) on the right-hand axis. Re (□) and Im (◊) of MICA water, and Re (×) and Im (o) of normal mineral water.
Recent Fourier-transform IR (FTIR) spectroscopic studies in the mid-IR region (Millo et al., 2005) have shown that breakage of hydrogen bonds generates at least two kinds of water species: molecules with broken O-H bonds, and molecules with broken lone-pair electrons. A spectral analysis of water in the mid-IR region (360-4000 cm-1, equivalent to 11-120 THz) showed that the enthalpy difference for the hydrogen bond obtained through OH-stretching analysis corresponds with the value calculated from bending data (Freda et al., 2005). Several recent studies have been conducted by using instrumentation with nonlinear optics. THz waves were generated by stimulated polariton scattering through phonon-polariton interaction in a lithium niobate (LiNbO3) crystal as a result of the action of the laser (Kawase et al., 1996). Parametric oscillation (0.3-2.5 THz) has also been generated by using a LiNbO3 crystal. (Kojima et al., 2003). Far-IR phonon-polariton dispersion has examined by means of TDS studies on the ferroelectric memory material Bi4Ti3O12. The coupling of propagating electromagnetic waves to polar optical phonons has recently been a popular topic of research. To explain the relationships underlying nonlinear dispersion, Kojima took account of phonon-polariton propagation in crystals, as determined from the phase delay. The transmittance of energy to atoms in solids, gases, and liquids occurs through interaction with electromagnetic waves of MICA-electrons. Let us consider a substance to which energy has been imparted by MICA electrons. The real part of the relative permittivity εr will change. The dielectric loss (tan δ) is introduced, and the loss factor (εr‘ ) is given as follows:
[2] εr‘= εr tanδ
Furthermore, εr‘ and εr are often described in terms of a complex number:
[3] εr* = εr – iεr‘
where εr* is the complex relative permittivity. The real part εr is the relative permittivity (dielectric constant Re) and the second (imaginary) term of the complex value (dielectric constant Im) corresponds to the loss factor.
The fact that there is a decrease in εr and/or εr* means that the loss factor and/or the relative permittivity decreases; as a result, the energy of MICA electron will be effectively transmitted to other substances. The results are shown in Fig. 2: the values of εr (Re) for normal water and MICA water at 1 THz are 4.5 and 4.2, respectively. Furthermore, the values of εr* (Im) are 2.7 and 2.4 for normal water and MICA, respectively. These facts show that, because of its lower dielectric constant εr‘, MICA water is electrically more conductive than conventional mineral water, and the lower value of εr* also shows a smaller loss factor or lower energy loss from the medium leading to the greater phase velocity of MICA electrons than that of normal water:
ν = c/√ε
where c is the velocity of light, and ε is the dielectric constant. The energy of an electron will increase by about 5-6% as the result of the MICA processes. This has been demonstrated by means of Raman spectroscopy, which showed that the energy was shifted to values about 5% higher than that in untreated substances. The intensity of the peak was also higher in the MICA-treated plastic. The difference in energy between the spectrum of the control sample and that of the activated one is calculated to be about 0.6 meV (0.15 THz), although MICA energy is essentially weak. We measured the frequency dispersion of the reflected electromagnetic wave in the THz region produced by MICA electrons. The substances to which MICA-energy was imparted were aluminum metal, Portland cement, and an Fe3O4-based magnet, and these were analyzed by THz spectroscopy, as shown in Fig. 3. Both treated and untreated samples of aluminum showed almost the same results. However, THz waves were reflected more by MICA-treated cement than by conventional cement. The magnet consisted of an alloy and Fe3O4, and was therefore partially ceramic in nature, so its reflectance was intermediate between that of cement (which is 100% ceramic) and that of aluminum (which is 100% metal). THz radiation cannot usually penetrate metals or water. In general, THz electromagnetic waves are reflected by the electrons in a metal that has a plasma frequency. Terahertz surface plasmon (TSP) propagation on a metal sheet has been reported by Jeon et al., (2006) in discussing TSP pulse propagation dispersion.
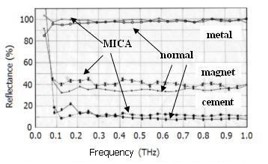
Figure 3: THz frequency dependences of the reflectance of normal and activated samples of a metallic aluminum plate, Portland cement, and a magnet; Al: normal (□) and MICA-treated (o); magnet: normal (☆) and MICA-treated (×); and Poland cement: normal (□) and MICA-treated (※).
