A Study on the Changes in Physical Properties of Distilled Water Put In Contact with Porous Hydrophilic Materials: Experimental Evidence on Neapolitan Yellow Tuff
Vittorio Elia1, Elena Napoli1, Roberto Germano*2, Daniele Naviglio3, Martina Ciaravolo1, Giovanni Dal Poggetto4, Domenico Caputo3, Rosario Oliva1, Tamar A. Yinnon5,6
1 Department of Chemical Sciences, University ‘‘Federico II’’, Complesso Universitario di Monte Sant’Angelo, Via Cintia, I-80126 Napoli, Italy
2 PROMETE Srl, CNR Spin off, P.le V. Tecchio, 45 – 80125 Napoli, Italy
3Department of Chemical, Material and Industrial Production Engineering, University “Federico II”, P.le V. Tecchio, 80 80125 Napoli, Italy
4 ECORICERCHE Srl, Via Principi Normanni 81043 Capua (CE), Italy
5 K. Kalia, D.N. Kikar Jordan, 90666, Israel.
6 Reedmace Lake, Enot Tsukim Nature Reserve at Kalia, Israel.
*Corresponding author e-mail: germano@promete.it
Published: July 24, 2022
Abstract
A series of experimental measurements were performed on Milli-Q water samples in which a piece of Neapolitan Yellow Tuff (NYT) was immersed. As time changes, the liquid acquires new physicochemical properties that make it experimentally very different from the starting liquid. In particular, the values of a large number of physicochemical parameters change: electrical conductivity, pH, density, and heat of mixing with acids and bases. The measurements of Thermogravimetry (TGA), Differential Thermal Analysis (DTA), and the infrared absorbance spectra performed on the solid (Xerosydryle) obtained by lyophilization, show unusual but experimentally certain behaviors. The freeze-drying process of these liquids produces weight quantities of solid. The liquids obtained and on which the various experiments were carried out were decanted and filtered to eliminate Tuff particles that inexorably detach from the piece under examination. This extraordinary new phenomenology induced by an inorganic system, such as Tuff, has close similarities and some peculiarities with respect to the phenomenology that occurs by perturbing the water with organic, natural, or synthetic insoluble polymers.
Introduction
Over the past ten years, our research group (Elia et al. 2013a; Elia et al. 2014; Capolupo et al. 2014; Germano 2015; Zheng et al. 2006; Zheng and Pollack 2006; Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2017; Yinnon et al. 2016; Elia et al. 2015; Elia et al. 2014; Elia et al. 2013c; Elia et al. 2013d; Elia and Napoli 2011; Signanini et al. 2019) and another research group (Lo et al. 1996; Lo et al. 2009; Ho 2014) have produced a substantial number of experimental works published in physicochemistry journals of aqueous solutions on the emerging properties of water. The physical perturbations that produced these variations were induced by an iterative process of successive hydrations and dehydrations of both synthetic (Nafion) and natural hydrophilic insoluble polymers (cotton wool, paper filter, cellophane, hemp, silk and wool) (Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2017; Yinnon et al. 2016; Elia et al. 2015; Elia et al. 2014; Elia et al. 2013c; Elia et al. 2013d; Elia and Napoli 2011).
In particular, an iterative process of successive vacuum filtrations produces a very similar phenomenology (Elia et al. 2013a; Elia et al. 2014; Capolupo et al. 2014). What this procedure has in common is the iteration of a low-energy physical process. The extraordinary nature of the results is reproducible and involves variations in physicochemical parameters that have undergone variations up to almost four orders of magnitude. What is reported in this work is to our knowledge the first ever case of numerical variations of various physicochemical parameters by simple immersion in pure water of a piece of rock, such as a Neapolitan Yellow Tuff (NYT), a lithified pyroclastic material produced by the largest, in terms of thickness and areal extension, and most powerful eruption of “Campi Flegrei,” a large volcanic area situated to the west of Naples, Italy (de’ Gennaro et al. 1990; de’ Gennaro et al. 2000).
This is the first case of extension to inorganic systems to what has already been documented with the use of insoluble organic polymers! One of the strangest peculiarities is linked either to the experimental fact that a simple freeze-drying process of these liquids produces a solid whose physicochemical characteristics depend on the substance that disturbs the liquid. It should be emphasized that the solids obtained by lyophilization of the perturbed liquids have a chemical composition not yet definitively ascertained. However, they are profoundly different from perturbing polymers and from Tuff itself.
Experimental Design
The parent phillipsite-rich rock came from a quarry in Chiaiano (Naples, Italy), belonging to the huge formation of NYT (de’ Gennaro et al. 1983). This phillipsite-rich material in Marano (Naples, Italy) occurs in the NYT deposit, the product of one of the largest, in terms of thickness and areal extension, and most powerful eruptions of Campi Flegrei. A substantial portion of this pyroclastic rock (50% in volume) was involved in diffuse zeolitization processes that determined the crystallization of phillipsite and, subordinately, chabazite and analcime (Iucolano et al. 2005). Zeolitization in NYT took place soon after eruption in a well-insulated thermal system in the presence of hot aqueous solutions of hydromagmatic origin, whose ionic composition was controlled by equilibrium glass hydrolysis (Perić et al. 1999). The investigated outcrop shows thicknesses of about 30 m with quite variable zeolite content, always higher than 50% wt. Italy (Europe) – Marano, (Naples, Italy) – Private quarry, owner Savanelli.
A sample of NYT coming from a quarry located in Marano (Napoli, Italy) was used. A piece of NYT measuring approximately 10x15x10 cm (1500 cm3) was immersed in quantities of Milli-Q water ranging between 1 and 0.1 Liters of very pure water (Milli-Q). To eliminate any soluble fraction of the component minerals, the Tuff was subjected to a preliminary prolonged washing in bidistilled water. Over time, some of its physicochemical properties were measured: electrical conductivity, pH, and density. Electrical conductivity is the most frequently used measure to follow the evolution of the properties of the liquid. With the measurement of electrical conductivity, increases of up to three orders of magnitude were found. When the volume of the liquid is excessively reduced, whether for reasons of consumption, evaporation or whatever, it is topped off with new Milli-Q water. There are no other interventions. The physicochemical parameters vary in an absolutely measurable and reproducible way. This statement is supported by the linear trends of the graphs of pH vs log χ, density vs χ, where χ is the electrical conductivity.
