 Vigorous Shaking Enhances Voltage and Power Generation in Polar Liquids due to Domain Formation as Predicted by QED
Vigorous Shaking Enhances Voltage and Power Generation in Polar Liquids due to Domain Formation as Predicted by QED
Authors: Poonam Bandyopadhyay1,2, Debbethi Bera1, Kaushik Das1, Biplab Kumar Paul1, Sukhen Das1,2, Durga Shankar Bhar1, Raj Kumar Manchanda3, Anil Kumar Khurana3, Debadatta Nayak3, Ruma Basu1, Papiya Nandy1*
1Centre for Interdisciplinary Research and Education, 404 B Jodhpur Park, Kolkata-700 068, India
2Department of Physics, Jadavpur University, 188 Raja S. C. Mallik Road, Kolkata-700 032, India
3Central Council for Research in Homeopathy, 61-65 Institutional Area, Janakpuri, New Delhi 110058, India
*Corresponding author: pnandy00@gmail.com
Received January 2, 2017; Revised May 6, 2017; Accepted May 9, 2017; Published: August 19, 2017;
Available online: August 19, 2017
Abstract
Using a U-shaped glass tube where one arm contains bi-distilled water and the other arm ethyl alcohol (91%) separated by a platinum foil, the generated voltage across two platinum electrodes and a DC power of the order of nanoW were measured. The generated voltage lasted for many hours. The magnitude of both the voltage and power generated increased with vigorous shaking of the alcohol.
Considering the absence of any significant quantity of ionic solutes in this system, voltage generation from two different polar liquids separated by a metal separator is an interesting phenomenon in the context of classical electrochemistry and seems to imply some kind of non-ionic conduction. A qualitative explanation of this phenomenon has been offered here based on the principle of Quantum Electrodynamics.
Keywords
Polar liquid; voltage-power generation; domain formation; succussion; quantum electrodynamics.
Introduction
Driven by the modern lifestyle’s growing demand for energy and significant pressure to protect the environment, newer technologies for getting electrical energy from eco-friendly alternative energy sources have been one of the major objectives of present day research.
Photo-voltage generation using solar power has been studied intensively for several decades. The limitations of conventional silicon technology based on photo-voltage have led to the use of varieties of nanoparticles (NPs) as the new building blocks to construct light energy harvesting assemblies (Gratzel, 2005; Nakayama et al., 2008; Chou et al., 2008; Vansark et al., 2012; Kamat, 2007). New initiatives like use of biomimetic systems to simulate natural photosynthesis (Choi et al., 2004; Das et al., 2004; Rybtchinski et al., 2004; Gratzel, 2010) and fabrication of hybrid solar cells by using nanoparticles have also been very promising (O’Regan et al, 1991; Gunes et al., 2010; Baxter et al., 2005; Martinson et al., 2007; Greene et al., 2007; Beek et al., 2004).
Recently, it was shown that power can be generated by perturbing polar liquids (water) with Nafion membranes in the presence of H2O2 and K2CO3 (Germano et al., 2012). The experiment was followed by another one, where electricity was extracted from bi-distilled water in the presence of Nafion and H2O2 (Germano et al. 2013). According to the authors, electricity extraction in the absence of any significant quantity of ionic solutes is an astonishing phenomenon in the frame of classical electrochemistry and it seems to imply some kind of non-ionic conduction. The authors have explained the phenomena within the context of Quantum Electrodynamics (QED).
In the present work, it is shown that electricity could be generated by using a U- shaped glass tube, whose one arm contained 91% ethyl alcohol and the other bi-distilled water, separated by a platinum foil. Two platinum electrodes were placed symmetrically in the two chambers. Electricity has been generated in this system in the absence of any significant ion source. Both the voltage (~ mV) and the power (~ nW) output, though small, reached a plateau region and continued for many hours.
It was further observed that the magnitude of the above two quantities increased with vigorous shaking of the alcohol. This process of vigorous shaking is technically termed as “succussion,” which imparts energy to the system. An estimation of the impact of the force applied by succussion in a specific setting has been calculated (Shah, 2016).
This extraction of electricity using only two different polar solvents and without any external agent to provide charge carriers, is in itself a unique phenomenon from the perspective of classical electrochemistry. Using QED as applicable in the case of polar liquids, an attempt has been made here to explain this observed enhancement of electrical energy with increase in succussion.