Reduction of Automobile Exhaust Gases
Catalytic converters in automobile exhaust gas systems function only after combustion of gasoline in the engine. Pt, Pd, and Rh are all used as catalysts to control emissions of NOx in Japan (1997), but few reports of data obtained by using real automobiles are available. Recently, there has been a trend to install catalytic converters closer to the engine, which ensures that the catalyst functions immediately when the engine starts. The Toyota and Daihatsu group has developed new materials comprising perovskite oxides, such as LaFe0.57Co0.38Pd0.05O3 (Nishihata et al., 2002). Although these super-intelligent catalysts, in which Pd is regenerated, represent the state of the art for petrol engines, the catalysts still use precious metals, and perovskite oxides are complicated materials. In terms of other exhaust gases, Tanabe et al. (2002) reported that a catalyst consisting of Pt supported on a thermally stable oxide (zeolite and CeO2-ZrO2) on which hydrocarbons become adsorbed and migrate to the Pt surface. Previously, oxides such as Co3O4-NiO-Cr2O3 have also been reported to be active catalysts, and Kalantar Neyestanaki et al. (1995) studied the catalytic combustion of propane and natural gases on metal oxides and platinum-activated metal oxides. Below, we describe the applications of MICA in photochemical removal of pollutants from automobile exhausts and in deodorization of air. Our pollution-reduction system for automobile exhaust gases has two distinct characteristics: the first is that it does not require any particular catalyst, and the second is that it is positioned ahead of the engine (on or inside the air filter), resulting in a change in the properties of the air taken in by the engine. We found that a Cu-plated plastic (CPP) device (5×5 cm) activated by MICA energy reduced the emissions of CO2 and NOx from an automobile. In separate experiments, we also found that a MICA-activated fluorescent lamp (FLL) deodorized organic solvents in an automobile paint plant and in a perfume company.
Activated nitrogen (N2*) in air activated by MICA device is introduced into the engine through the intake. The various hydrocarbons (HC) in the gasoline are burned by the air. The bulk of typical gasoline consists of hydrocarbons with between 5 and 12 carbon atoms per molecule, for example:
C8H18→CnHm , CO2, CO, H2O, NOx
If we designate hHC as higher hydrocarbons, lHC as lower hydrocarbons, and (hHC)N2* as N2– activated hydrocarbons, then:
i) lHC + hHC + O2 + N2 →(hHC)N2* + lHC + CO + CO2 + NOx + H2O
ii) (hHC)N2* + O2 → (C or lHC) + N2 + H2O
iii) (hHC)N2*+ CO → (C or lHC)+ N2 + O2 + H2O
iv) (hHC)N2* + CO2 + NOx → (C or lHC) + N2 + O2 + H2O.
In our system, N2* can form higher hydrocarbons (hHC)N2* instead of NOx, and these compounds react with CO2, NOx etc. ,when burned to form lHC; this is followed by the formation of N2 and H2O. In other words, both reduction and oxidation occur in the engine space. This is a much simpler way of reducing emissions of CO2 and NOx than, for example, the method proposed by Nishihata et al. (2002). As shown in Table 1, levels of CO2 were reduced by almost 27%, and those NOx of were reduced by 40%. Furthermore, the humidity inside the tent was 65% compared with 50% in the tent for the automobile without the MICA device. The total nitrogen content of the water in the pail was 4.1 mg/L for the automobile with the CPP device and 7.4 mg/L for automobile without the device. Those results suggest that gasoline was burned nearly completely in the engine of the automobile fitted with the device. Table 2 showed data for a second vehicle. Remarkable reductions of CO2 and other gases were also observed in this case. We can therefore air pollution by reducing automobile exhaust gases by using a MICA device with electromagnetic energy.
Table 1(a): Results of testing a CPP device in a tractor.
| CO2 (%) |
O2 (%) |
CO (%) |
HC (ppm) |
NOx (ppm) |
|
| Normal | 4.9 | 14.9 | 0.20 | 51.9 | 22.8 |
| CPP | 4.5 | 15.0 | 0.14 | 52.0 | 21.8 |
| Activated CPP |
3.6 | 16.8 | 0.08 | 19.2 | 13.6 |
| Change(%) | -26.5 | 12.8 | -60.0 | -63.0 | -40.3 |
Table 1(b): Results of testing a CPP device in second vehicle.
| CO2 (%) |
O2 (%) |
CO (%) |
HC (ppm) |
NOx (ppm) |
|
| Normal | 4.0 | 15.4 | 0.21 | 42.0 | 22.0 |
| CPP | 3.4 | 16.9 | 0.17 | 22.0 | 15.0 |
| Activated CPP | 2.8 | 17.5 | 0.08 | 12.0 | 1.00 |
| Change (%) | -30.0 | 13.6 | -62.1 | -71.0 | -95.4 |
Deodorization
The odor of animals can be reduced by the use of MICA-activated fluorescent lamps and MICA-activated water sprays. In an automobile painting company, volatile organic compounds (VOC) were reduced by 50-90% on the basis of xylene, 25-40% on the basis of ammonia, and by 60% on the basis of heavy and higher carbon compounds.