Density Measurements
The solution densities were measured using a vibrating tube digital density meter (model DMA 5000 by Anton Paar, Austria) with a precision of ±1×10-6 g cm-3 and an accuracy of ±5×10-6 g cm-3. The temperature of the water around the densitometer cell was controlled to ±0.001 K. The densitometer was calibrated periodically with dry air and pure water.
The density measurements of the liquid obtained by immersing the “brick” of NYT in Milli-Q water were carried out. As can be seen from the tables and graphs, there are significant increases in the density of the water that has been in contact with the piece of Tuff. As mentioned in the Introduction, significant variations of some chemical and physical parameters are obtained by prolonged holding of the piece of Tuff in contact with water. The increase in density records a linear trend as a function of electrical conductivity, χ. Naturally, given the friable nature of the Tuff, the iteratively perturbed water (IPW) registers the presence of solid particles of the zeolitic material. To eliminate this drawback that would affect the density values, the liquid is left to decant and subsequently filtered with suitable paper filters. The density, pH and electrical conductivity measurements are carried out on the liquid thus obtained.
The density ρ (g/cm3) vs the specific electrical conductivity χ (µScm-1) is linear. See Figure 1 and Table 1.
The described trend is a demonstration of the reproducibility of the phenomenon, as easily detectable by the high numerical values of the mean square deviation resulting from the interpolation of the experimental data.
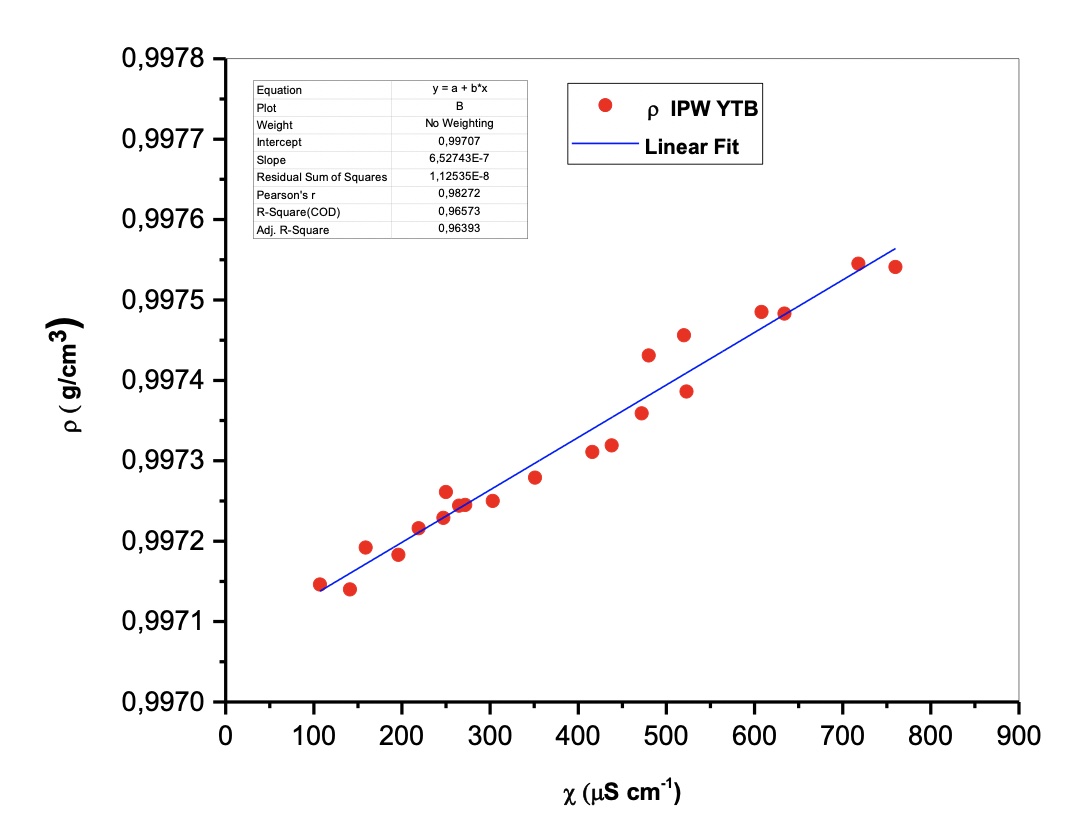
Figure 1. The density ρ (g/cm3) vs the specific electrical conductivity χ (µScm-1)
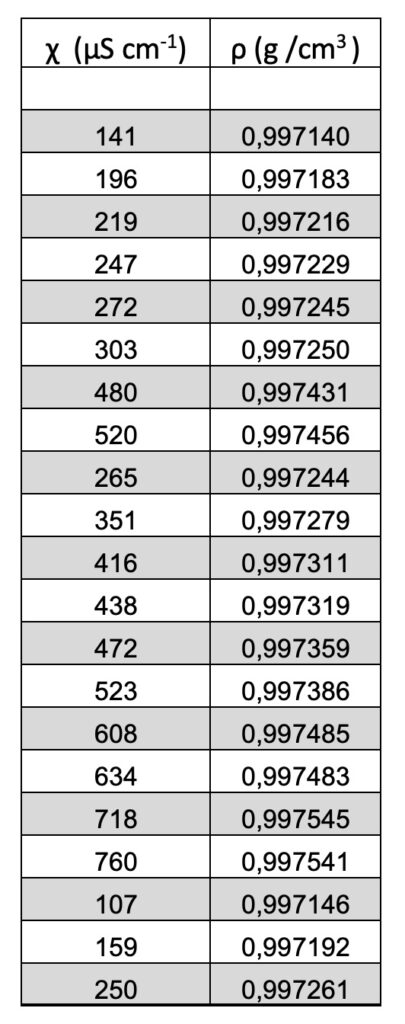
Table 1. The density ρ (g/cm3) vs the specific electrical conductivity χ (µScm-1).
