SPECIAL EDITION
Water and Environment
Interference of Water and Environmental Variables on Lead Chloride Toxicity in Artemia salina Model
Mohammad SN1, Pinto AAG1, Nagai MYO1, Coimbra EM1,
Suffredini IB1, Peres GB1, Bernardi MM1, Bonamin LV1 *
1] Graduate Program in Environmental and Experimental Pathology – Universidade Paulista (UNIP). Rua Dr. Bacelar, 1212 – 4th floor. ZIP-CODE: 04026-002 – São Paulo, São Paulo, Brazil.
Authors’ e-mails: Mohammad SN (suhamnowrooz@gmail.com); Pinto AAG (andreia.agpe@hotmail.com), Nagai MYO (mynagai@yahoo.com.br), Coimbra EN (ednarcoimbra@hotmail.com), Suffredini IB (ivana.suffredini@docente.unip.br), Peres GB (giovani.peres@docente.unip.br), Bernardi MM (maria.bernardi@docente.unip.br), Bonamin LV * (leoni.bonamin@docente.unip.br; leonibonamin@gmail.com).
Keywords: ecotoxicology, microcrustacean, circalunar, water agitation, succussion
• Received: October 30, 2021
• Revised: December 15 2021
• Accepted: February 21, 2021
• Published: April 25, 2022
Highlights
Low concentrations of lead chloride increase Artemia salina cysts’ hatching and nauplii mobility.
This effect is influenced by the moon phase and is evident during the full moon.
The addition of succussed water into the seawater impaired this effect.
The circalunar cycle and products of water agitation modulate the adaptive control of hatching in Artemia salina exposed to lead chloride.
Abstract
An experimental model was established based on the premise that environmental and water conditions can influence the effects of toxic agents on living beings. To verify if circalunar phases and water agitation can modify the toxicity of lead chloride on in vitro Artemia salina, cysts were exposed to seawater containing 0.04% of lead chloride (equal to EC10 or 10% effective concentration) in 96-well culture plates. Thirty-six experimental repetitions were performed in four series to observe the possible effects of adding stirred water, the so-called succussed water, and of moon phases on toxicity. The cysts were recorded after 48 hours using a digital microscope (1000x magnification) to identify hatching percentage, nauplii viability and mobility. The exposure of cysts to lead chloride (PbCl2) led to an increase in the hatching rate, and this was more evident during the full moon. Addition of succussed water into the seawater medium reduced this effect to baseline levels. An increase in mobility was seen in nauplii born from exposed cysts during the full moon, but this effect was not affected by treatment with succussed water. The organization of nano and microbubbles generated after the succussion of water is supposed to be related to this protective effect. In conclusion, environmental factors, such as the circalunar cycle and products of water agitation, can modulate the adaptive control of hatching in Artemia salina exposed to lead chloride at EC10.
Introduction
Lead is a toxic heavy metal commonly used in civil construction, steel batteries, and electrical cables (Wani et al. 2015; Obeng-Gyasi, 2019). It is one of the most common heavy metals in the environment, thus, recycling, and conscientious use of the element must be done judiciously (Yuan et al. 2016). When individuals are exposed to lead, the main route of exposure is the respiratory and gastrointestinal tract; its toxicity mainly affects the nervous and hematopoietic system, including aspects related to genotoxicity (Banfalvi et al. 2012; Yuan et al. 2016). Inorganic lead is not metabolized but distributed and deposited in soft and hard tissues and then directly excreted, since this metal does not undergo biotransformation. After reaching hard tissues such as bones and teeth, lead remains stored for a long time. In the central nervous system, lead poisoning occurs after damage to the blood-brain barrier (Souza et al. 2018).
Recently, sustainable resources have been sought for the removal of solid particles (Kalogerakis et al. 2015; Stanovych et al. 2019) and heavy metals in suspension from water (Orden et al. 2021), including lead (Tang et al. 2020). The insertion of nanobubbles in a specific volume of water has been seen as an excellent debugging resource, both alone (Agarwal et al. 2011) or in association with other resources (Atkinson et al. 2019; Wu et al. 2021). The possible applications include alleviation of heavy metal toxicity in aquatic organisms (Fan et al. 2020).
On the other hand, Artemia salina, also known as brine shrimp, has been used as a sensitive and low-cost model for toxicity studies (Ntungwe et al. 2020). Artemia salina cysts are formed by a rigid, semi-permeable shell, with morphology adapted to resist UV radiation, which gives the embryo a first line of defense (MacRae, 2016; Tan and MacRae, 2018). Trehalose is a structural shell sugar responsible for membrane and protein preservation during desiccation. The sugar substitutes the water molecules and contributes to the vitrification observed in the cysts. The vitrification and the presence of certain embryogenic proteins, such as heat shock proteins (HSP), and chaperones allow the cyst to survive for long periods in quiescence, or diapause. HSPs help newly synthesized proteins to avoid irreversible denaturation during stress. Such effects are present at various levels of the phylogenetic scale. All these structures within cysts work together under stress and provide them with resistance to different aggressor stimuli such as extreme temperatures, radiation, desiccation, anoxia, oxidation, and others (MacRae, 2016; Tan and MacRae, 2018). This degree of tolerance gives Artemia spp. a remarkable characteristic, which defines it as an excellent experimental model for studies on bioresilience (Pinto et al. 2021).
Nanosized superstructures are produced after vigorous shaking and gas incorporation of aqueous solutions and can be identified by nuclear magnetic resonance (Demangeat, 2015; Pan et al. 2016). Thus, we questioned whether the insertion of vigorously succussed water into the aquatic microenvironment could modify the effects of lead chloride (PbCl2) on the hatching of Artemia salina cysts as a model of bioresilience, according to previous studies performed by our group (Pinto et al. 2021), and whose results could be easily transported to field conditions, given the natural movement of seawater.
Among several environmental parameters likely to interfere in the toxicity process, the circalunar variations on the intoxication of cysts were evaluated by 36 experimental repetitions, carried out over a month, under controlled temperature, light, and humidity conditions. The inclusion of this variable in the study was motivated by recent literature, which shows the characterization of biochemical oscillations in different metabolic functions according to circadian and lunar cycles, both in aquatic and terrestrial species (Andreatta and Tessmar-Raible, 2020; Kaiser et al. 2016; Oldach et al. 2017; Payton and Tran, 2019; Schenk et al. 2019; Ugolini et al. 2016).