Materials and Experimental Set-up:
91 % Ethyl alcohol was prepared from pure ethyl alcohol (E. Merck, India) and was succussed by using the standard procedure for preparing homeopathic liquids, i.e., by striking a bottle, 2/3 filled with alcohol, on a hard but elastic body§. There was no dilution after that. The alcohol samples were succussed individually and then poured in the one arm of the U-tube. The bi-distilled water was poured in the other arm. The experiments were performed within 2-3 days after the succussion, while the samples were kept under ambient conditions in normal glass bottles.
The ethyl alcohol and bi-distilled water of conductivity ~1.5 µS/cm and ~5.5 µS/cm respectively were used throughout the experiment.
The following samples were used:
Sample A : unsuccussed,
Sample B : succussed 60 times
Sample C : succussed 300 times
Sample D : succussed 2000 times
The experimental cell was a glass U-tube containing ethyl alcohol in one arm and bi-distilled water in the other arm, the two arms being separated by a platinum foil. (Fig. 1)
Using a 1 MΩ resistance, the open circuit voltage and current were measured with a digital multimeter (87 V Fluke) and a Keithley Electrometer (DM 196). The measurements were made for several hours even after reaching the saturation values.
§Homeopathic Pharmacopoeia of India, 1971, published by Ministry of Health, Govt. of India. The Homeopathic Pharmacopoeia of the United States (on-line version).
Figure 1. Experimental cell (R: resistor, A: Ammeter, E: Electrometer, S: separator)
Dimensions of different parts used in the experimental set up were as follows:
Dimension of the cell:
Diameter of the connecting tube: 1.27 cm (d1)
Diameter of each arm: 1.83 cm (d2)
Length of each arm: 11.0 cm (L1)
Length of the connecting tube: 3.4 cm (L2)
Dimension of the rectangular platinum electrode (pt E):
Length: 5.8 mm
Width: 4.9 mm
Thickness: 0.27 mm
Dimension of the platinum separator (S):
Circumference: 126.6 sq. mm.
Thickness: 0.3 mm
Results
The voltage and the power generated in the experimental cell using two different liquids and in the absence of any source of ions was observed and these effects were enhanced when alcohol was succussed. The time variations of open circuit voltage and power are shown in Fig. 2 and Fig. 3 respectively. For each sample the experiment was repeated at least three times. The process was found to be qualitatively reproducible. The standard deviations were less than 10 percent of the mean values of voltage and power. The data reported here are the average of three repetitions.
Figure 2. Variation of open circuit voltage with time. A: unsuccussed, B: succussed 60 times, C: succussed 300 times, D: succussed 2000 times.
The highest magnitude of open circuit voltage for Sample D is 114 mV (Fig. 2). For the same sample, the voltage drop across the resistance (maximum value ~ 48.3 mV), the current flowing through the circuit (maximum value ~ 0.013 µA ) and the power ( maximum value ~ 0.62 nW, Fig. 3 ) decreased very slowly for several hours.
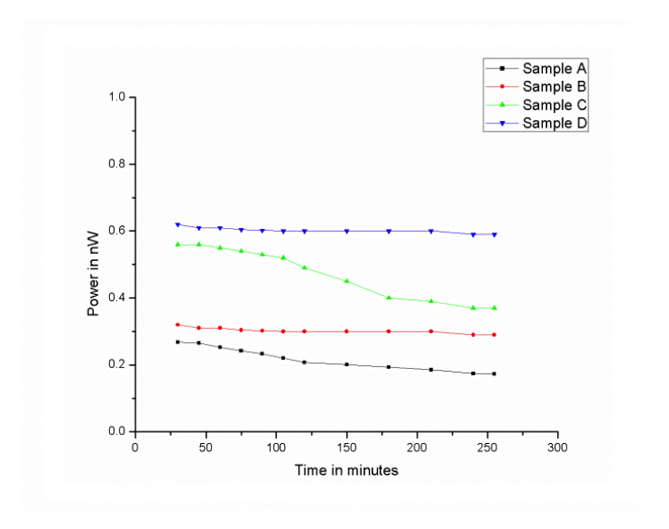
Figure 3. Variation of power with time. A: unsuccussed, B: succussed 60 times, C: succussed 300 times, D: succussed 2000 times.