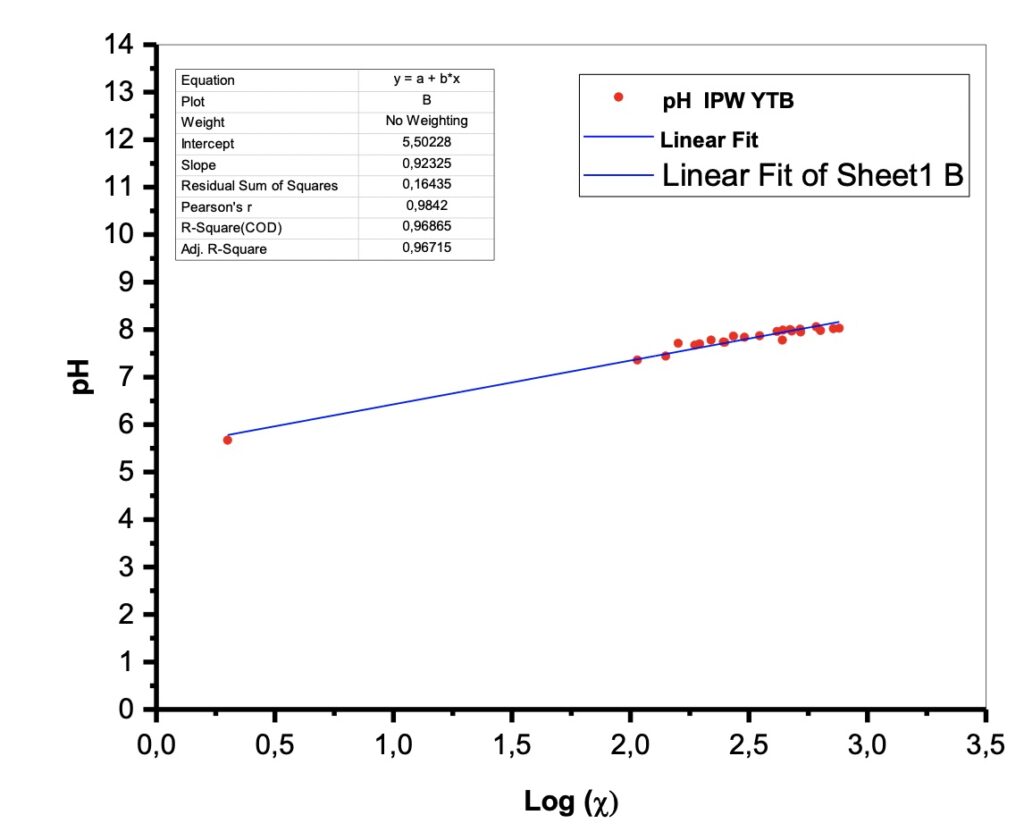
Figure 2. pH vs the conductivity log.
pH Measurements
The pH measurements were carried out with a pH meter (Crison GLP 21-22), with a resolution of ± 0.01 pH units, equipped with a pH electrode for microsamples, model 52 09. The electrode specifications are: asymmetry potential < ±15 mV, pH sensitivity 4…7 (at 25° C) > 98 %.
There were pH measurements made on the liquid samples. The samples exhibit values in the range of alkaline solutions with pH values around 8.
Experimentally it appears that the pH (vs the conductivity log) is also linear. See Figure 2.
This result has been interpreted in the literature as a proof of a quantum behavior that can be described by fractal mathematics. See Table 2. In fact, recently, the “fractal behavior” of these dissipative structures was experimentally evidenced (Capolupo et al. 2014) and this seems to confirm even further their coherent quantum nature, also discussed in Germano (2015). An isomorphism exists between the observed scale-free, self-similar properties of the physical “modified” liquid water and the deformed coherent state formalism. The fractal dimension, in fact, provides a measure of a dynamical “deformation,” so that the observed scale-free law relating, e.g., pH and electric conductivity, is the macroscopic manifestation of dissipative local deformations at a microscopic level. This extremely interesting isomorphism was first theoretically evidenced in its whole generality only a few years ago, in a seminal work by G. Vitiello (2012), in which he demonstrated that fractal self-similarity properties can be described in terms of coherent states, and consequently quantum dissipation, through quantum deformation or squeezing of coherent states, and seems to be at the origin itself of the self-similarity properties at a macroscopic level.
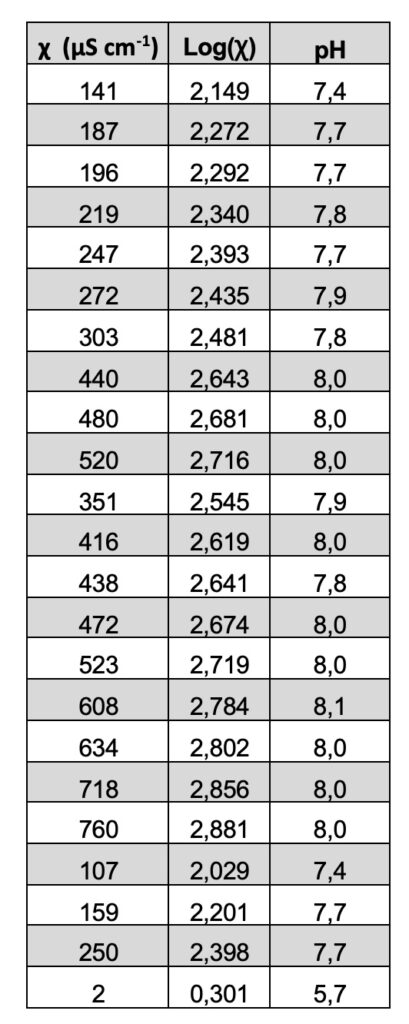
Table 2.