Material and Methods
Preparation of Succussed Water
Water samples subjected to agitation (succussion) were prepared on the eve of the experiments. An aliquot of 20 ml of pure, sterile water was obtained by the Direct-Q3 purification system, using SmartPak Direct Q3 and Biopak filters (MERCK – MILLIPORE, Darmstadt, Germany). Then, it was sterilized by autoclavation and filtered through a 0.22 µm mesh filter (MILLIPORE, Burlington, USA) right before its use to avoid any microbial contaminant. After the sterile bottles were closed inside the laminar flux hoven, they were subjected 100 times to automatic vertical succussion in a mechanical arm (Denise® – AUTIC, São Paulo, Brazil). The control was made with purified sterile water not submitted to succussion. All preparation, storage procedures and handling were performed under sterile conditions in a laminar flow hood.
Preparation of Artificial Marine Water
One liter of distilled water and 30 g of sea salt were used for each experimental week, with constant stirring with a magnetic stirrer. Then, 50 mg of yeast (Saccharomyces cerevisiae) was added for every 10 ml of artificial seawater as food for newborn nauplii to avoid confounding starvation variables concerning toxicity (Pillard and Tapp, 2021).
Pilot Experiment to Define PbCl2 EC10
The effective concentration capable of producing 10% lethality (EC10) in nauplii exposed from cysts’ hatching was determined as described. EC10 was chosen to mimic the ordinary conditions of environmental contamination, in which small concentrations of toxic agents are found in water.
Thus, 75 g of cysts were immersed in 200 ml of artificial seawater for hatching, being kept at constant temperature (23 to 24oC), humidity (60 to 65%), and artificial light (1700 lm) over 48 hours for hatching induction. Then, 5 to 8 nauplii were transferred to a 96-well plate, then, PbCl2 was added to each 12-well row in a half-fold dilution, starting from 1M. The 10% viability was determined from a linear curve obtained from the live/dead nauplii ratio for each tested concentration, considering the total population of nauplii obtained from the sum of the 12 wells. The control of the environmental conditions was essential, given the system’s sensitivity to the variables under test (Lin et al. 2021).
Finally, the calculated EC10 was equivalent to 4×10-4M or 0.04%. EC10 was the concentration used to challenge the cysts for hatching in the main experiment.
Artemia Salina Cysts’ Hatching as a Function of Moon Phases
Ethics. The present study did not use vertebrate animals. According to national (CONCEA) and international (EU Directive 2010/63/EU for animal experiments) legislation, analysis of the project by the ethics committee was unnecessary in this case.
Study design. The study was conducted in four one-week long series, between November 26th, 2018, and March 1st, 2019, with each week corresponding to a specific phase of the moon. During one week, three experimental series were performed in triplicate. Thus, nine microplates were evaluated for each moon phase, totaling 36 microplates. Each series was scheduled to cover the whole moon phase period (Figure 1).
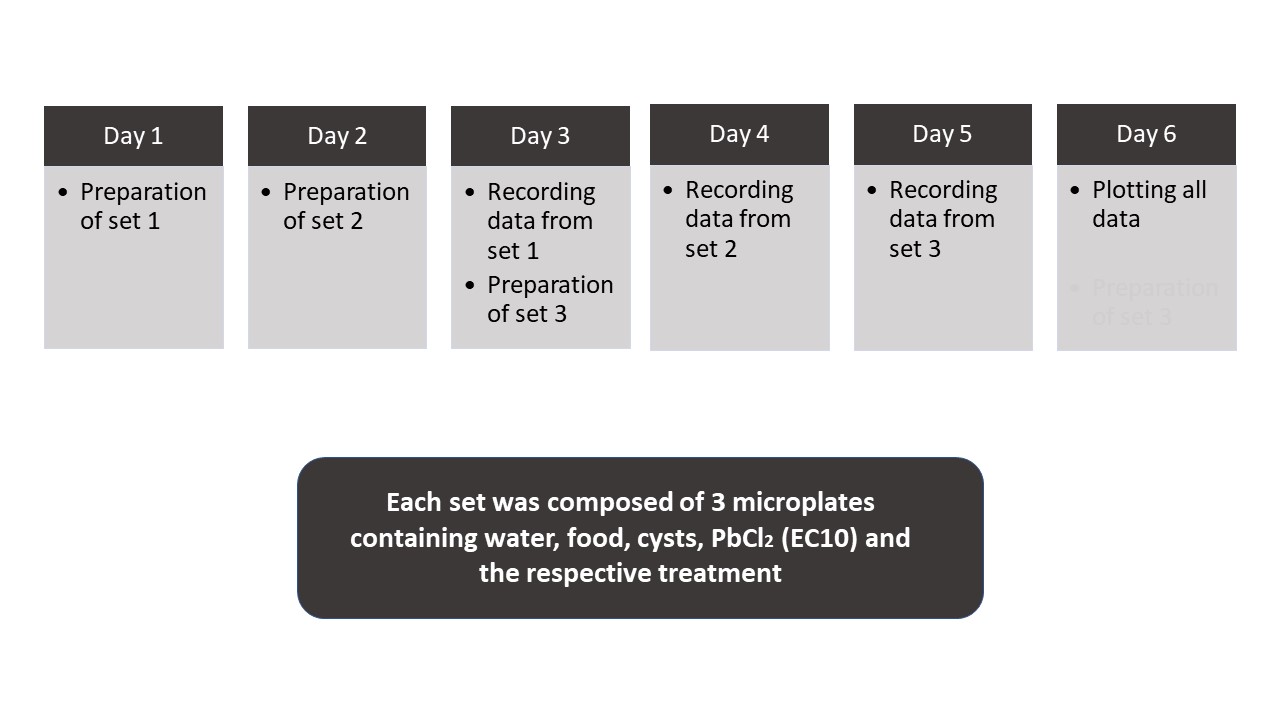
Figure 1. Diagram presenting the timeline of the experiments (experimental design) for each moon phase.
Three experimental groups were organized:
Unchallenged group (cysts born in seawater with food, no other interference)
Water or challenged control group (cysts born in seawater with food containing 0.04% PbCl2 and treated with non-succussed water)
Succussed or experimental water group (cysts born in seawater with food containing 0.04% PbCl2 and treated with succussed water).
The control group supported evaluating the effects caused by the PbCl2 exposure under the same osmolarity conditions as the experimental group. The unchallenged group worked as the baseline for all parameters.
About 5 to 8 cysts were inserted per well to induce hatching. Cysts were suspended in 100 µl of artificial marine water containing food. Thus, 10 µl of the respective treatment (pure water or pure succussed water) and 90 µl of an aqueous solution of PbCl2 were added, reaching the EC10 after considering the dilution factor of each well. Thus, the final volume in each well was 200 µl.
