Optimization of Vortex-Assisted Dispersive Liquid-Liquid Microextraction for Preconcentration of Copper and Lead Prior to Determination by UV-Vis in Drinking Water
Zimila HE1,2*, Chibindje RHJ1, Mandlate JS1
1Department of Chemistry, Eduardo Mondlane University, Julius Nyerere Av. # 3453, P.O. Box 257, Maputo, Mozambique
2Department of Chemical and Environmental Engineering, The University of Arizona, Tucson, AZ 85721, United States
*Corresponding author: herciliozimila@gmail.com
Keywords: vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME), UV-Vis spectrophotometry, copper, lead, drinking water
Submitted: October 16, 2023
Revised: August 2, 2024
Accepted: August 14, 2024
Published: December 21, 2024
Abstract
Several methods have been developed for the analysis of heavy metals in water; however, the majority are unaffordable for many laboratories in developing countries. This work aimed at optimizing and validating the vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME) tandem UV-Vis spectrophotometry for Cu2+and Pb2+ determination in drinking water. The method involves the complexation of Cu2+ and Pb2+ with 8-hydroxyquinoline and dithizone, respectively, followed by extraction and preconcentration under appropriate conditions. VA-DLLME operating conditions (volume of solvents, extraction time, pH, agitation frequency) were screened using the fractional factorial design, and the most important factors were optimized using the response surface approach based on the central composite design. Optimum conditions for Cu2+ determination include ethanol volume of 2000 μL, tetrachloromethane volume of 500 μL, 1 min vortex stirring at 3200 rpm, 5% NaCl, pH 5, and centrifugation time of 8 min. For Pb2+ analysis, the optimum conditions include a methanol volume of 2000 μL, chloroform volume of 350 μL, 1 min vortex stirring at 3200 rpm, pH 5, and centrifugation time of 5 min. The methods offer good linearity with R2 > 0.99, limits of detection and quantification of 0.016 and 0.048 ppm for Cu2+, and 0.004 and 0.016 ppm for Pb2+, respectively. Accuracy assessed as recovery ranged between 90 – 113% for both Cu2+ and Pb2+. The RSD values ranged from 3 – 6% for Cu2+ and 4% – 9% for Pb2+. The methods are simple, selective, precise, and accurate for cost-effective monitoring of Cu2+ and Pb2+ in drinking water.
Introduction
Heavy metals can have both beneficial and detrimental effects on the body, depending on their concentration and chemical form. Copper is an essential mineral that is required for the proper functioning of metalloenzymatic systems involved in energetic metabolism, the scavenging of deleterious oxygen radicals, and the biogenesis of melanin and hormones (Scheiber et al., 2013). Nonetheless, doses above the Recommended Dietary Allowance (RDA) of 900 μg day-1 can cause severe liver damage and neuromuscular disorders, such as Alzheimer’s and Wilson’s diseases (Everett et al., 2021; Zheng et al., 2017). Lead, in turn, is classified as a probable human carcinogen (group B2) and may lead to impaired neurodevelopment in children, slow nerve conduction in adults, and chronic nephropathy in the kidneys (ATSDR, 2007).
Drinking water is among the primary exposure pathways to heavy metals. The maximum permissible levels of copper and lead in drinking water established by the World Health Organization (WHO) are 2.0 mg L-1 and 10 µg L-1, respectively (WHO, 2022). Complying with such stringent guidelines demands the development of sensitive and accurate techniques for estimating trace concentrations of these heavy metals.
Flame atomization atomic absorption spectrophotometry (FAAS) and graphite furnace atomic absorption spectrometry (GFAAS) (Mandlate et al., 2017; Sixto et al., 2019), inductively coupled plasma mass spectrometry (ICP-MS) (Li et al., 2015; Seeger et al., 2017; Xing et al., 2019), and inductively coupled plasma optical emission spectrophotometry (ICP OES) (Ahmad et al., 2021) are the most commonly used techniques for copper and lead analysis in several matrices, including water. However, these methods are costly and inaccessible in many developing countries like Mozambique. Conversely, Ultraviolet-Visible (UV-Vis) spectrophotometry is widely available in analytical chemistry laboratories, but its sensitivity to trace concentrations and proneness to interference when studying complex matrices can limit its effectiveness (Ali and Shakrani, 2014). Therefore, researchers have been striving to develop simple, reliable, faster, and environmentally friendly sample preparation methods that could enable the quantification of contaminants using more economical techniques.