With rise in temperature, the value of current increased and so did the voltage drop across the resistance 1.0 MΩ. The magnitude of all the parameters, viz., voltage, current and power were highest for Sample D. At 75oC the respective values of these quantities are ~56.2 mV, ~0.061 µA and ~ 3.43 nW as compared to ~48.3 mV and ~0.013 µA and 0.62 nW at temperature 30oC. The variation of power with temperature is shown in Fig. 4.
Figure 4. Variation of power with temperature. A: unsuccussed, B: succussed 60 times, C: succussed 300 times, D: succussed 2000 times.
Discussion:
The two surfaces of the platinum foil are in contact with two liquids of different dipole moments (91 % ethyl alcohol ~ 1.66 D and water ~1.87 D). The measured open circuit voltage decreased initially and reached a steady value and thereafter retained that value for several hours (Fig. 2). The side in contact with ethyl alcohol showed negative polarity. These observations can be explained as follows.
At each surface of the platinum foil, adsorption of molecular dipoles takes place, water on one side and ethyl alcohol on the other. For adsorption of water molecules on platinum it is known that the “flipped-up” orientation (the negatively charged oxygen end of the dipole towards the electrode surface) is slightly more favoured than the “flopped-down” orientation (the positively charged hydrogen end towards the surface) (Bockris et al., 1970). This has been rationalized by the asymmetry of the water molecule by which the flipped-up orientation leads to stronger image as well as dispersion interactions due to closer placement of the dipole to the electrode surface, than the flopped-down orientation. Also, the electrostatic interaction with the flipped-up water dipoles is stronger because the negative charge, residing on the oxygen atom, is somewhat localized while, for flopped-down dipoles, the positive charge is spread over the two hydrogen atoms thereby decreasing the charge density.
For ethyl alcohol dipoles the same mechanism can be proposed, but in this case the negative charge on the oxygen end is higher compared to that in water due to the electron-releasing inductive effect of the ethyl group which is reflected by higher acidity of water than ethyl alcohol (Morrison et al., 1969). At the same time, in ethyl alcohol the positive charge is spread over a larger region than in water, covering both the hydrogen atom and the ethyl group. Therefore, for ethyl alcohol the flipped-up orientation is expected to be considerably more stable than for water. This makes the difference between the number densities of flipped-up and flopped-down dipoles to be greater in ethyl alcohol than in water. Though the electric fields generated by adsorbed dipoles on two sides of the platinum separator act in opposition to each other, the net effect is a more negative potential at the side of the metal surface that is in contact with ethyl alcohol. In this way the appearance of the voltage, the direction of the potential drop and its stability with time can be accounted for.
Here, we attempt to explain our results within the context of QED, the fundamental quantum field theory of matter. The QED model predicts that under certain conditions self organization may take place in water and other polar liquids (Arani et al., 1995; Del Giudice et al., 2006; Bono et al., 2012). Evidence of large supramolecular clusters in polar liquids has been observed (Ho, 2014; Sedl’ak et al., 2013; Elia et al., 2013; 2014; Konovalov et al., 2014). According to QED, the molecules may distribute over a coherent phase (CP) and a non-coherent phase (NCP). In the CP, all molecules coherently oscillate. Part of these CP molecules are organized in domains wherein they coherently oscillate between their ground electronic state |a> and a well-defined excited state |b>. In the |a> state, the electrons are firmly bound. In the |b> state, the electrons are nearly free i.e., quasi-free electrons. Here we denote these coherent domains composed of electronically excited molecules as CDelec. Only a small amount of energy is sufficient to free an electron from the quasi free state (Germano et al., 2012; 2013; Bono et al., 2012). Therefore, the CDelec is a reservoir of quasi-free electrons. These electrons can be released under appropriate condition (Del Giudice et al., 2010; 2013).
The other part of the CP molecules coherently oscillates between two rotational states (Del Giudice et al., 2006). These molecules organize in coherent domains (CDrot), wherein their electric dipoles are aligned, i.e., these molecules are ferroelectric ordered. Therefore, CDrot have a net dipole moment. In the NCP, molecules reside in the ground electronic state |a> and the rotational energy distribution is that of the Boltzmann distribution. Experimental evidence for CP and NCP phases has accumulated during last decade (Kononov et al., 2015; Yinnon et al., 2009; 2012; 2015a,b; 2016; Elia et al., 2015; 2016; De Ninno et al., 2013; ). In particular, in water and other polar liquids adjacent to interfaces, stabilization of domains with properties of CDelec and CDrot have been observed. Also in serial diluted succussed polar liquids, such stabilization has been identified.