For each microplate, a row of 12 wells was used for each experimental group.
The evaluation of the hatching rate was carried out after 48 hours using a digital magnifying microscope (Digital Microscope with Zoom 1000x R Camera 2.0 Mega Pixels USB 6 LEDs, Beijing, CHINA). Data was recorded using image recording software (AMCAP, Beijing, CHINA). Nauplii mobility was also evaluated at this time.
In short, the following parameters were recorded per well:
Number and stage of nauplii born at all stages (alive, dead, and umbrella stage)
Number of unhatched cysts
13-second video recording for further analysis of mobility
The recorded data were further analyzed, considering each microplate, so that the sum of unhatched cysts and each stage of nauplii obtained from the 12 wells of the same row was considered the experimental unit, resulting in an N = 9 per moon phase. Each row corresponded to a specific treatment.
The working schedule allowed the analysis of 650 to 700 nauplii per moon/treatment or about 8000 to 8500 nauplii in the entire experiment.
Mobility of nauplii. All nine plates used during the full moon were selected for the mobility analysis. Images were recorded in AVI files, and the mobility of all nauplii in each well was automatically calculated by Image J software, Plug-in Trackmate. For this analysis, among the plates observed during the full moon, three plates observed on the same day were selected because they express the most representative results concerning the average of the nine repetitions. The program was adjusted for color reading in RGB, and the yellowish coloration of stage V nauplii was used as a reference to identify the mobility points. All images were transformed to 8-bit binary resolution and the analysis method chosen was Phansalkar. After the image analysis using the Trackmate Plug-in, the data generated in the Track Statistics spreadsheet were compiled into an Excel® spreadsheet. Statistical analysis was performed using the parameters Track Stop and Track Displacement, in which pixel values were normalized as a percentage to the mean of the unchallenged group and then graphically expressed. This method was previously described elsewhere (Pinto et al. 2021).
Statistical Analysis
The calculation of relative hatching was normalized by the mean of the unchallenged group, using the following formula:

Two-factor analysis of variance (ANOVA) with mixed models was used to investigate the putative interference of treatments and/or moon phases on PbCl2 challenging outcomes, emphasizing the hatching rate of the cysts.
To evaluate the effect of the treatment on the locomotion rate of nauplii and the number of stops, a one-way Analysis of Variance (ANOVA) was used. The results were expressed as the number of pixels per nauplii, per plate, in each treatment.
In both cases, the comparison between means was made by the Tukey test. Observed power was also calculated as an additional analysis.
The assumptions of the models were investigated, respecting normality and homogeneity. When necessary, outliers were removed, using the Tukey criterion. In this case, the rule was applied if the values exceeded the limits of 1.5 times the interquartile range above the third quartile or 1.5 times the interquartile range of the group below the first quartile. The level of significance α was set at 5%, considering the effects obtaining a chance of error p<0.05 as significant.
The programs used for statistical analysis were: Statistica v 12.0; IBM SPSS v 21.0; Excel 2019 v 16.0.6742.2048; GraphPad Prism 8.0.1.
Results
Analysis of Cysts’ Hatching as a Function of the Moon Phase
When analyzing the treatments separately, regardless of the moon phase, the cysts’ hatching rate was higher after the challenging with lead chloride EC10, as seen in the water control group (treated with non-succussed water and challenged) than in the other conditions (p=0.0055, Figure 2). By observing the effect of the different phases of the moon independent of the treatment, there was a reduction of the hatching rate during the crescent moon, in comparison with the other moon phases (p=0.0001, Figure 3). The peak of hatching was seen during the full moon.
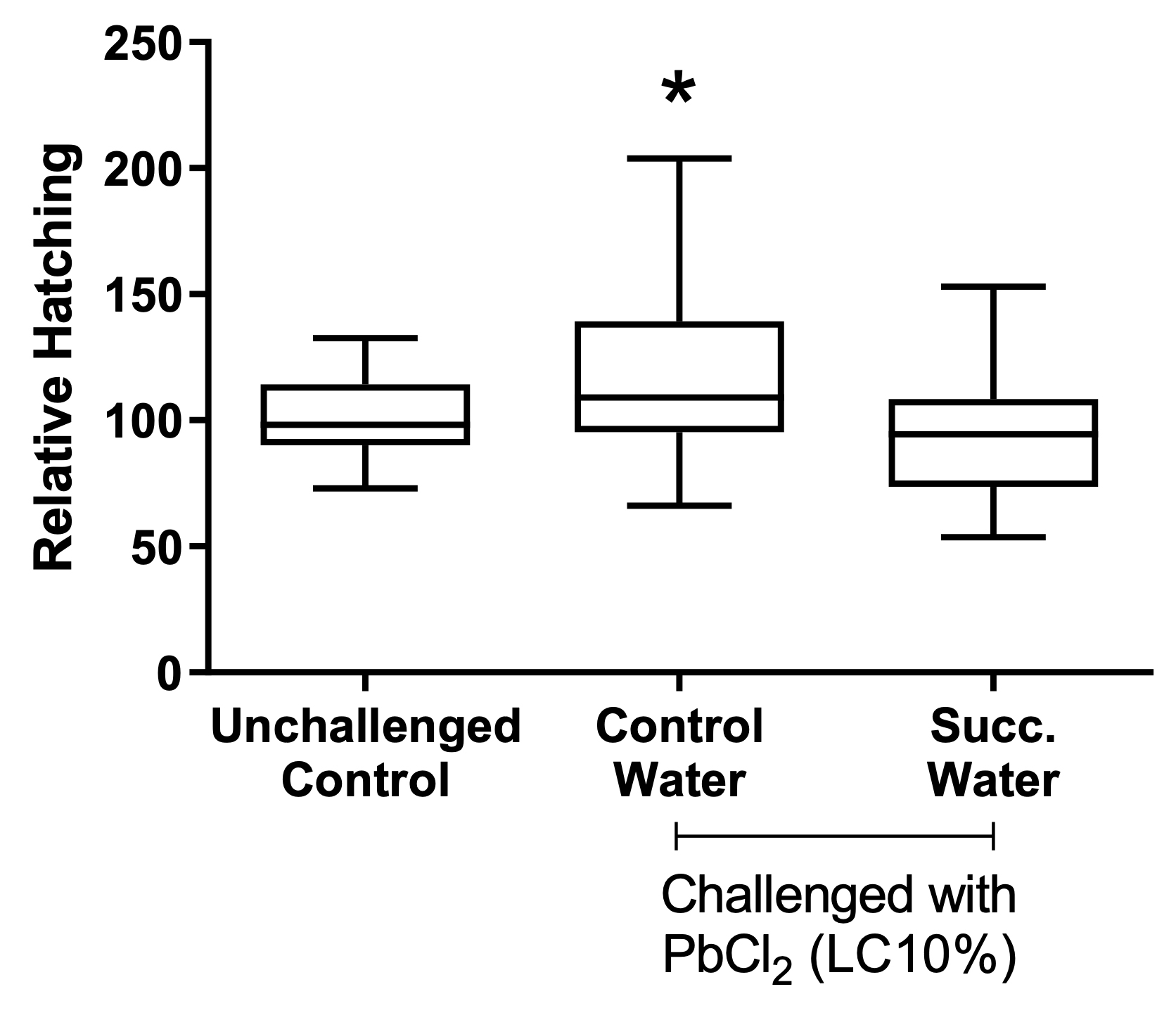
Figure 2. Effect of treatments on cysts’ hatching. Values represent a mean ± 95% confidence interval. Two-factor ANOVA of mixed models (F(2; 86) = 8,2092, p=0.0055, ŋ2= 0.160). Observed power = 0.955. The Tukey test revealed differences between control water and the other groups. Succ. Water = succussed water.