Since its development by Rezaee et al., (2006), dispersive liquid-liquid microextraction (DLLME) has become one of the most efficient high-throughput miniaturized methods for sample preparation and preconcentration of heavy metals from complex matrices aqueous samples. However, very few studies have explored the combination of DLLME with UV-Vis for copper and lead analysis in aqueous samples. Wen et al., (2011) and Faraji et al., (2019) developed the DLLME-UV-Vis for the determination of copper in water using diethyldithiocarbamate and (4-hydroxy-2-oxo-2H-chromen-3-yl) methyl pyrrolidine-1-carbodithioate as
ligands, respectively. While Wen et al., (2011) used tetrachloromethane and ethanol, Faraji et al., (2019) employed a mixture of tetrachloroethane and acetone as extracting and dispersing solvents, respectively. Likewise, Nejad et al., (2017), Faraji and Helalizadeh (2017), and Maciel et al., (2014) applied DLLME-UV-Vis to quantify lead and Fe in water, urine, and wine samples, respectively, using O-diethyl dithiophosphoric acid and ammonium pyrrolidine dithiocarbamate (APDC) as chelating agents.
The slow mass transfer rate of the analyte is the major bottleneck of DLLME. The high surface tension and the interfacial area between the aqueous and organic phases can prompt an ineffective and irreproducible transfer of the analyte to the non-polar organic phase, especially when the mixing step is done manually. Vortexing can improve the efficiency of DLLME by providing mechanical energy to break the organic phase into fine droplets with a large surface area, allowing for a faster mass transfer of the analyte from the aqueous sample to the organic phase (Zhong et al., 2014).
In the present study, the vortex-assisted DLLME coupled with UV-Vis spectrophotometry (VA-DLLME-UV-Vis) has been optimized for the determination of Cu and Pb in water samples. Factors affecting VA-DLLME were optimized through fractional factorial design followed by central composite design, which allows for investigation of the effect and interactions of multiple factors at once with a few experiments.
Materials and Methods
Instrumentation
A UV/Vis spectrophotometer (Jenway 6850, France), with a scan range between 190 and 1100 nm with a quartz cuvette, was used to determine the elements in extracts and samples. To estimate the accuracy of the method, the analytes were also determined by ICP-OES (Model ICPE–9820, Shimadzu Corporation, Japan). The instrument is equipped with a concentric nebulizer, a cyclonic spray chamber, and a quartz mini-torch with an injector tube. Radiofrequency power was set at 1200 W and argon (99.999%, Afrox, Mozambique) was used for plasma generation. The plasma, auxiliary, and nebulizer gas flow rates were set at 10, 0.6, and 0.7 L min-1, respectively.
The pH was measured using a pH meter (model Edge pH, Hanna Instruments, USA) calibrated with an aqueous solution of HNO3 or NH4OH. The salinity was measured in a Hanna conductivity meter (model Edge EC, Hanna Instruments, USA). ThermoScientific LPVortex Mixer 0 – 3200 rpm was employed for sample stirring. A centrifuge (model Thermo ALC 4206, Thermo Electron Industries SAS, France) was used for the phase separation during the DLLME procedure. The volume of reference solutions and water samples was measured using a Gilson micropipette of 200–1000 µL.
A mixture of solvents was injected into the sample using 250 – 1000 µL microsyringes (Hamilton, USA). All solutions and standards were prepared with ultrapure water (resistivity of 18.2 MΩ/cm) in a Milli-Q system (Direct 8.0, Millipore, Japan).
Chemicals
Standard solutions of Cu (II) and Pb (II) at 1000 mg L-1 were purchased from SMM Instruments, South Africa. Sodium hydroxide (98%), hydrochloric acid (37%), and the analytical grade chelating agents, 8-hydroquinoline (8HQ) and dithizone (Dz), were supplied by Merck, South Africa. All the solvents used in this study were of analytical grade and supplied by Merck, South Africa, except for the HPLC grade methanol, which was purchased from Skylabs, South Africa. To adjust the ionic strength, NaCl (Merck, South Africa) solutions were prepared by dissolving adequate amounts in ultrapure water.
Dispersive Liquid-Liquid Microextraction Procedure
Aliquots of 10 mL of water (or a solution of 2 mg L-1 Cu2+ or 0.01 mg L-1 Pb2+) and 1000 μL of chelating solution (1.25 mM 8HQ or 0.1875 mM Dz) were placed in a 15 mL polypropylene vial. The pH was adjusted with solutions of HCl or NaOH. A mixture containing the dispersive and extracting solvent was quickly injected into the sample using a 2.5 mL microsyringe. A cloud-like white dispersion was formed in the solution. The mixture was vortexed for 1 min and centrifuged, and a drop containing the complexed analyte and the extracting solvent settled at the bottom. The drop was washed with ultrapure water to eliminate the salts and reduce the interferences. The aqueous phase was removed, and the extract was further diluted up to 2 mL with the dispersion solvent for subsequent Cu2+ and Pb2+ determination by UV/Vis.
All statistical analyses were performed through the Minitab 17.0 software package at a 95% confidence interval, and the results were considered significant at p < 0.05.