The relative abundance of CP and NCP molecules is temperature dependent. The abundance of CP decreases with temperature (Preparata, 1995). No domains stabilize in the absence of the electromagnetic field as predicted by QED and experimentally verified (Preparata, 1995; Konovalov et al., 2014; Ryzhkina et al., 2015). For non-polar liquids, QED does not predict stabilization of CDrot (Del Giudice et al., 2006).
The effects of succussions on CDelec and CDrot has been explained by Yinnon and Yinnon (2011), Yinnon and Elia (2013) and Yinnon and Liu (2015). Succussions break up these domains. The broken pieces of CDelec very quickly (on a mesoscopic time scale) reassemble. The dynamics of the broken pieces of CDrot is more complicated. It takes place on a macroscopic time scale (minutes-months). In these broken pieces, the molecules are ferro-electrically ordered. Thus these broken domain pieces can be regarded as Electric Dipole Aggregates (EDA). These EDA can enhance stabilization of CDelec. Thus based on the explanation of Yinnon et al. (2011; 2013; 2015a,b), succussions break up CDrot, create EDA and therefore enhance stabilization of
CDelec, the source of quasi-free electrons. We speculate that the quasi-free electrons of these CDelec might have contributed to our observed enhancement in the voltage and power generation of succussed alcohol versus unsuccussed alcohol.
Conclusion
The extraction of electrical energy using two different polar liquids in the absence of any external ion source is a very interesting phenomenon from the perspective of classical electrochemistry and implies another kind of conduction rather than ionic conduction. The difference in polarity of molecules in the two chambers creates a potential barrier across the platinum barrier. We have explained this extraction of electrical energy within the context of QED.
QED predicts that under certain conditions two phases may stabilize in polar liquids, i.e., a coherent phase (CP) and a non-coherent phase (NCP). The CP, under certain conditions, is composed of two types of domains (CDelec and CDrot). In these domains, the molecules perform coherent oscillations. The molecules in CDelec contain quasi-free electrons. It is our conjecture that these electrons underlie the voltage and power generation phenomenon reported in this paper, i.e., these electrons flow across the platinum barrier. Adjacent to some interfaces, the CP grows. Initially it grows at a rate on the order of microns per second, but later its growth rate diminishes. Succussions excite or break up the domains. The CDrot break up into Electric Dipole Aggregates (EDA). These EDA stabilize additional CDelec. The dynamics of EDA take place over macroscopic time scales. Hence stabilization of CDelec by EDA is a slow process. Stabilization of additional CDelec increases the number of quasi-free electrons. This explanation rationalizes why the voltage persists over macroscopic times.
Acknowledgements
The authors are thankful to the Central Council for Research in Homeopathy (CCRH), the Ministry of AYUSH, Govt. of India for providing the financial assistance. The study was undertaken in joint collaboration between Centre for Interdisciplinary Research and Education (CIRE), Kolkata and CCRH, New Delhi.
The authors express their sincere gratitude to the learned reviewers for their suggestions to improve the quality of this manuscript.
Papiya Nandy expresses her heartfelt appreciation to Dr. T. A. Yinnon for her continuous support and encouragement.
References
Arani R, Bono I, Del Giudice E, Preparata G (1995). QED coherence and the thermodynamics of water. Int J Mod Phys B 9:1813-1841.
Baxter JB, Aydil ES (2005). Nanowire-based dye-sensitized solar cell. Appl Phys Lett 86: 053114.
Beek WJE, Wienk MM, Janssen RAJ (2004). Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugate polymer. Adv Mater 16: 1009-1013.
Bockris J O’M, Reddy AKN (1970). Modern Electrochemistry, Plenum Press, New York.
Bono I, Del Giudice E, Gamberale L, Henry M (2012). Emergence of the coherent structure of liquid water. Water 4: 510-532.
Choi JW, Fujihira M (2004). Molecular-scale biophotodiode consisting of a green fluorescent protein/cytochrome c self-assembled heterolayer. Appl Phys Lett 84: 2187-2189.