Electrical Conductivity Measurements
Specific electrical conductivity, χ (µS cm-1), measurements were performed with a YSI 3200 Conductivity Meter, model 3200. The cell was periodically calibrated by determining the cell constant K (cm-1). The specific conductivity, χ (µS cm-1), was then obtained as the product of the cell constant and the solution conductivity. For a given conductivity measuring cell, the cell constant was determined by measuring the conductivity of a KCl solution with a specific conductivity known with great accuracy, at several concentrations and temperatures. All conductivities were measured in a room at controlled temperature of 25±1°C and temperature-corrected to 25°C, using a pre-stored temperature compensation for pure water.
Extremely high electrical conductivity measurements χ (µS/cm) were registered, with increases compared to those of the Milli-Q water used greater than two orders of magnitude (Table 2).
It is necessary to emphasize that, given the insoluble nature of the NYT, it is not possible to interpret the changed physicochemical picture of the liquid as due to the release from the Tuff of substances, electrolytic or not, into the water.
For insoluble organic polymers (Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2014; Elia et al. 2013c), the increases in physicochemical parameters have been interpreted in the literature with the presence of aggregates of water molecules. It was highlighted by optical, fluorescence, electron and atomic force microscopy measurements (Elia et al. 2013b; Elia et al. 2017).
The increase in χ is consistent with the hypothesis of aggregates of water molecule formation that increase the contribution of the proton jump mechanism (Elia et al. 2014).
Variations in pH are supported by calorimetric measurements that determine the value of the binding constant of the proton or hydroxyl ion through calorimetric titration measurements (Elia et al. 2013d).
The density variations, based on the experimentally determined presence of aggregates of water molecules, are more easily understood.
In the literature there are experimental works in which it is unequivocally demonstrated that the lyophilization process on perturbed liquids (IPW) leads to the obtaining of solids with physical properties clearly distinct from those of perturbing substances (insoluble hydrophilic polymers of various kinds) (Elia et al. 2013a; Elia et al. 2014; Capolupo et al. 2014; Germano 2015, Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2017; Yinnon et al. 2016; Elia et al. 2015; Elia et al. 2014; Elia et al. 2013c; Elia et al. 2013d; Elia and Napoli 2011).
A first aspect to check for the peace of mind of the operators is the reproducibility of the described phenomena. The linear trends of the parameters pH, density and electrical conductivity, of the so-called log/log diagrams are a first demonstration of this. The linearity exhibited is an indication that phenomenology was produced by a single cause.
In the reported research, we show for the first time that this new phenomenology belongs, therefore, also to inorganic materials such as the NYT. Great prospects for study are developing in the field of organic and inorganic substances, including the materials produced by volcanic eruptions!
Freeze Drying
Freeze drying has been carried out using a Manifold Freeze Dryer MFDQ 2002, with a tabletop unit having a condensing capacity of 3 to 4 kg/24 h, reaching a temperature of -80°C. The instrument was equipped with 8 trays with a bulk capacity of 2.4 l, allowing the freeze drying of any sample shape and size. The lyophilization (water removal by sublimation) was performed by freezing the samples and reducing subsequently the environmental pressure so that the frozen water sublimates, converting directly from solid to gaseous state without passing through the liquid phase.
IR Spectra
We recorded IR spectra of RIPW in a KCl dispersion medium, using an FT-IR Jasco-FT-IR-430 spectrophotometer. The recording conditions for each FT-IR spectrum were: 64 scans, scanning speed of 2 mm/sec, a triangular apodization function and a resolution of 2 cm-1.
To characterize this new class of solid materials, Xerosydryle, produced by lyophilization of perturbed liquids (IPW), experimental measurements of absorbance spectra in the infrared IR were performed (Figure 3) on it.
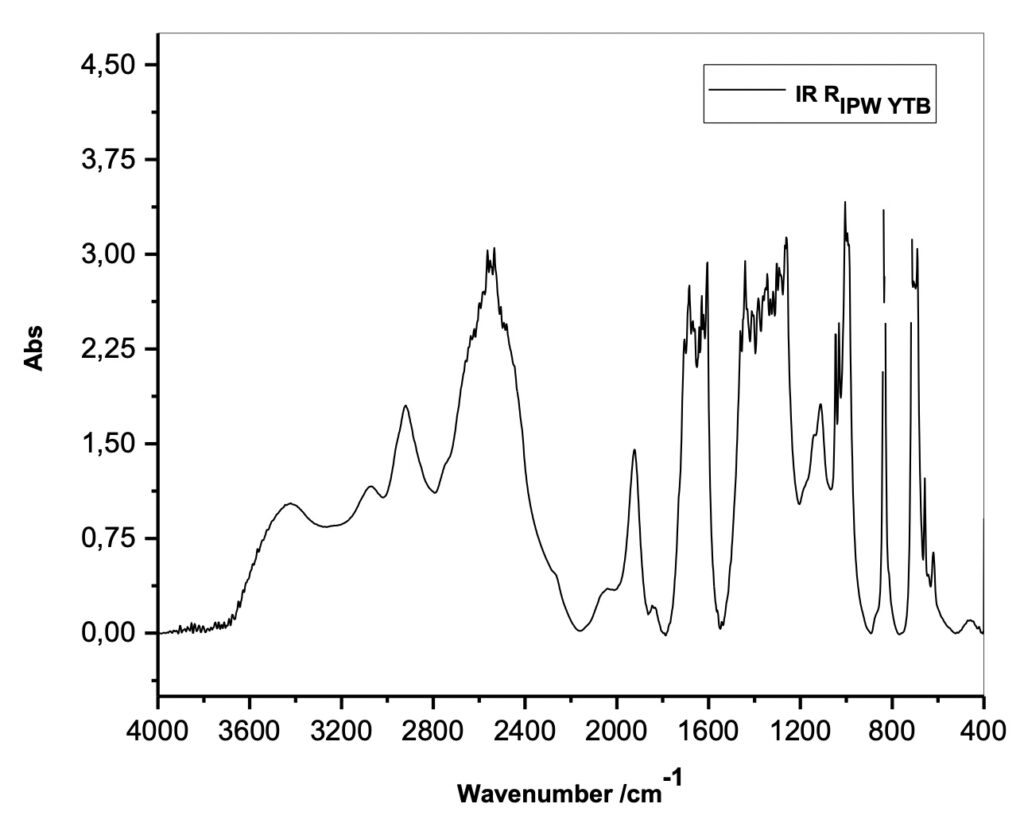
Figure 3. Absorbance spectra in the infrared range (IR) of the Xerosydryle (RIPW).