Results and Discussion
VA-DLLME is a mechanistically complex process whose efficiency is affected by a wide range of factors, including the pH, temperature, ionic strength, centrifugation time, stirring rate, and time, aside from the type and concentration/volume of ligand, dispersing and extracting solvents (Andruch et al., 2012; Mandlate et al., 2017). Prior to the optimization of VA-DLLME of copper and lead analysis in water, preliminary experiments were performed to find the most appropriate ligands and their respective concentrations. The results demonstrated that 1.25 mmol L-1 8-hydroxyquinoline (8HQ) is a suitable ligand for 2 mmol L-1 Cu2+ and the resulting Cu-8HQ complex exhibited a maximum absorption at 352 nm. On the other hand, 0.1875 mmol L-1 dithizone (Dz) was appropriate for Pb2+ determination (0.01 mmol L-1) and formed a purple Pb-Dz complex with a maximum absorption wavelength of 578 nm (spectra not shown).
Apart from the ligand studies, the preliminary step is also aimed at finding the best pair extracting-dispersing solvents that can attain appreciable extraction efficiency and enrichment factor. While extracting solvents are typically hydrophobic and denser than water, with high affinity with the complexed analyte, the dispersing solvents are miscible in both the extracting solvent and the aqueous phase (Andruch et al., 2012; Bagherian et al., 2019). The performance of several combinations of 250 μL of extracting solvent (chloroform, tetrachloromethane, dichloroethane, and dichlorobenzene) and 1000 μL of dispersing solvent (methanol (MeOH), ethanol (EtOH), and tetrahydrofuran (THF)) was investigated. The selection criteria included cloudy dispersion formation, sediment formation and size, and absorbance. Tetrachloromethane–ethanol and methanol–chloroform were the best-performing solvent systems for Cu2+ and Pb2+ preconcentration (results not shown), respectively, and were selected for further studies.
Optimization of VA-DLLME of Copper Analysis
Screening of Variables
A 2 III (6 – 3) fractional factorial design of a total of 18 random runs (two replicates of an 8-run design + 1 center point) was conducted to simultaneously screen out the key variables affecting the VA-DLLME performance. For Cu2+ analysis, such variables included the volume of ethanol (500 – 2000 μL) and volume of tetrachloromethane (200 – 500 μL), pH (1 – 6), ionic strength (0 – 10% w/v NaCl), vortex stirring frequency (1500 – 3200 rpm), and centrifugation time (3 – 8 min). For Pb2+ analysis, the following factors were studied: methanol volume (500 – 2000 μL), chloroform volume (200 – 500 μL), pH (1 – 5), vortex stirring frequency (1500 – 3200 rpm), centrifugation time (3 – 8 min), and ionic strength (5 – 10%, w/v NaCl). The factor levels were selected based on our preliminary studies.
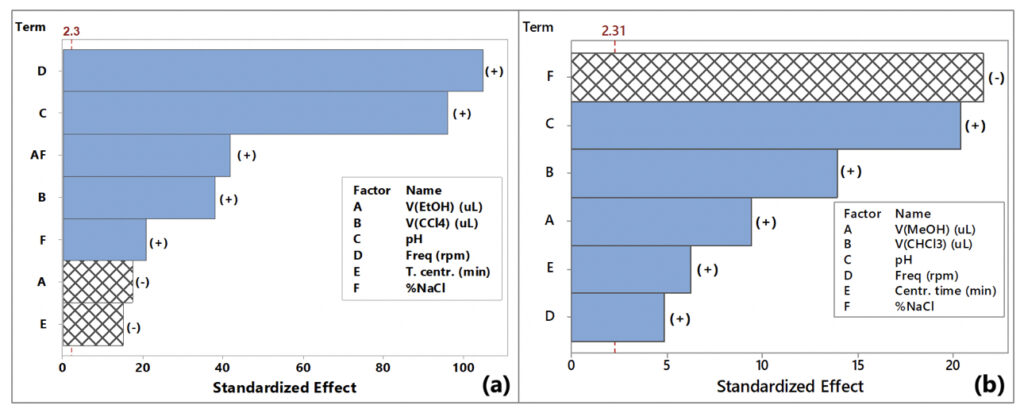
The data was evaluated through the Analysis of Variance (ANOVA), at the 5% significance level, and visualized as the Pareto chart of standardized effects (Figure 1).
Effects whose bars extend beyond the reference line of 2.3 on the Pareto chart are statistically significant at the 95% confidence level. Hence, all the studied variables significantly affect the preconcentration of both Cu2+ and Pb2+. Vortex stirring frequency and pH are the most important factors for the VA-DLLME of Cu2+, whereas the ionic strength and pH are the most significant variables for the VA-DLLME of Pb2+. Furthermore, all the studied variables for the VA-DLLME of Cu2+ exhibited positive effects on the absorbance, except for the volume of ethanol (extraction solvent) and the centrifugation time. For Pb2+, only ionic strength showed a negative effect on the response.