Chou TP, Zhang Q, Russo B, Cao G (2008). Enhanced light-conversion efficiency of titaniumdioxide dye-sensitized solar cells with the addition of indium-tin-oxide and fluorine-tin-oxide nanoparticles in electrode films. J Nanophotonics 2: 023511 (1 -11).
Das R, Kiley PJ, Segal M, Norville J, Yu AA, Wang L, Trammell SA, Reddick LE, Kumar R, Stellacci F, Lebedev N, Schnur J, Bruce BD, Zhang S, Baldo M (2004). Integration of photosynthetic protein molecular complexes in solid state electronic devices. Nano Lett 4: 1079-1083.
Del Giudice E, Vitiello G (2006). Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transition. Physical Rev A 74: 022105.
Del Giudice E, Spinetti PR, Tedeschi A (2010). Water dynamics at the root of metamorphosis in living organism. Water 2: 566-586.
Del Giudice E, Tedeschi A, Vitiello G, Voeikov V (2013). Coherent structures in liquid water close to hydrophilic surfaces. J Phys: Conf Ser 442: 012028.
Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M (2013). Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with nafion polymer membrane. Water 5:16-26.
Elia V, Ausanio G, Gentile F, Germano R, Napoli E, Niccoli M (2014). Experimental evidence of stable water nanostructures in extremely dilute solutions, at standard pressure and temperature. Homeopathy 103: 44-50.
Elia V, Germano R, Napoli E (2015). Permanent dissipative structures in water: The matrix of Life? Experimental evidences and their quantum origin. Curr Top Med Chem 15: 559-571.
Elia V, Yinnon TA, Oliva R, Napoli E, Germano R, Bobba F, Amoresano A (2016). Chiral micron-sized H2O aggragates in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water. Water 8 (in press).
Germano R, Tontodonato V, Hison C, Cirillo D, Tuccinardi FP (2012). Oxhydroelectric effect: Electricity from water by twin electrodes. Key Engg Mater 495: 100-103.
Germano R, Del Giudice E, De Ninno A, Elia V, Hison C, Napoli E, Tontodonato V, Tuccinardi FP, Vitiello G (2013). Oxhydroelectric effect in bi-distilled water. Key Engg Mater 543: 455-459.
Gratzel M (2005). Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 44: 6841-6851.
Gratzel M (2010). Molecular photovoltaics that mimic photosynthesis. Pure Appl Chem 73: 459-467.
Greene LE, Law M, Yuhas BD, Yang P (2007). ZnO-TiO2 core-shell nanorod/P3HT solar cells. J Phys Chem C 111: 18451-18456.
Gunes S, Marjanovic N, Nedeljkovic JM, Sariciftci NS (2010). Photovoltaic characterization of hybrid solar cells using surface modified TiO2 nanoparticles and poly(3-hexyl) thiophene. Nanotechnology 19: 424009 p-5.
Ho M-W (2014). Large Supramolecular water clusters caught in camera – A Review. Water 6: 1-12.
Kamat PV (2007). Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J Phys Chem C 111: 2834-60.
Kononov L (2015). Chemical reactivity and solution structure: On the way to paradigm shift? RSC Adv 5: 46718-46734.
Konovalov AI, Ryzhkina IS (2014). Reviews: formation of nanoassociates as a key to understanding of physicochemical and biological properties of highly dilute aqueous solutions. Russ Chem Bull Int Ed 63: 1-14.
Martinson ABF, Elam JW, Hupp JT, Pellin MJ (2007). ZnO nanotube based dye sensitized solar cell. Nano Lett 7: 2183-2187.
Morrison RT, Boyd RN. In Organic Chemistry, Prentice Hall of India, New Delhi, 2nd ed., 1969, p-532.
Nakayama K, Tanabe K, Atwater HA (2008). Plasmonic nanoparticle enhanced light absorption in GaAs solar cells. Appl Phys Lett 93: 121904 (1-3).
O’Regan B, Gratzel M (1991). A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 thin films. Nature 353: 737-740.
Preparata G (1995). QED coherence in matter. World scientific, Singapore, New Jersey London, Hong Kong.
Rybtchinski B, Sinks LE, Wasielewski MR (2004). Combining light harvesting and charge separation in a self-assembled artificial photosynthetic system based on perylenediimide chromophores. J Am Chem Soc 126:12268-12269.