Differential Thermal Analyses (DTA)
We did the DTA analyses with a Netzsch thermoanalyzer model STA 409 Luxx. We weighed samples of about 10 mg. We heated the samples on alumina pans from 25 to 950 °C. The heat rate was 20 K/min. We heated the samples in air or in a N2 flow. We used α-Al2O3 as a reference material.
In this phase of studying chemically still unknown and unpredictable substances according to the current dominant paradigms, we decided to verify the reproducibility of the TGA experiments. For this purpose, three IPW samples of different electrical conductivity were produced in subsequent times, even months away from each other (see Figure 4), respectively of 166, 227, and 490 χ (µS/cm) and comparable volumes of liquids of the order of 100-200 ml. Each preparation of the perturbed liquid was subjected to the lyophilization process obtaining three solids called RIPW (Xerosydryle).
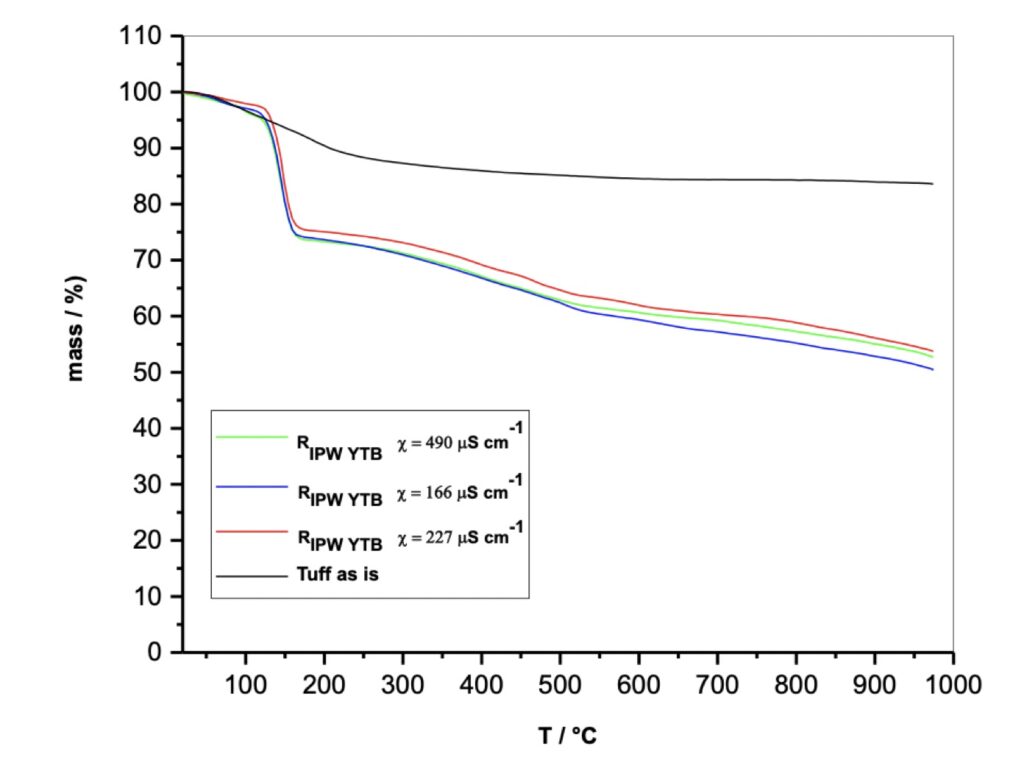
Figure 4. Thermogravimetric analysis, TGA of the Tuff as it is and of the relative Xerosydryle (RIPW) .
Three distinct Gravimetric Thermal Analysis experiments were performed on the three Xerosydryle samples, reaching temperature values close to 1000°C (see Figure 4). They show a weight loss of about 25% around 150°C and a subsequent further loss between 150°C and 1000°C. On the same graph you can see the experiment obtained with the use of NYT powder as it is. Also, in this case, there is a first weight loss of around 10% in the first 150°C and a flat trend up to 1000°C.
Beyond details, numerically of secondary importance, the difference in behavior with respect to the thermal stability of the Tuff as it is and that of the three solid samples obtained by freeze-drying is extremely clear. The simplest and most indisputable difference between the perturbing material (Tuff) and the relative product for lyophilization, (Xerosydryle), is represented by the insolubility of the Tuff and the solubility of the Xerosydryle in water. In turn, the three samples deriving from three different IPWs are practically reproducible. With these experiments, what has already been achieved previously with the use of insoluble hydrophilic polymers of organic nature are confirmed polymers (Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2014; Elia et al. 2013c). We have also extended the phenomenology of the DTA for the three samples mentioned above to the description of the TGA. Also, for this technique there is a remarkable reproducibility if the nature of the experiments is taken into account. With the DTA method, it can be seen that around 150°C, in correspondence with the weight loss of about 25%, there is a considerable endothermic phenomenon that is probably attributable to the de-structuring of aggregate water and therefore more stable than normal hydration water which, as known, is eliminated around 100°C. In fact, the experiments carried out on Tuff powder as such show a normal elimination of water around 100°C. It can be seen that the Tuff exhibits a completely different behavior from the RIPW (Xerosydryle). These are different substances, and phenomena are experimentally reproducible and not explained by current theories on water.