Apart from the main effects, interaction plots provide useful insights into how the influence of a certain variable on the response is affected by the level of other factors. Figure 2 displays the two-way interaction plots of the studied variables in the VA-DLLME of Cu2+.
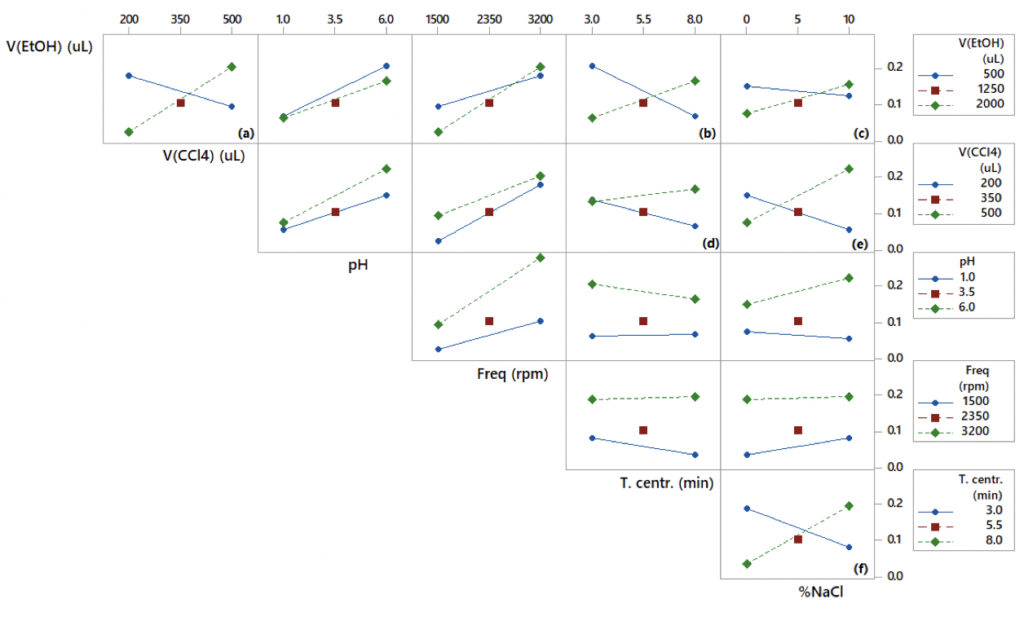
According to Figure 2, the most prominent interactions (interactions in which a certain variable shows opposite effects depending on the level of the other variable) are those of volume of ethanol vs. volume of tetrachloromethane (Figure 2a), volume of ethanol vs. centrifugation time (Figure 2b), volume of ethanol vs. % NaCl (Figure 2c), volume of tetrachloromethane vs centrifugation time (Figure 2d), volume of tetrachloromethane vs. % NaCl (Figure 2e), and centrifugation time vs. % NaCl (Figure 2f).
Figure 2a indicates that if the volume of ethanol is kept at the lowest level of 500 μL, the absorbance of the Cu-8HQ complex increases as the volume of tetrachloromethane decreases; the opposite trend is observed when the volume of ethanol is kept at the highest level (2000 μL). This is attributed to the deficient formation of a stable cloudy dispersion at low ethanol volumes (dispersing solvent), which hampers the formation of fine tetrachloromethane droplets necessary to collect the analyte from the aqueous phase (Bagherian et al., 2019). Conversely, large volumes of ethanol allow for the formation of a stable cloudy solution and high surface area tetrachloromethane droplets, hence better extraction efficiency (Bagherian et al., 2019). Under these conditions, a longer centrifugation time (Figure 2b) is needed to precipitate all the tetrachloromethane droplets dispersed across the solution. Furthermore, high ionic strength (Figure 2c) is crucial to enhance the salting-out effect and enrichment performance (Wang et al., 2022).
Since the best extraction was found at the maximum frequency of the vortex, this factor was kept at 3200 rpm for further studies. Accordingly, the independent variables for the response surface design were pH, the volume of tetrachloromethane, and ionic strength. The centrifugation time and volume of ethanol were fixed at their highest levels of 8 min and 2000 μL, respectively.
The interaction plots for the studied variables in the VA-DLLME of Pb2+ are shown in Figure 3.
Like VA-DLLME of Cu2+, the increase in the volume of chloroform is beneficial for the Pb2+ extraction efficiency at a high volume of methanol (2000 μL) and a deleterious effect at a small volume of methanol (Figure 3a). Using a methanol volume of 2000 μL, a good Pb2+ extraction is favored by the increase in the pH and centrifugation time, as well as the decrease in the stirring frequency (Figure 3b) and ionic strength. Therefore, the volume of methanol and vortex stirring frequency were fixed at 2000 μL and 3200 rpm, respectively, and sodium chloride was excluded from the surface design. Although the decrease in the vortex stirring frequency enhanced the extraction efficiency at high methanol volume, the highest level of 3200 rpm was employed in the response surface because its overall effect was positive. Subsequently, the three factors studied in detail for the response surface approach are the volume of chloroform (350 – 600 μL), centrifugation time (5 – 10 min), and pH (5 – 10).