Ryzhkina IS, Murtazina LI, Kiseleva YU, Konovalov AI (2015). Self-Organization and Physicochemical properties of aqueous solutions of the antibodies to interferon gamma at ultrahigh dilution. Dokl Phys Chemi 462: 110-114.
Sedl´ak M, Rak D (2013). Large-scale Inhomogeneities in Solutions of Low Molar Mass Compounds and Mixtures of Liquids: Supramolecular Structures or Nanobubbles? J Physical Chem B 10: 2495-2504.
Shah R (2016). Standardization of the potentizing machine and quantification of the impact of potentization. Ind J Res Hom 10: 126-132.
Van Sark WGJHM, Meijerink A and Schropp REI (2012). Solar Spectrum Conversion for Photovoltaics Using Nanoparticles. Fthenakis V, Editor. Third Generation Photovoltaics, INTECH: E-Publishing Inc; p. 1-28.
Yinnon CA, Yinnon TA (2009). Domains in aqueous solutions: Theory and experimental evidence. Mod Phys Lett 23: 1959-1973.
Yinnon TA, Yinnon CA (2012). Domains of solvated ions in aqueous solutions, their characteristics and impact on electrical conductivity: theory and experimental evidence. Mod Phys Lett 26: 1150006-1 to 1150006-14.
Yinnon TA, Liu ZQ (2015a). Domains formation mediated by electromagnetic fields in very dilute aqueous solutions: 2. Quantum electrodynamic analyses of experimental data on strong electrolyte solutions. Water 7: 48-69.
Yinnon TA, Liu ZQ (2015b). Domains formation mediated by electromagnetic fields in very dilute aqueous solutions: 3. Quantum electrodynamic analyses of experimental data on solutions of weak electrolytes and non-electrolytes. Water 7: 70-95.
Discussion with Reviewers
Reviewer 2:
Power generation by a system containing a polar liquid (alcohol) perturbed by succussions, to the best of my knowledge, is a novel approach. Several years ago, it was shown that power can be generated by perturbing polar liquids (water) with Nafion membranes [Germano et al. (2012). Oxhydroelectric effect: Electricity from water by twin electrodes. Key. Eng. Mater.495:100–103; Germano et al. (2013).Oxhydroelectric effect in bi-distilled water. Key. Eng. Mater. 543: 455–459.] Like the authors, Germano et al. in their 2012 paper claim: “The experimental evidence of electricity extraction from water by twin electrodes (the same metal) mediated by oxygen molecules, that we report in this paper, is an astonishing phenomenon in the framework of the classical electrochemistry. Nevertheless, we will show that it has a theoretical background in the modern quantum electrodynamic (QED) description of water.” While in their 2013 paper, they claim: “Electricity extraction from bi-distilled water by twin electrodes reported in this work is an astonishing phenomenon in the frame of the classical electrochemistry because – considering the absence of any significant quantity of ionic solutes in this pure water system – it seems to imply some kind of “other than ionic” conduction.
I would like the authors to point out to the readers the similarities and differences between:
i. Their experimental techniques and those of Germano et al. (2012, 2013);
ii. Their data and that obtained by Germano et al. (2012, 2013), including details about the differences in maximal power generation and its duration in their system and both systems described in the papers by Germano et al.(2012, 2013);
iii. Their QED based interpretations of the experimental results and those of Germano et al. (2012, 2013);
iv. The relative technological advantage of their technique compared to the techniques Germano et al. (2012, 2013).
Authors:
i) The authors’ experimental techniques and those of Germano et al.
The components of the experimental system of Germano et al., (2012) consist of a transparent plastic beaker, inside which a cylindrical symmetry anode-cathode structure was positioned. The inner electrode (anode), made of a platinum wire tightly coiled around a strongly hydrophilic material, Nafion® film, glued on the external surface of a plastic bobbin, was used as anode-cathode holder. The outer electrode (cathode), made of an identical Pt wire, was coiled and fixed around a bearing structure, consisting of thin wooden sticks, glued on the bobbin flanges. The electrolyte was a saturated solution of potassium carbonate (K2CO3) in water (H2O), providing an alkaline background (pH >10). (Details given in the paper and please see the accompanying figure.)