The reproducibility of the phenomenology produced was strengthened by having obtained a linear trend between the increase in the weight of the Xerosydryle obtained by freeze drying the perturbed liquid, RIPW as a function of χ. The good linear trend is highlighted by the high value of the mean square deviation R2.
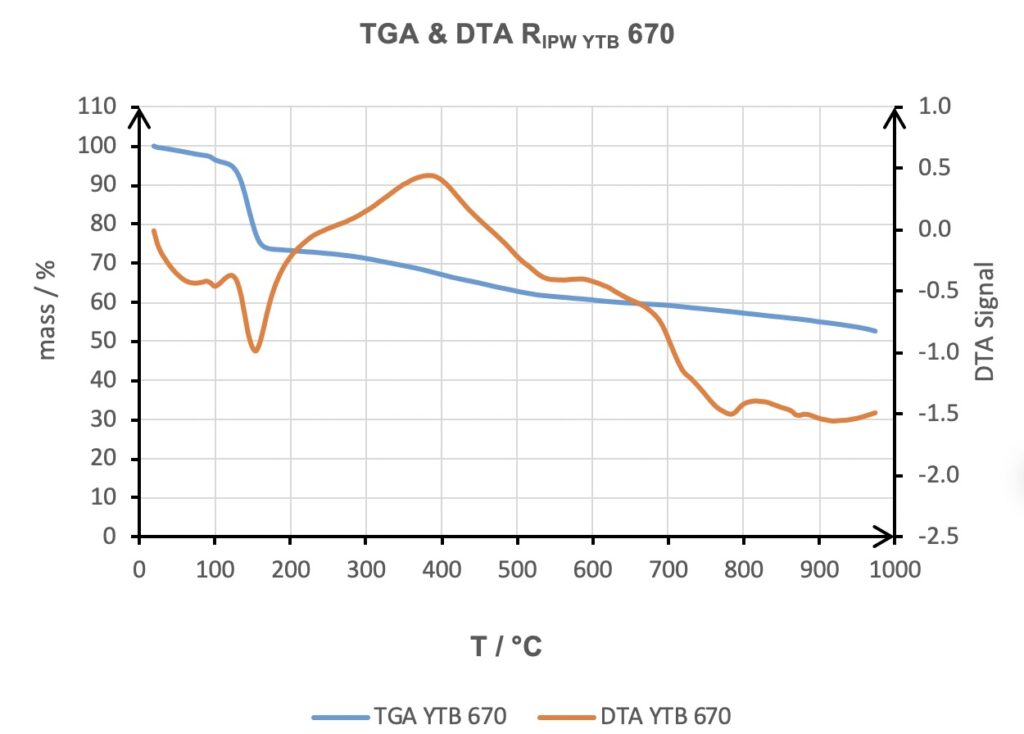
Figure 5. Thermogravimetric Analysis and Differential Thermal Analysis (DTA) of the Xerosydryle (RIPW) obtained by water in contact with Tuff.

Figure 6. Photo of the macroscopic Xerosydryle, 129 mg, obtained by lyophilization of 250 ml of pure water put in contact with Tuff, after it had reached the Electrical Conductivity of 453 µS/cm.
Conclusions
The phenomenology described in this work relates to the ability to modify the physicochemical properties of water with iterative procedures of a physical nature and low-energy content (Elia et al. 2013a; Elia et al. 2014; Capolupo et al. 2014; Germano 2015; Elia et al. 2013b; Elia et al. 2018; Elia et al. 2019; Elia et al. 2022; Elia et al. 2020; Elia et al. 2017; Yinnon et al. 2016; Elia et al. 2015; Elia et al. 2014; Elia et al. 2013c; Elia et al. 2013d; Elia and Napoli 2011; Signanini et al. 2019).
The use of insoluble organic polymers and, for the first time of an insoluble inorganic system, Tuff, identify new and interesting problems to be addressed. The common factor is water. Once again, the most studied substance in the world turns out to be an unknown capable of surprising us.
Discussion With Reviewers (DWR)
Reviewer: The work of Elia et al. describes very interesting phenomena related to the changes in physicochemical properties of water in contact with the inorganic material. Previous works of the authors on the effect of organic polymers on water are known to me, so this work is of particular significance as far as the reproducibility of the phenomena in different systems is considered. Nevertheless, pulling apart previous results, I have some concerns regarding the effect of the inorganic material now studied.
Neapolitan Yellow Tuff (NYT) is composed mainly of zeolites, which are highly porous aluminosilicate minerals with very high cation-exchange capacity. It means that inside of their insoluble three-dimensional negatively charged framework they accommodate a very high concentration of mobile cations that can be easily exchanged with ions from aqueous media.
Could the authors comment on the possibility that the observed changes in water in contact with such material can be related to the ability of the latter to release ions to water and exchange them for protons from water molecules? This would agree with the observed increase in pH of water in contact with the solid (protons are removed so pH shifts toward more basic) as well as with the significant conductivity increase.
In the description of the water imprinting method with the use of Tuff material, the methodology of the bidistilled water treatment is described. From this, it is clear that any ion exchange phenomena, assuming that they can occur between the Tuff material and the double distilled water, which is extremely unlikely given the concentration of ions in the double distilled water, should be exhausted in the washing procedure. On the contrary, the phenomenology of increased conductivity and pH continues for months of immersion of the Tuff in double-distilled water. Any ionic exchanges between the ions of the Tuff and the double distilled water (liquid practically free of ions) is really difficult to hypothesize!
I guess it is experimentally not accessible for the authors at the moment, but at some point, I would like to see analysis of cations present in the perturbed liquid, e.g., studied by atomic absorption spectroscopy.
Authors: It is necessary to underline that the washing operations with bidistilled water are interrupted when, in subsequent washing operations to eliminate the water-soluble chemical components, they no longer produce significant variations in electrical conductivity. This phase indicates that the soluble components of the Tuff have been eliminated.