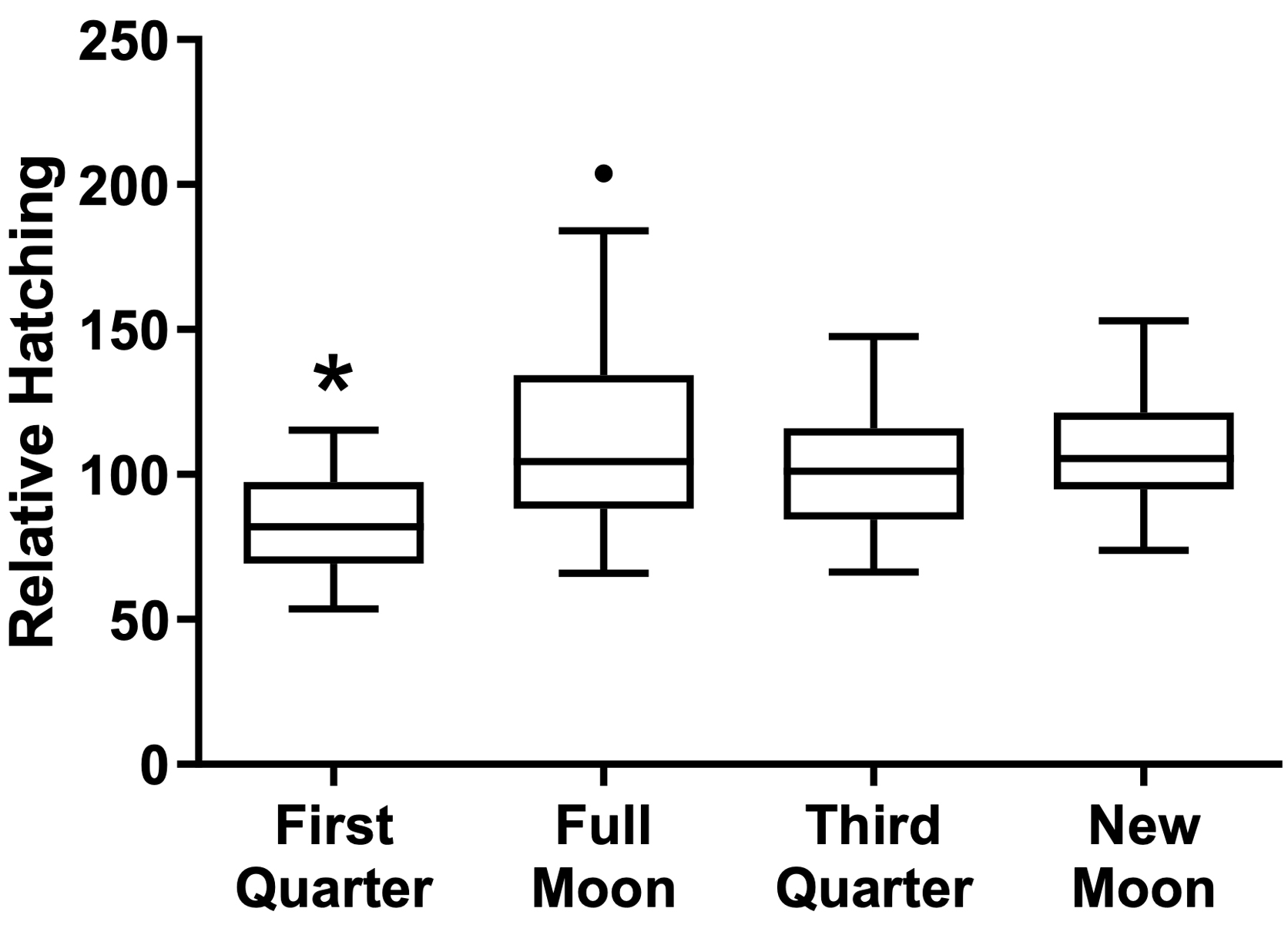
Figure 3. Effect of moon phases on cysts’ hatching. Values represent a mean ± 95% confidence interval. Two-factor ANOVA of mixed models (F(3; 86) = 7.6183, p=0.00014, ŋ2 = 0.210). Observed power = 0.984. Tukey test revealed differences between the first quarter moon versus other moon phases. The dot point is an outlier not considered for statistics.
Treatment of the cysts with succussed water reduced the hatching rate to levels comparable to the unchallenged, or baseline, group (Figure 2). The statistical interaction between moon phases and treatments is presented in Figure 4.