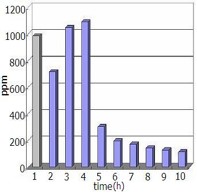
Figure 4: Measurement of VOC emissions in automobile exhaust fumes before and after installing a Cu-plated plastic device activated by the MICA process. In the first two hours after installation of the device, adhered soot or microparticles in the engine are eliminated by the MICA-treated air. A steady state exists subsequently.
Conclusions
By using MICA-treated devices, we have achieved a remarkable reduction in CO2 levels and the elimination of NOx as a result of improved combustion of gasoline. We also succeeded in deodorization and reduction of VOCs. The underlying concept of the process is related to the presence of electromagnetic energy in substances activated by MICA energy. We have also identified effects of MICA on deodorization, and fuel consumption; we will describe these in a subsequent paper.
Acknowledgements
We thank Mrs. K. Fukushima, N. Tetsuka, and Ms. H. Furui at the Terahertz project group of Tochigi Nikon for recording the THz spectra, Dr. K. Miura at Canon Co. Ltd for computational discussions, and Mrs. T. Iino, K. Hokora, and Y. Nagasaka for acquiring data on fuel consumption in everyday use.
References
Hatanaka K (1991). EP 0421563; (1990). JP 1786552; (1993). US 5034138; (1991).
Mishima OR, Stanley HE (1998). The relationship between liquid, supercooled and glassy water. Nature 392: 164–168.
Elington JR, Dehencedetti PG (2001). Relationship between structural order and the anomalies of liquid water. Nature 409: 318–321.
Novoa JJ, Mota F, Perez del Valle C, Plana M (1997). Structure of the first solvation shell of the hydroxide anion: A model study using OH–(H2O)n (n = 4, 5, 6, 7, 11, 17) clusters. J. Phys. Chem. A 101: 7842–783.
Millo A, Raichlin Y, Katzir A (2005). Mid-infrared fiber-optic attenuated total reflection spectroscopy of the solid–liquid phase transition of water. Appl. Spectrosc. 59: 460–466.
Freda M, Pilluso A. Santucci A, Sassi P (2005). Transmittance Fourier-transform infrared spectra of liquid water in the whole mid-infrared region: temperature dependence and structural analysis. Appl. Spectrosc. 59: 1155–1159.
Kawase K, Sato M, Taniuchi T, Ito H (1996). Coherent tunable THz-wave generation from LiNbO3 with monolithic grating coupler. Appl. Phys. Lett. 68: 2483–2485.
Kojima S, Tsumura N, Takeda WM, Nishizawa S (2003). Far-infrared phonon–polariton dispersion probed by terahertz time-domain spectroscopy. Phys. Rev. B: Condens. Matter Mater. Phys. 67: 035102.
Nagai N (2005). Analysis of the industrial materials by THz spectroscopy. Rev. Laser Eng. 33: 848–854 (in Japanese).
Nagai N, Fukasawa R (2004). Abnormal dispersion of polymer films in the THz frequency region. Chem. Phys. Lett. 388: 479–482.
Jeon T-I, Grischkowsky D (2006) THz Zenneck surface wave (THz surface plasmon) propagation on a metal sheet. Appl. Phys. Lett. 88: 061113.
Ueda K (Ed.) (1997), Handbook of Energy and Resources, Japan Society of Energy and Resources, Tokyo, pp. 1229–1231 (in Japanese).
Nishihata Y, Mizuki J, Akao T, Tanaka H, Uenishi H, Kimura M, Okamoto T, Hamada N (2002) Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418: 164–166.
Tanabe T, Hatanaka T, Tuji M, Shinjo R (2002). NOx selective catalytic reduction over Pt supported catalyst promoted by zeolite and CeO2–ZrO2. R & D Rev. Toyota CRDL: 37(3), 25–30.
Kalantar Neyestanaki A, Lindfors L-E (1995). Catalytic combustion of propane and natural gas over silica-fibre supported catalysts. Combust. Sci. Technol. 110: 303–320.