Central Composite Design
The response surface approach based on central composite design (CCD) combines statistical and mathematical methods for in-depth investigation of the optimum experimental conditions for the critical variables (Asadollahzadeh et al., 2014; Bahrami et al., 2021; Ebrahimi-Najafabadi et al., 2019). It consists of factorial (corner), central, and axial points that allow for investigating the relationship between the response and the independent variables as well as the interaction between the variables. Since CCD requires a large number of experiments, it is typically performed for the critical variables identified in the screening fractional factorial design.
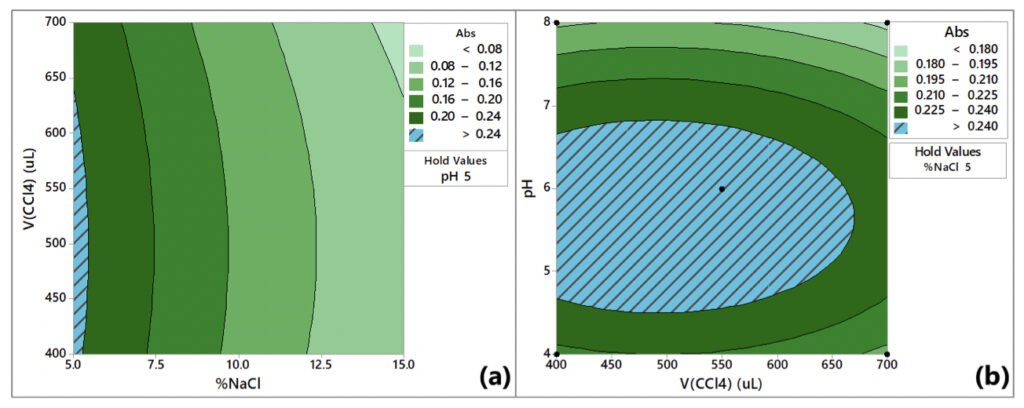
A 40-run CCD was employed to optimize the VA-DLLME of Cu2+ and Pb2+. As previously mentioned, for extraction, the pH, volume of tetrachloromethane, and ionic strength were considered for the design, and the contour plots are shown in Figure 4.
According to Figure 4, the desirable absorbance values (>0.24) can be attained using up to 5% NaCl, a tetrachloromethane volume between 400 and 600 μL, and a pH between 5 to 6. Therefore, the optimum parameter levels for Cu2+ determination by VA-DLLME are ethanol volume of 2000 μL, tetrachloromethane volume of 500 μL, 1 min vortex stirring at 3200 rpm, 5% NaCl, pH 5, and centrifugation for 8 min.
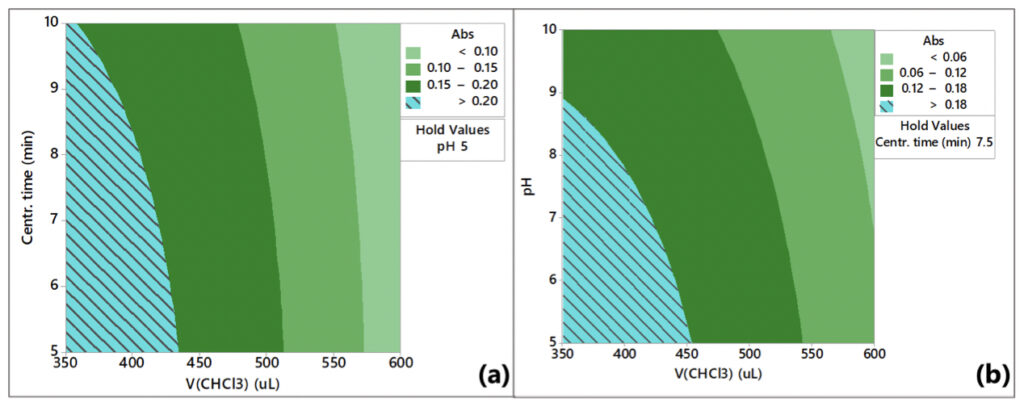
The VA-DLLME of Pb2+ was optimized for the volume of chloroform, pH, and centrifugation time, and the respective response surface plots are presented in Figure 5.
The contour plots in Figure 5 indicate that the highest response is achieved at a pH between 5 and 8, a volume of chloroform between 350 and 425 μL, and a centrifugation time between 5 to 9.5 min. Therefore, the optimum conditions for VA-DLLME of Pb2+ are a methanol volume of 2000 μL, chloroform volume of 350 μL, 1 min vortex stirring at 3200 rpm, pH 5, and centrifugation for 5 min.