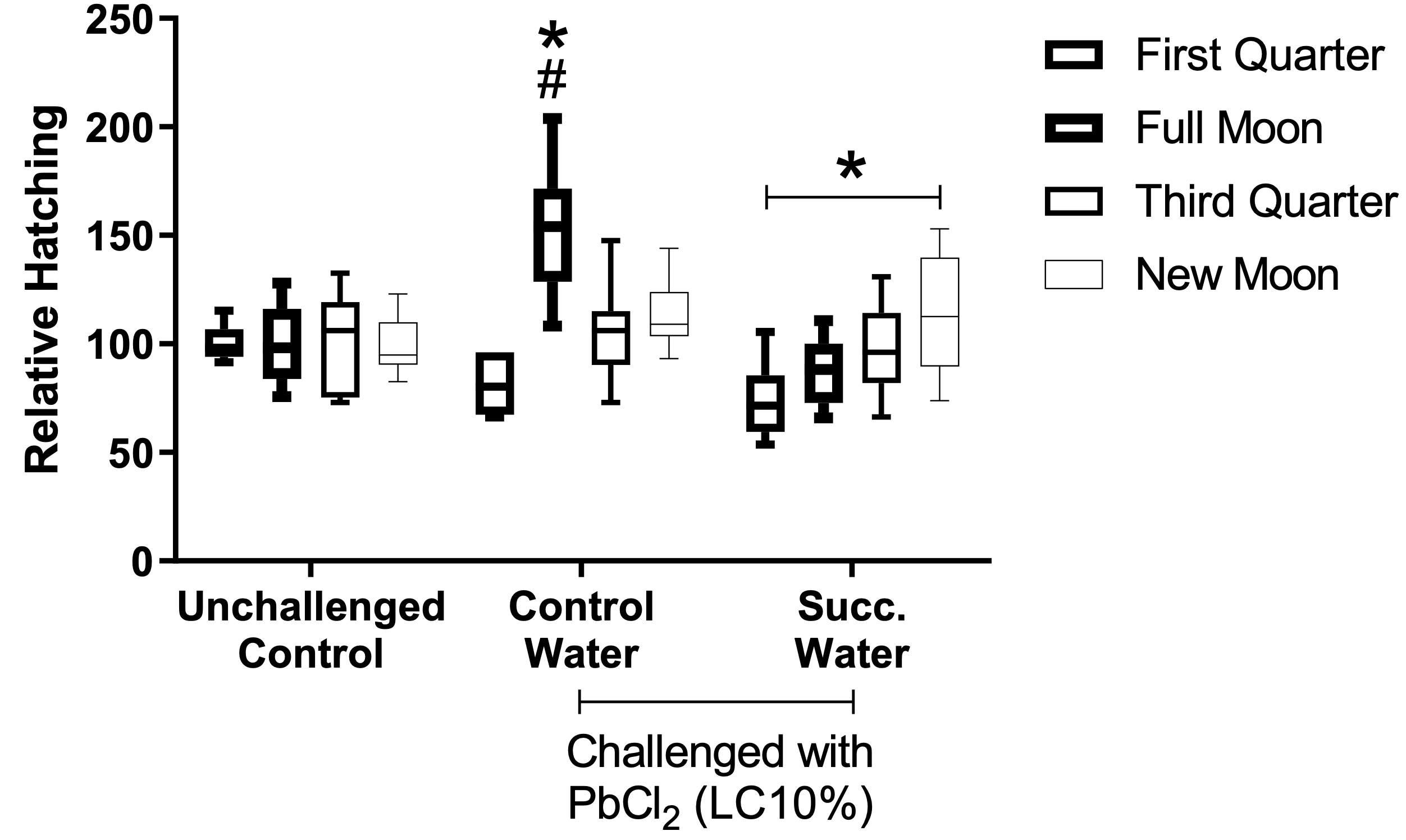
Figure 4. Effect of interaction between treatments and moon phases. According to two-factor ANOVA with mixed models, there was statistical interaction between treatments and moon phase on the relative hatching rate (F(6, 86) = 6.8218, p=0.00001, ŋ2 = 0.323). Observed power = 0.999. Tukey’s test revealed differences between moons (*) and between treatments (#).
There was statistical interaction between the moon phase and exposure of the cysts to PbCl2 EC10 regarding the hatching rate. The interaction occurred so that the effects of intoxication per se were more evident during the full moon, with a significant increase in outbreaks compared to the other conditions. Such interaction was nullified by treating the cysts with succussed water (p=0.0001, Figure 4). The samples exposed to PbCl2 presented progressive differences in the hatching rate in function to the different phases of the moon, with the lowest rate during the crescent moon. Such progressive differences were not seen in the unchallenged group (Figure 4).
Analysis of the Mobility of Nauplii During the Full Moon
Due to the higher hatching of nauplii challenged with PbCl2 during the full moon phase, the behavior of nauplii born in 48 hours was analyzed using Image J software – Trackmate plug-in. The number of stops and locomotion (in pixels) were quantified. Locomotion is understood as the distance traveled by nauplii/well over 13 seconds (Figure 5). Note that both groups challenged with PbCl2 EC10 showed increased mobility compared to the unchallenged group. The introduction of succussed water did not affect this variable.
Figure 6 presents the number of stops of the hatched nauplii during the full moon. All animals challenged with lead chloride showed an increase in arrests compared to the group that did not receive intoxication.
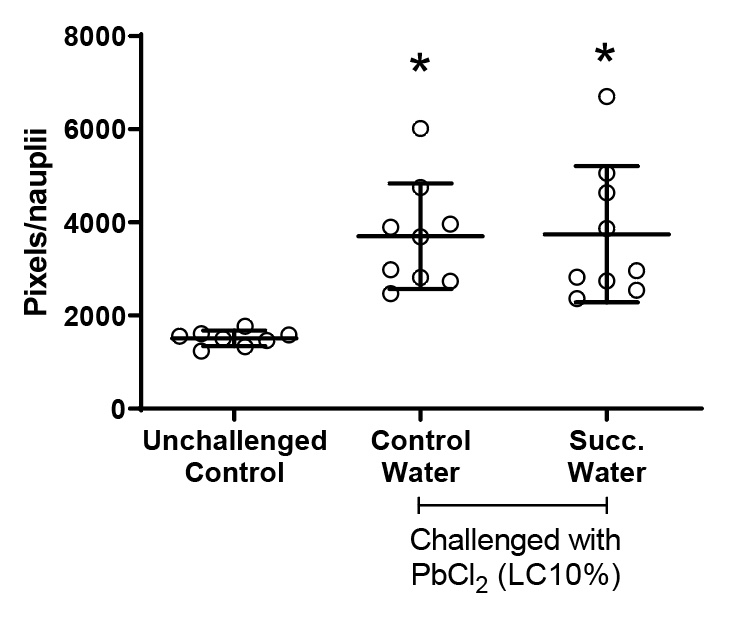
Figure 5. Nauplii locomotion. Values represent a mean ± 95% confidence interval. Two-factor ANOVA of mixed models (F(2; 23) =11.332, p=0.00038, ŋ2 = 0.496). Observed power = 0.984. Points represented by circles correspond to outliers. Tukey’s post-test indicated differences between both groups challenged with PbCl2 and the unchallenged control group.