At this point the systematic imprinting of the water begins. It consists of adding bidistilled water to the container where the piece of Tuff is located. The procedure is “iterated” simply by leaving the piece of Tuff immersed in the bidistilled water. Naturally, when the “imprinted” water is taken to carry out the necessary operations of measurement of the parameters described in the manuscript, other virgin water is added.
The various recorded phenomena are numerically very significant, as can be deduced from the reported data up to three orders of magnitude for the electrical conductivity. The “imprinted” water is consumed substantially in significant quantities in freeze-drying operations. To obtain weighable quantities of Xerosydryle, it is necessary to consume a few hundred milliliters of imprinted water. However, by adding new double-distilled water to the container where the Tuff is, the phenomenology is repeated!
It should be emphasized that the idea of iterative freeze drying does not correspond to the procedure adopted. The iteration in this procedure consists of adding new water and waiting for the phenomenology to repeat itself. Once “imprinted” water has been eliminated, for example because a certain ponderable quantity of solid (Xerosydryle) has been produced, to obtain more imprinted water we just add double distilled water to the container where the piece of Tuff is located.
Time will allow us to produce quantities, which can be assumed to be infinite, by repeating the addition of bidistilled water.
This is discussed in more detail in our Experimental Design and Electrical Conductivity Measurements Sections.
Reviewer: In the Electrical Conductivity Measurement section, the NYT is described as insoluble. Since this information is important in understanding the conductivity data, it is recommended to show the solubility data of the Tuff in water.
Authors: A typical mineralogical composition of a zeolitic tuff such as the NYT coming from Marano (Na) is here reported:
Phillipsite=46%; chabazite=5%; smectite=10%, analcime 9%, feldspar= 26% and minor amounts of pyroxene and mica. (Iucolano et al. 2005)
So, the main mineralogical phases are zeolites (phillipsite, chabazite and analcime). As reported in literature (Peric et al. 1999), the hydrolysis of natural zeolites in doubly distilled water is a very rapid process (in a few tens of minutes).
Therefore, a prolonged washing in bidistilled water is enough to eliminate any soluble fraction of the component minerals and stabilize the zeolitic phases, as demonstrated by the very low values of the electrical conductivity of the washing water (very close to the value of the used doubly distilled water).
Reviewer: In Figure 3, you show the absorbance spectra of IPW YTB. Since this material, IPW YTB, was obtained from water through iterative lyophilization, it will be very helpful to see the distinctive difference if the IR absorption of water is shown together.
Authors: As previously stated, the iterative freeze-drying operation has never been used for any of the produced Xerosydryle(s).
As described in the procedure for preparing water imprinted with Tuff, we have never written anything that could generate this hypothesis. We reiterate that the various freeze-drying procedures refer to different imprinted liquids by varying the immersion times of the piece of Tuff in bidistilled water. As you can see, this is a different procedure from that of imprinted liquids with the use of natural or synthetic insoluble polymers. In this case the iteration consists of immersing the polymer in water and after about 12-24 hours extracting it from the liquid by squeezing it. The polymer will then be left to dry. The iteration consists of repeating the drying operation and immersion of the polymer in the imprinted water.
Finally, it is not possible to obtain any solid residue by lyophilization of pure water on which one is performing an IR absorbance measurement.
Reviewer: Figure 4 was obtained from Xerosydryle material that was obtained from the mixture of water and Tuff. If the origin of Xerosydryle is purely from 100% water, it would be very difficult to explain the curves of Figure 4. It seems that the water-like portion of the materials evaporated or dissociated at 150 0C. This graph shows that there was something left after heating the Xerosydryle even at 1000 0C. What is the composition of that residue? It is necessary to confirm this residue. The residue may be debris or fine powder from the Tuff. Possibly, this residue may be a hydrophilic component from Tuff and acting as a kind of embryo for the formation of Xerosydryle.
Authors: This experimental result is totally in agreement with our previous dozens of papers on Xerosydryle, including our last one in which we baptize this new class of materials with this name, confirms totally this kind of behavior (Elia et al. 2022).
Reviewer: In Figure 5, the DTA signal around 400 0C shows the exothermic reaction is occurring. Can you discuss the origin of this behavior in the manuscript? No explanation is given. Could it be related to the hydrophilic component from Tuff?
Authors: As we showed in many papers about Xerosydryle, it is comprised of a certain percentage of carbon component, ranging from almost zero to more than 50%. This peak is clearly coming from this component of the Xerosydryle. The Tuff cannot be involved.
References
Capolupo A, Del Giudice E, Elia V, Germano R, Napoli E, Niccoli M, Tedeschi A, Vitiello G (2014). Self-similarity properties of Nafionized and filtered water and deformed coherent states. Int. J. Mod. Phys. B 28, 1450007. https://doi.org/10.1142/S0217979214500076
de’ Gennaro M, Colella C, Franco E, Aiello R (1983). Italian zeolites 1. Mineralogical and technical features of Neapolitan yellow tuff. Ind. Miner. 186, 47.
de’ Gennaro M, Petrosino P, Conte MT, Munno R, Colella C (1990). Zeolite chemistry and distribution in a Neapolitan yellow tuff deposit. Eur. J. Mineral., 2, 779-786. https://doi.org/10.1127/ejm/2/6/0779
de’ Gennaro M, Cappelletti P, Langella A, Perrotta A, Scarpati C (2000). Genesis of zeolites in the Neapolitan Yellow Tuff: geological, volcanological and mineralogical evidence. Contrib. Mineral. Petrol., 139 (1), 17-35. https://doi.org/10.1007/s004100050571
Elia V, Napoli E (2011). Nanostructures of water molecules in iteratively filtered water. Key Eng. Mater. 495, 37-40. https://doi.org/10.4028/www.scientific.net/KEM.495.37
Elia V, Marchettini N, Napoli E, Niccoli M. (2013a). Calorimetric, conductometric and density measurements of iteratively filtered water using 450, 200, 100 and 25 nm Millipore filters. J. Therm. Anal. Calorim. 114, 927-936. https://doi.org/10.1007/s10973-013-3046-y
Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M (2013b). Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with a Nafion® polymer membrane. Water 5, 16-26.