Analytical Merit Figures
Method validation was performed against selected merit figures [linearity, limits of detection (LOD) and quantification (LOQ), repeatability, recovery, and tolerance limits] to evaluate the suitability of the proposed methods for the intended purposes.
Linearity was determined by 10-point calibration curves over the analytical ranges of 0.08 – 0.8 mg L-1 and 0.002 – 0.02 mg L-1 for Cu2+ and Pb2+, respectively. Both methods showed strong and positive correlations between the concentration and the absorbance with correlation coefficients (r2) greater than 0.99. It should be noted that the correlations obtained in this work are suitable for the UV-Vis technique.
The method limits of detection (LODm) and quantification (LOQm) were determined at three and ten times the absorbance of the blank, respectively. VA-DLLME of Cu2+ exhibited LODm and LOQm values of 0.016 mg L-1 and 0.048 mg L-1, respectively. VA-DLLME of Pb2+ showed LODm and LOQm values of 0.004 mg L-1 and 0.016 mg L-1, respectively.
The accuracy was evaluated by determining the analytical recovery and by comparing the results with the ICP-OES method, whereas the repeatability of the methods was determined as a percent of the relative standard deviation (RSD) of six sample replicates. Three concentration levels of Cu2+ (0.5, 1.0, and 1.5 mg L-1) and Pb2+ (0.1, 0.2, and 0.3 mg L-1), 6 replicates for each level, were analyzed by the optimized procedures. The recoveries fell within the range of 90% – 113% for both Cu2+ and Pb2+. Additionally, the results were compared against those of ICP-OES, and agreements of 96% and 101% were obtained for Cu and Pb, respectively. Regarding repeatability, the RSD values ranged from 3 – 6% and 4 – 9% for VA-DLLME of Cu2+ and Pb2+, respectively.
Analysis of Real Samples
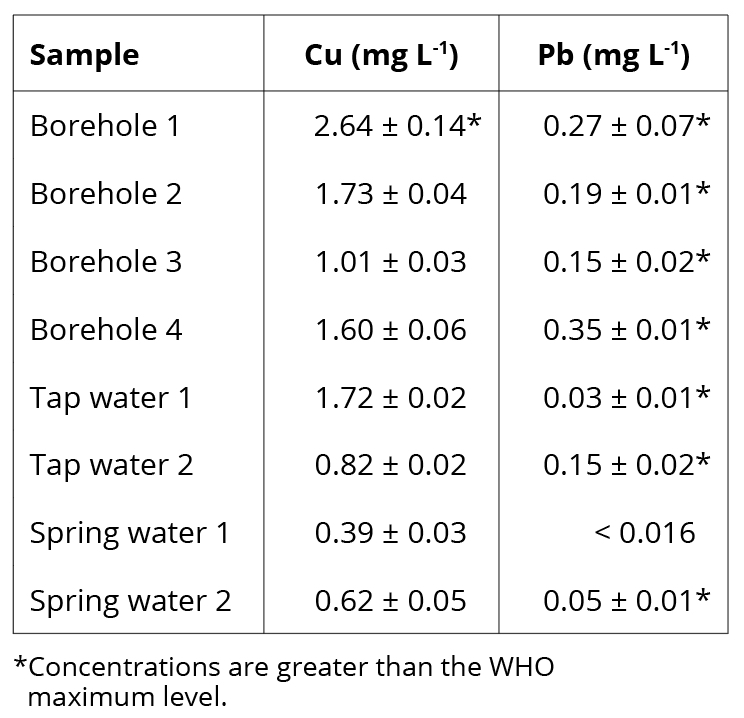
The developed methods were applied to 4 water samples from private boreholes, 2 tap water samples from the public distribution systems, and 2 spring water samples. The quantification of Cu2+ and Pb2+ was performed in triplicate through the optimized method and the results are shown in Table 1.
Different concentrations of Cu2+ and Pb2+ were found for the analyzed samples, which are related to the sample characteristics. Only the water sample from borehole 1 exceeded the WHO guideline for copper (2 mg L-1). However, Pb2+ concentrations were greater than the established limit (0.01 mg L-1) in all samples except for the spring water 1.
Conclusions
Simple, rapid, cost-effective, and environmentally friendly approaches for monitoring Cu2+ and Pb2+ have been optimized and validated. The methods involve complexing the heavy metals with 1.25 mM 8-hydroxyquinoline or 0.1875 mM dithizone, extraction, and preconcentration with vortex-assisted dispersive liquid-liquid microextraction and analyzed by UV-Vis spectrophotometry. The methods exhibited satisfactory accuracy, repeatability, sensitivity, selectivity, and linearity over the concentration ranges found in drinking water. This fact demonstrates the applicability of VA-DLLME tandem UV-Vis spectrophotometry for monitoring Cu and Pb in drinking water. These results suggest that there is a potential health risk for consumers relying on the studied water sources, as the levels of toxic heavy metal contents are above WHO standards.