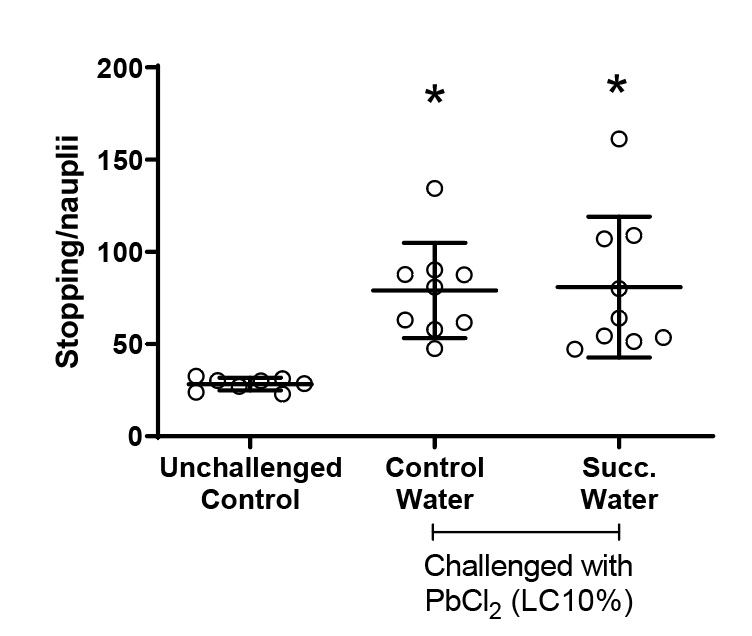
Figure 6. Number of nauplii stops during the full moon. Values represent a mean ± 95% confidence interval. Two-factor ANOVA of mixed models (F(2;23)=9.9470, p=0.00077, ŋ2=0.464). Observed power = 0.970. Points represented by circles correspond to outliers. Tukey’s post-test indicated differences between both groups challenged with PbCl2 and the unchallenged control group.
Discussion
The impact of experimental conditions on toxicity assessment has been addressed in recent studies, primarily involving aquatic organisms (Lin et al. 2021). The correlation between toxicity and environmental stimulus in the Artemia salina model has been reported in recent years (Asadi et al. 2019; Pillard and Tapp, 2021). However, two critical variables for marine organisms have not yet been explored enough: circalunar variations and events from the agitation of the water. In this study, these parameters were evaluated, using the hatching of Artemia salina cysts exposed to lead chloride as an experimental model. The EC10 obtained for PbCl2 was chosen as a challenge to mimic concentrations usually found in contaminated natural environments (Pillard and Tapp, 2021).
The increase in the hatching rate was the main effect of the intoxication per se, this effect being evident during the full moon, with statistical interaction between exposure to PbCl2 and the phases of the moon. These findings corroborate data obtained in previous studies (Pinto et al. 2021). They are following the fact that certain marine organisms are sensitive to lunar variations regardless of environmental lighting conditions due to the activation of clock genes such as those from the cryptochrome (Cry) family (Andreatta and Tessmar-Raible, 2020; Raible et al. 2017), or even due to epigenetic control of other adaptive processes (Schenk et al. 2019; Kaiser and Neumann, 2021), which seems to be a universal phenomenon, found even in plants and terrestrial animals (de Mallo Gallep and Robert, 2021). For instance, in corals, clock gene expression patterns vary according to the lunar phase. Notably, Cry 1 and Cry 2 alternate their expressions between crescent and full moons (Brady et al. 2016), impacting spawning behavior. Clock genes were also recently described in the crustacean Talitrus saltator (O’Grady et al. 2016), allowing us to infer the existence of similar mechanisms in microcrustacean Artemia salina. These mechanisms would be capable of favoring the toxic activity of PbCl2 during the full moon.
On the other hand, the effects of succussed water on hatching were higher in the new moon, suggesting greater susceptibility of the cysts to oxidative processes in this phase. It is known that cysts are resistant forms of Artemia salina (MacRae, 2016; Tan and MacRae, 2018; Pinto et al. 2021), which functions are controlled by molecular mechanisms related to oxidation-reduction processes (Chen et al. 2021; Pecoraro et al. 2021). In other crustacean species such as Litopenaeus vannamei, the total hemocyte counts (THC) and the production of superoxide anion (O2-) is higher in the new moon (Bautista-Covarrubias et al. 2020).
Hatching delay is a protective adaptive process against the presence of harmful agents in water and, therefore, can be considered an experimental model of bioresilience (MacRae, 2016; Tan and MacRae, 2018; Pinto et al. 2021). Herein, succussed water promoted this protective effect on the hatching of cysts exposed to PbCl2, which suggests the participation of nano and microbubbles in this process, which form during agitation (Agarwal et al. 2011; Atkinson et al. 2019; Fan et al. 2020; Pan et al. 2016; Shang et al. 2019; Wu et al. 2021) especially when done in a repeated and rhythmic way (Demangeat, 2015). Recently, Xiao et al. (2018) demonstrated that nanobubbles inhibit the crystallization of SPA−Pb (styryl phosphoric acid-Pb) and increase the flotation of precipitated particles. Thus, they can separate these contaminants from the bulk of water. It is speculated whether a similar effect of nucleation and flotation could occur with lead chloride crystals dissolved in saline water, reducing its bioavailability to the diapause embryo. This hypothesis is supported by the fact that the presence of NaCl in the artificial seawater used is above 0.1M since, under these conditions, the size of the nanobubbles and the surface charge density increase, favoring flotation (Meegoda et al. 2019).
Nauplii not submitted to any challenge or treatment had lower overall activity compared to nauplii exposed to PbCl2. Therefore, in addition to increasing the hatching rate, exposure during the full moon also produced behavioral changes, suggesting neurotoxic effects even at low concentrations (EC10). Neurotoxic effects of lead are known and described in several species (Li et al. 2013; Souza et al. 2018; Wani et al. 2015).
These experimental results point to a critical phenomenological aspect that can be transported to field situations. It deserves to be considered in planning sustainable corrective actions for the purification and decontamination of seawater, that is, the observation of lunar cycles and water movement as factors allied to optimizing the results. Water agitation, by means of at least nano and microbubbles production, could also contribute favorably as a simple additional resource for depollution (Agarwal et al. 2011; Atkinson et al. 2019; Wu et al. 2021).
In short, this study refers to a translational approach in which the knowledge of usually neglected variables, which were systematically observed in laboratory conditions, can be applied in field situations. The aim is to help the development of simple, cheap, and clean technologies able to deal with pollution from the understanding of fundamental water and environmental properties. The sensitivity of Artemia salina to circalunar variations must be highlighted, considering that it can potentially interfere with the outcomes in toxicological studies. This bridge between basic and applied research on water particularities follows the proposal of this special issue.