Elia V, Napoli E, Niccoli M (2013c). Physical-chemical study of water in contact with a hydrophilic polymer: Nafion. J. Therm. Anal. Calorim. 112, 937-944. https://doi.org/10.1007/s10973-012-2576-z
Elia V, Napoli E, Niccoli M (2013d). Calorimetric and conductometric titrations of nanostructures of water molecules in iteratively filtered water. J. Therm. Anal. Calorim. 111, 815-821. https://doi.org/10.1007/s10973-011-2164-7
Elia V, Lista L, Napoli E, Niccoli M (2014). A Thermodynamic characterization of aqueous nanostructures of water molecules formed by prolonged contact with the hydrophilic polymer Nafion. J. Therm. Anal. Calorim. 115, 1841-1849. https://doi.org/10.1007/s10973-013-3371-1
Elia V, Ausanio G, De Ninno A, Germano R, Napoli E, Niccoli. M (2014). Experimental evidences of stable water nanostructures at standard pressure and temperature obtained by iterative filtration. Water 5, 121-130.
Elia V, Germano R, Napoli E (2015). Permanent dissipative structures in water: the matrix of life? Experimental evidences and their quantum origin. Curr. Top. Med. Chem. 15, 559-571. https://doi.org/10.2174/1568026615666150225102531
Elia V, Yinnon TA, Oliva R, Napoli E, Germano R, Bobba F, Amoresano A (2017). Chiral micron-sized H2O aggregates in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water. Water 8, 1-29.
Elia V, Oliva R, Napoli E, Germano R, Pinto G, Lista L, Niccoli M, Toso D, Vitiello G, Trifuoggi M, Giarra A, Yinnon TA (2018). Experimental study of physicochemical changes in water by iterative contact with hydrophilic polymers: A comparison between Cellulose and Nafion. J. Mol. Liq. 268, 598-609. https://doi.org/10.1016/j.molliq.2018.07.045
Elia V, Napoli E, Germano R, Oliva R, Roviello V, Niccoli M, Amoresano A, Naviglio D, Ceravolo M, Trifuoggi M, Yinnon TA (2019). New chemical-physical properties of water after iterative procedure using hydrophilic polymers: the case of paper filter. J. Mol. Liq., 296, 111808. https://doi.org/10.1016/j.molliq.2019.111808
Elia V, Napoli E, Germano R, Roviello V, Oliva R, Niccoli M, Amoresano A, Toscanesi M, Trifuoggi M, Fabozzi A, Yinnon TA (2020). Water perturbed by Cellophane: Comparison of its physicochemical properties with those of water perturbed with cotton wool or Nafion. J. Therm. Anal. Cal. 146, 2073-2088. https://doi.org/10.1007/s10973-020-10185-0
Elia V, Napoli E, Germano R, Naviglio D, Ciaravolo M, Dal Poggetto G, Caputo D, Oliva R, Yinnon TA (2022). New physicochemical properties of liquid water resulting from recurrent contact with hydrophilic polymers. Characteristics of the resulting supramolecular aggregates: the Xerosydryle. Water 12, 72-85.
Germano R (2015). Water’s Permanent Dissipative Structures Quantum Origin And Life. Electromagnetic Biology and Medicine 34 (2), 133-137. https://doi.org/10.3109/15368378.2015.1036074
Ho MW (2014). Large Supramolecular Water Clusters Caught on Camera – A Review. Water 6, 1-12.
Iucolano F, Caputo D, Colella C (2005). Permanent and safe storage of Ba2+ in hardened phillipsite-rich tuff/cement pastes. Applied Clay Science 28 (1-4), 167-173. https://doi.org/10.1016/j.clay.2004.01.011
Lo S-Y, Lo A, Chong LW, Tianzhang L, Hui Hua L, Geng X (1996). Physical properties of water with IE structures. Modern Physics Letters B 10, 921-930. https://doi.org/10.1142/S0217984996001048
Lo S-Y, Geng X, Gann D (2009). Evidence for the existence of stable-water-clusters at room temperature and normal pressure. Physics Letter A 373, 3872-3876 https://doi.org/10.1016/j.physleta.2009.08.061
Perić J, Trgo M, Ćurković L (1999). Monitoring of hydrolysis in natural zeolite-H2O systems by means of pH and electrical conductivity measurements. Studies in Surface Science and Catalysis 125, 761-767. https://doi.org/10.1016/S0167-2991(99)80284-5
Signanini P, Vessia G, Elia V, Napoli E, Germano R (2019). A study on the changes in physical properties of demineralized water put in contact with porous hydrophilic materials: experimental evidences on metabrick material. Journal of Porous Media 22 (12), 1609-1625. https://doi.org/10.1615/JPorMedia.2019026816
Vitiello G (2012). Fractals, coherent states and self-similarity induced noncommutative geometry. Phys. Lett. A 376, 2527-2532. https://doi.org/10.1016/j.physleta.2012.06.035
Yinnon TA, Elia V, Napoli E, Germano R, Liu Z-Q (2016). Water ordering induced by interfaces: an experimental and theoretical study. Water 7, 96-128.
Zheng JM, Pollack GH (2006). Solute exclusion and potential distribution near hydrophilic surfaces. In: GH Pollack, IL Cameron, DN Wheatley (Eds.), Water and the Cell, Springer, Netherlands, Dordrecht.
Zheng JM, Chin WC, Khijniak E, Khijniak Jr. E, Pollack GH (2006). Surfaces and interfacial water: evidence that hydrophilic surfaces have long-range impact. Adv.Colloid Interf. Sci. 127, 19-27. https://doi.org/10.1016/j.cis.2006.07.002