References
Ahmad H, Zhao L, Liu C, Cai C, Ma F (2021). Ultrasound assisted dispersive solid phase microextraction of inorganic arsenic from food and water samples using CdS nanoflowers combined with ICP-OES determination. Food Chem 338: 128028
Ali MF, Shakrani SA (2014). A comparison of ICP-OES and UV-Vis spectrophotometer for heavy metals determination in soil irrigated with secondary treated wastewater. Int J Civ Environ Eng 14: 8-15.
Andruch V, Kocúrová L, Balogh IS, Škrlíková J (2012). Recent advances in coupling single-drop and dispersive liquid-liquid microextraction with UV-Vis spectrophotometry and related detection techniques. Microchem J 102: 1-10.
Asadollahzadeh M, Tavakoli H, Torab-Mostaedi M, Hosseini G, & Hemmati A (2014). Response surface methodology based on central composite design as a chemometric tool for optimization of dispersive-solidification liquid-liquid microextraction for speciation of inorganic arsenic in environmental water samples. Talanta 123: 25-31.
ATSDR. (2007). Toxicological Profile for Lead.
https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf
Bagherian G, Chamjangali MA, Evari HS, & Ashrafi M (2019). Determination of copper(II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid-liquid microextraction technique. J Anal Sci Technol 10: 1-11.
Bahrami M, Shabani AMH, Dadfarnia S, Moghadam MR, Baneshi M (2021). Experimental design optimization of supramolecular dispersive liquid-liquid microextraction of nickel and its spectrophotometric determination. J Anal Chem 76: 442-451.
Ebrahimi-Najafabadi H, Pasdaran A, Rezaei Bezenjani R, and Bozorgzadeh E (2019). Determination of toxic heavy metals in rice samples using ultrasound assisted emulsification microextraction combined with inductively coupled plasma optical emission spectroscopy. Food Chem 289: 26-32.
Everett J, Lermyte F, Brooks J, Tjendana-Tjhin V, Plascencia-Villa G, Hands-Portman I, Donnelly JM, Billimoria K, Perry G, Zhu X, Sadler PJ, O’Connor PB, Collingwood JF, Telling ND (2021). Biogenic metallic elements in the human brain? Sci Adv 7: 1-10.
Faraji H, Helalizadeh M (2017). Lead quantification in Urine samples of athletes by coupling DLLME with UV-Vis spectrophotometry. Biol Trace Elem Res 176: 258-269.
Faraji M, Pourmohammad M, Aryanasab F, Shabanian M (2019). (4-Hydroxy-2-oxo-2H-chromen-3-yl)methyl pyrrolidine-1-carbodithioate as a novel, highly selective and sensitive ligand for determination of copper in water and food samples by dispersive liquid-liquid microextraction coupled with microvolume UV-Vis spectr. J Iran Chem Soc 16: 1579-1589.
Li Y, Peng G, He Q, Zhu H, Al-Hamadani SMZF (2015). Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim Acta A Mol Biomol Spectrosc 140: 156-161.
Maciel JV, Soares BM, Mandlate JS, Picoloto RS, Bizzi CA, Flores EMM, Duarte FA (2014). Simple and fast method for iron determination in white and red wines using dispersive liquid-liquid microextraction and ultraviolet-visible spectrophotometry. J Agric Food Chem 62: 8340-8346.
Mandlate JS, Soares BM, Seeger TS, Vecchia PD, Mello PA, Flores EMM, Duarte FA (2017). Determination of cadmium and lead at sub-ppt level in soft drinks: An efficient combination between dispersive liquid-liquid microextraction and graphite furnace atomic absorption spectrometry. Food Chem 221: 907-912.
Nejad MG, Faraji H., Moghimi A (2017). Monitoring Pb in aqueous samples by using low density solvent on air-assisted dispersive liquid-liquid microextraction coupled with UV-Vis spectrophotometry. Bull Environ Contam Toxicol 98: 546-555.
Rezaee M., Assadi Y., Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006). Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116: 1-9.
Scheiber I, Dringen R, Mercer JFB (2013). Copper: Effects of deficiency and overload. Springer Science+Business Media Dordrecht. 360-374.
Seeger TS, Vecchia PD, Machado EQ, Reinke K, Mesko MF, Duarte FA (2017). Feasibility of DLLME for the extraction and preconcentration of As and Cd in sugar for further determination by ICP-MS. J Braz Chem Soc 28: 1691-1697.
Sixto A, Mollo A, Knochen M (2019). Fast and simple method using DLLME and FAAS for the determination of trace cadmium in honey. J Food Compost Anal 82: 1-6.