Conclusion
Intoxication of Artemia salina cysts by PbCl2 (EC10) in microplates produces more significant cyst hatching and more significant general activity of nauplii in their early stages of development up to 48 hours. This effect is particularly evident during the full moon. The introduction of water subjected to succussion in the system produced protective results on hatching, and such effects were also highlighted during the full moon. These are variables often neglected by scientists but must be observed in light of their importance to the new approaches for water pollution mitigation.
Acknowledgments
We thank Wilton Pereira for his technical support during the experimentation period.
Conflict of Interest and Funding
There is no conflict of interest related to this article. This work was supported by CAPES–PROSUP [88882.366859/2019-1; 88887.475302/2020-00].
Discussion with Reviewer – DWR
Reviewer: Can the circalunar phase impact any experiment with this theme?
Authors: No, considering different sensitivities according to each species. The Artemia salina model seems to be very sensitive, considering our previous results (Pinto et al. 2021). Anyway, this is an aspect that has been rarely observed in standard biological experiments, but a number of new studies, some of them cited in the text, have been raised in literature over the last few years. The magnitude of biological changes resulting from the circalunar cycle involves different molecular adaptive processes to environmental challenges (Schenk et al. 2019; Kaiser and Neumann, 2021). It is observed not only in aquatic animals but also in terrestrial animals and plants, even under standard laboratory conditions (de Mello Gallep and Robert, 2021). Thus, observing this variable in many biological experimental models will probably be considered mandatory in different methodological standards.
Reviewer: What would be the influencing mechanism of the circalunar phase on the mobility of nauplii?
Authors: Although no specific study can be found in the literature, the expected effect would probably be related to the intensity of swimming activity according to the light incidence. It is known that nauplii are very reactive to light, and part of the effect of circalunar effects on aquatic animals is related to light incidence during the full moon phase (Rosenberg et al. 2019).
References
Agarwal A, Ng WJ, Liu Y (2011). Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84(9):1175-80. DOI: 10.1016/j.chemosphere.2011.05.054.
Andreatta G, Tessmar-Raible K (2020). The Still Dark Side of the Moon: Molecular Mechanisms of Lunar-Controlled Rhythms and Clocks. J Mol Biol. 432(12):3525-3546. DOI: 10.1016/j.jmb.2020.03.009. 27.
Asadi Dokht Lish R, Johari SA, Sarkheil M, Yu IJ (2019). On how environmental and experimental conditions affect the results of aquatic nanotoxicology on brine shrimp (Artemia salina): A case of silver nanoparticles toxicity. Environ. Pollut. 255(Pt 3): 113358. DOI: 10.1016/j.envpol.2019.113358.
Atkinson AJ, Apul OG, Schneider O, Garcia-Segura S, Westerhoff P (2019). Nanobubble Technologies Offer Opportunities to Improve Water Treatment. Acc Chem Res. 52(5):1196-1205. DOI: 10.1021/acs.accounts.8b00606.
Banfalvi G, Sarvari A, Nagy G (2012). Chromatin changes induced by Pb and Cd in human cells. Toxicol In Vitro 26(6):1064-71. DOI: 10.1016/j.tiv.2012.03.016.
Bautista-Covarrubias JC, Zamora-Ibarra PA, Apreza-Burgos E, Rodríguez-Ocampo AN, Peraza-Gómez V, López-Sánchez JA, Pacheco-Vega JM, González-Hermoso JP, Frías-Espericueta MG (2020). Immune response and oxidative stress of shrimp Litopenaeus vannamei at different moon phases. Fish Shellfish Immunol. 106:591-595. DOI: 10.1016/j.fsi.2020.08.040.
Brady AK, Willis BL, Harder LD, Vize PD (2016). Lunar phase modulates circadian gene expression cycles in the broadcast spawning coral Acropora millepora. Biol Bull. 230(2):130–42. DOI:10.1086/BBLv230n2p130.
Chen B, Chu TW, Chiu K, Hong MC, Wu TM, Ma JW, Liang CM, Wang WK (2021). Transcriptomic analysis elucidates the molecular processes associated with hydrogen peroxide-induced diapause termination in Artemia-encysted embryos. PLoS One 16(2): e0247160. DOI: 10.1371/journal.pone.0247160.
Demangeat JL (2015). Gas nanobubbles and aqueous nanostructures: the crucial role of dynamization. Homeopathy 104(2):101-15. DOI: 10.1016/j.homp.2015.02.001.
Fan W, Zhang Y, Liu S, Li X, Li J (2020). Alleviation of copper toxicity in Daphnia Magna by hydrogen nanobubble water. J Hazard Mater 389:122155. DOI: 10.1016/j.jhazmat.2020.122155.
Kaiser TS, Neumann J (2021). Circalunar clocks-Old experiments for a new era. Bioessays 43(8): e2100074. DOI: 10.1002/bies.202100074.
Kaiser TS, Poehn B, Szkiba D, Preussner M, Sedlazeck FJ, Zrim A, Neumann T, Nguyen LT, Betancourt AJ, Hummel T, Vogel H, Dorner S, Heyd F, von Haeseler A, Tessmar-Raible K (2016). The genomic basis of circadian and circalunar timing adaptations in a midge. Nature 540(7631): 69-73. DOI: 10.1038/nature20151.
Kalogerakis N, Arff J, Banat IM, Broch OJ, Daffonchio D, Edvardsen T, Eguiraun H, Giuliano L, Handå A, López-de-Ipiña K, Marigomez I, Martinez I, Øie G, Rojo F, Skjermo J, Zanaroli G, Fava F (2015). The role of environmental biotechnology in exploring, exploiting, monitoring, preserving, protecting, and decontaminating the marine environment. N Biotechnol. 32(1):157-67. DOI: 10.1016/j.nbt.2014.03.007.
Li WH, Shi YC, Tseng IL, Liao VH (2013). Protective efficacy of selenite against lead-induced neurotoxicity in Caenorhabditis elegans. PLoS One 8(4): e62387. DOI: 10.1371/journal.pone.0062387.
Lin F, Baillon L, Langlois VS, Kennedy CJ (2021). Environmental modulators of diluted bitumen effects in juvenile pink salmon (Oncorhynchus gorbuscha). Mar Environ Res. 169:105392. DOI: 10.1016/j.marenvres.2021.105392. Online ahead of print.
MacRae TH (2016). Stress tolerance during diapause and quiescence of the brine shrimp, Artemia. Cell Stress Chaperones 21(1):9-18. DOI: 10.1007/s12192-015-0635-7.