Discussion with Reviewers
Anonymous Reviewer: Why are the catalysts applied to automobiles called “Catalyst Water?”
Sunao Sugihara: The energy stored in MICA water acts as a “catalyst and, as a result, we do not require actual catalyst materials because the air and/or gasoline is altered by electrons and electromagnetic waves in the near-infrared through THz region that are emitted as MICA energy from an activated device. In our system, the subject is originally water, but devices that have been activated by water with broken hydrogen bonds effect not only reductions in exhaust gases, but also deodorization, retention of the freshness of meat and vegetables, and purification of water.
Reviewer: The definition of MICA could be developed.
Sugihara: MICA is defined as Minimal Catalyst water and is obtained by modifying ordinary water through a special process that involves high-pressure treatment. MICA water can be produced from tap water or any other type of water by high-pressure treatment (0.5 to 3 MPa). During this process, hydrogen bonds between the water molecules gradually break, and the substance contains free electrons that are considered to be in a plasma-like state (see Rev.2-3). Materials activated by MICA water can behave as catalysts by transferring the energy of the water with the broken hydrogen bonds.
Reviewer: Your statement — “MICA water can activate solids, liquids or gases” — could be developed in more details.
Sugihara: From the results of computational calculations, we propose the existence of free electrons in a plasma-like state in MICA water containing broken hydrogen bonds. The altered molecular structure of water results in a lower energy state and the electrons still have spin
(magnitude of (1/2)ħ, ħ = h/2π, where h is Planck’s constant
These plasma-like electrons (collective electrons) contain energy that can be transferred as radiation, and when MICA water approaches the surface of a solid or liquid, the atoms of the substance absorb energy from the MICA water through radiation or, if contact occurs, by conduction. The nature of this radiation is discussed below. The plasma frequency in our system is given by the expression ωp2 ≈ 4π n e2/(m* ε∞), and this calculation shows it to be equal to be approximately 1 THz (ε∞ is 4; other symbols are used conventionally: m* = m0 of a free electron, n = number of electrons).
Reviewer: The difference between “MICA water” and “ MICA energy” is not clear.
Sugihara: Basically they are same; “MICA water” is used when water itself is discussed, and “MICA energy” is used when space and/or a substance are apart from the water. In general, I tried to use the terminology “MICA energy.”
Reviewer: The concept of “electromagnetic waves” could be developed in more details.
Sugihara: The concept of “electromagnetic waves” corresponds to near-infrared (1015 Hz) through THz (1012 Hz); in the latter case the wavelength is in the order of μm. Electromagnetic waves in the THz region therefore have wavelengths that are between those of light and those of radio waves. This energy can resonate with other substances, for instance, the hydrogen bond of –COOH (the carboxyl radical) has a wavelength of 3 × 10–3 m, the rotation of water molecules has a wavelength of 2 × 10–4 m, and the angle between the atoms in NH2• (the amino radical) has a wavelength of 3 × 10–6 m, and these energies of radicals and molecules are resonant with MICA energy in the near-infrared to THz electromagnetic regions.
Reviewer: How did you assess the reduction of the odor level?
Sugihara: Volatile organic compounds (VOCs) were measured by using a flame-ionization detector (Horiba FV-250). Assessments referred to legal regulations are very difficult because these differ from area to area in different industries and for different substances; for example, in a car painting company, 900 ppm is one standard for paint on the basis of xylene or toluene; furthermore, this limit value will be changed soon. It is better, therefore, to compare our developed devices or new methods for reduction of VOC levels with existing ones. In our research, we achieved a reduction of 50–90% on the basis of xylene and 25–40% on the basis of ammonia. Besides the instrumental analysis, the odor was assessed subjectively by government-certified odor testers.
The mechanism for odor reduction in the car-painting company appears to be dissociation of methyl radicals bound to the benzene ring, and proposals for the mechanisms of chemical reactions in the engine bay of an automobile are described in the manuscript for the case of exhaust gases.
Reviewer: How were the reduction in CO2 levels and the elimination of NOx determined?
Sugihara: A Ferrer F16P 5 Gas Analyzer (GXT International, NY, USA) was used for the quantitative analysis. Exhaust gases from the automobile were measured in real time in the tent. Besides the reported research, we performed measurements with the same instrument after driving for a certain period before and after attaching the device; these data are not reported in the manuscript, but showed almost the same trends. The mechanism for the reduction of exhaust gases from the automobile is indicated in the text.