Wang L, Chen X, Han X, Ju B (2022). Determination of three pyrethroid insecticides in food by magnetic ionic liquid-based dispersive liquid phase microextraction (DLLME) with high-performance liquid-chromatography (HPLC). Anal Lett 56: 1229-1240
Wen X, Yang Q, Yan Z, Deng Q (2011). Determination of cadmium and copper in water and food samples by dispersive liquid-liquid microextraction combined with UV-Vis spectrophotometry. Microcheml J, 97, 249-254.
WHO (2022). Guidelines for drinking-water quality: Fourth edition incorporating the first and second addenda. https://iris.who.int/bitstream/handle/10665/352532/9789240045064-eng.pdf?sequence=1
Xing G, Sardar MR, Lin B, Lin JM (2019). Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS. Talanta 204: 50-56.
Zheng T-T, Zhao J, Fang Z-W, Li M-T, Sun C-Y, Li X, Wang X-L, Su Z-M (2017). A luminescent metal organic framework with high sensitivity for detecting and removing copper ions from simulated biological fluids. Dalton trans 46: 2456-2461.
Zhong Z, Li G, Wu R, Luo Z, Zhu B, Shao Y (2014). Rapid determination of trace phenols migrating into drinking water from plastic-based pipe materials and household water treatment equipment using vortex-assisted emulsification microextraction. Anal Methods 6: 3482-3489.
Discussion with Reviewers
Reviewer 1: What do you think about the use of the developed procedures for the analysis of other metals that pollute water and for the enhancement of other analysis techniques?
Authors: We believe that the protocol can be expanded to other toxic metals. However, the operational conditions and ligands should be studied/optimized for the new targets.
Reviewer 2: It seems that through vortexing is very essential for the transfer of analyte to the nonpolar organic phase. If manual mixing was mostly used so far, it should be included in the general extraction-dispersion protocol.
Authors: We appreciate the observation. The conventional DLLME uses manual shaking to disperse the droplets in the solution. As indicated in our paper, vortexing was introduced later to improve the dispersion of the extraction solvent droplets in water, thus reaching equilibrium conditions faster than manual mixing. Therefore, we opted for vortexing with its demonstrated effectiveness rather than manual mixing.
Reviewer 2: Fractional factorial design to screen out the key variables affecting VA-DLLME, and interaction plots of the variables, the central composite design, needs to be explained more easily for those who are not accustomed to these programs.
Authors: We thank you for the suggestion. We rephrased this in the paper to make it understandable for those who are not familiar with the methodology.
Reviewer 3: How did you control the vortexing conditions in your experiments? Or do vortexing conditions affect the outcomes of the measurements?
Authors: Our vortex device has frequencies indicated on it. As indicated in Figure 1, the higher the frequency the better the recovery of both mercury and lead.
Reviewer 3. How stable is this UV-Vis absorption reaction? How long can it last?
Authors: We have not studied the stability of these complexes; however, some studies (Corte-Real B, Hamad I, Hornero R et al. (2023). Sodium perturbs mitochondrial respiration and induces dysfunctional Tregs. Cell Metab 35(2): 299-315. https://pubs.acs.org/doi/full/10.1021/acs.inorgchem.3c01066) have demonstrated that these ligands form stable complexes that showed only 3% variation over a 24 h period.
Reviewer 3: If there are sufficient organics in the water samples (like many natural water samples), since most natural organic matter is amphiphilic, can this organic matter interfere with the measurements?
Authors: The effect of organic matter was not covered in this study, but it might affect the method’s efficiency depending on its content and nature.
Reviewer 3: What happens at higher pHs, like 9, or 10; the authors only tested lower pHs.
Authors: The effect of high pH is presented in Figures 4 and 5. Values of pH beyond the optimum range negatively affect the performance.
Reviewer (not specified): Can this protocol be used for seawater samples or higher salinity water samples?
Authors: We evaluated the effect of salts through salinity, whose overall effect was negative for lead quantification and positive for copper quantification (Fig. 1). A salinity of 5% was found to be optimum for copper quantification (Fig. 4). Based on that, we presume that the method would work to some extent for seawater, depending on the salinity and other species in water. However, we strongly recommend further studies to determine its performance in such conditions before the application.
Reviewer (not specified): Please estimate the total time needed to conduct this protocol. Is this protocol time efficient?
Authors: The protocols are time efficient. The overall time to execute these protocols is 10 min, which includes 1 min for pH adjustment, 1 min for adding the solvents, 1 min of vortexing, 5 min of centrifugation, and 2 min of absorbance readings. This time is compensated for by the other advantages that the method offers, particularly the cost-effectiveness in developing countries.
Reviewer (not specified): Will other divalent ions interfere with the outcomes, e.g., Ca2+, Zn2+, Mg2+, Mn2+?
Authors: Previous tolerance studies have indicated that the efficiency of DLLME is affected by divalent metals, especially heavy metals (https://www.sciencedirect.com/science/article/pii/S0308814614019979). So we believe that some of these species will interfere with the method’s efficiency. However, additional studies are needed to quantify the effect of each of these ions.