Meegoda JN, Hewage SA, Batagoda JH (2019). Stability of nanobubbles. Langmuir 35: 12100-12112. DOI: 10.1021/acs.Langmuir.9b01443.
de Mello Gallep C, Robert D (2021). Are cyclic plant and animal behaviours driven by gravimetric mechanical forcing? J Exp Bot. 2021: erab462. DOI: 10.1093/jxb/erab462. Online ahead of print.
Ntungwe N E, Domínguez-Martín EM, Roberto A, Tavares J, Isca VMS, Pereira P, Cebola MJ, Rijo P (2020). Artemia species: An Important Tool to Screen General Toxicity Samples. Curr Pharm Des. 26(24): 2892-2908. DOI: 10.2174/1381612826666200406083035.
Obeng-Gyasi E (2019). Sources of lead exposure in various countries. Rev Environ Health. 34(1):25-34. DOI: 10.1515/reveh-2018-0037.
O’Grady JF, Hoelters LS, Swain MT, Wilcockson DC (2016). Identification and temporal expression of putative circadian clock transcripts in the amphipod crustacean Talitrus saltator. Peer J. 4:e2555. DOI: 10.7717/peerj.2555.
Oldach MJ, Workentine M, Matz MV, Fan TY, Vize PD (2017). Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol Ecol. 26(9): 2514-2526. DOI: 10.1111/mec.14043.
Orden S, Macías F, Cánovas CR, Nieto JM, Pérez-López R, Ayora C (2021). Eco-sustainable passive treatment for mine waters: Full-scale and long-term demonstration. J Environ Manage. 280:111699. DOI: 10.1016/j.jenvman.2020.111699.
Pan G, He G, Zhang M, Zhou Q, Tyliszczak T, Tai R, Guo J, Bi L, Wang L, Zhang H (2016). Nanobubbles at Hydrophilic Particle-Water Interfaces. Langmuir 32(43):11133-11137. DOI: 10.1021/acs.Langmuir.6b01483.
Payton L, Tran D (2019). Moonlight cycles synchronize oyster behaviour. Biol Lett. 15(1):20180299. DOI: 10.1098/rsbl.2018.0299.
Pecoraro R, Scalisi EM, Messina G, Fragalà G, Ignoto S, Salvaggio A, Zimbone M, Impellizzeri G, Brundo MV (2021). Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microsc Res Tech. 84(3):531-536. DOI: 10.1002/jemt.23609.
Pillard DA, Tapp KL (2021). Influence of feeding an organism age on the acute toxicity of sodium bromide to Artemia salina. Ecotoxicology 30: 914–918. DOI: 10.1007/s10646-021-02417-2.
Pinto AAG, Nagai MYO, Coimbra EN, Mohammad SN, Silva JS, Von Ancken A, Pinto SAG, Aguiar MS, Dutra-Correa M, Hortellani MA, Miranda A, Sarkis JES, Suffredini IB, Peres GB, Bernardi MM, Cartwright SJ, Bonamin LV (2021). Bioresilience to mercury chloride of the brine shrimp Artemia salina after treatment with homeopathic Mercurius corrosivus. Homeopathy. DOI: 10.1055/s-0041-1729562. Online ahead of print.
Raible F, Takekata H, Tessmar-Raible K (2017). An Overview of Monthly Rhythms and Clocks. Front Neurol. 8:189. DOI: 10.3389/fneur.2017.00189.
Rosenberg Y, Doniger T, Harii S, Sinniger F, Levy O (2019). Demystifying Circalunar and Diel Rhythmicity in Acropora digitifera under Constant Dim Light. iScience 22: 477-488. DOI: 10.1016/j.isci.2019.11.040.
Schenk S, Bannister SC, Sedlazeck FJ, Anrather D, Minh BQ, Bileck A, Hartl M, von Haeseler A, Gerner C, Raible F, Tessmar-Raible K (2019). Combined transcriptome and proteome profiling reveals specific molecular brain signatures for sex, maturation and circalunar clock phase. E-life 8: e41556. DOI: 10.7554/eLife.41556.
Shang H, Wu B, Liang X, Sun Y, Han X, Leizhang, Wang Q, Cheng W (2019). Evaluation of therapeutic effect of targeting nanobubbles conjugated with NET-1 siRNA by shear wave elastography: an in vivo study of hepatocellular carcinoma bearing mice model. Drug Delivery 26(1):944-951. DOI: 10.1080/10717544.2019.1667450.
Souza ID, Andrade AS, Dalmolin RJS (2018). Lead-interacting proteins and their implication in lead poisoning. Crit Rev Toxicol. 48(5):375-386. DOI: 10.1080/10408444.2018.1429387.
Stanovych A, Balloy M, Olszewski TK, Petit E, Grison C (2019). Depollution of mining effluents: innovative mobilization of plant resources. Environ Sci Pollut Res Int. 26(19):19327-19334. DOI: 10.1007/s11356-019-05027-y.
Tan J, MacRae TH (2018). Stress tolerance in diapausing embryos of Artemia franciscana is dependent on heat shock factor 1 (Hsf1). PLoS One 13(7): e0200153. DOI: 10.1371/journal.pone.0200153.
Tang J, Su M, Wei L, Wei Y, Liang J, Liu Y, Luo Y (2020). Comprehensive evaluation of the effectiveness on metals recovery and decontamination from MSWI fly ash by an integrating hydrometallurgical process in Guangzhou. Sci Total Environ. 728:138809. DOI: 10.1016/j.scitotenv.2020.138809.
Ugolini A, Hoelters LS, Ciofini A, Pasquali V, Wilcockson DC (2016). Evidence for discrete solar and lunar orientation mechanisms in the beach amphipod, Talitrus saltator Montagu (Crustacea, Amphipoda). Sci Rep. 6: 35575. DOI: 10.1038/srep35575.
Wani AL, Ara A, Usmani JÁ (2015). Lead toxicity: a review. Interdiscip Toxicol. 8(2):55-64. DOI: 10.1515/intox-2015-0009.
Wu J, Zhang K, Cen C, Wu X, Mao R, Zheng Y (2021). Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express. 11(1):96. DOI: 10.1186/s13568-021-01254-0.
Xiao W, Ke S, Quan N, Zhou L, Wang J, Zhang L, Dong Y, Qin W, Qiu G, Hu J (2018). The Role of Nanobubbles in the Precipitation and Recovery of Organic-Phosphine-Containing Beneficiation Wastewater. Langmuir 34(21):6217-6224. DOI: 10.1021/acs.Langmuir.8b01123.
Yuan W, Yang N, Li X (2016). Advances in Understanding How Heavy Metal Pollution Triggers Gastric Cancer. Biomed Res Int. 2016:7825432. DOI: 10.1155/2016/7825432.