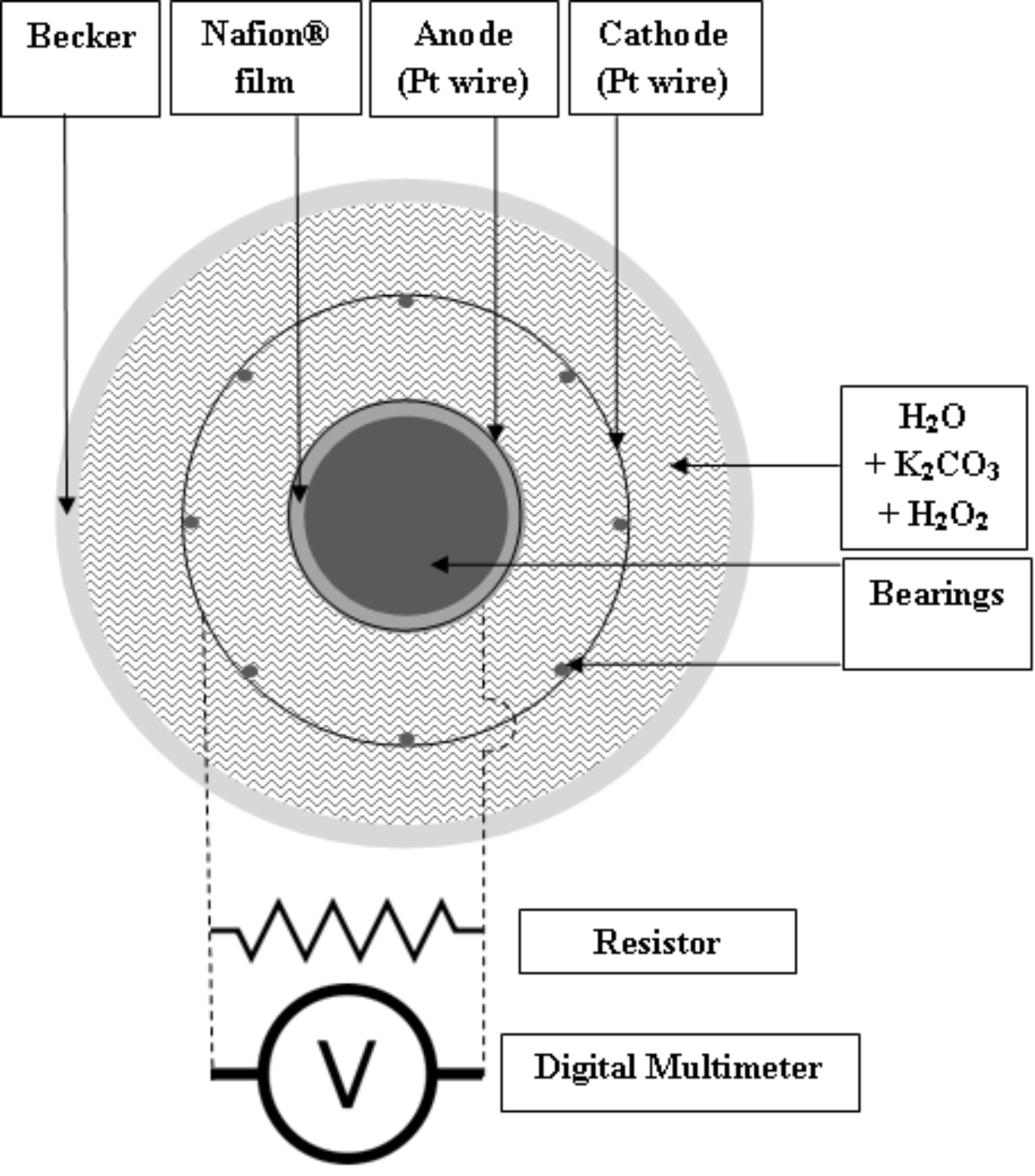
A DC power on the order of hundredths of nW was measured for days through a resistor connected to the twin Pt electrodes.
In the second set of experiment of Germano et al (2013) the system consists of two L-shaped glass semi-cells connected among them and provided with a 25 nm filter Millipore® positioned at their junction. The semi-cells are filled with bi-distilled water and in each of them a platinum wire electrode is immersed.
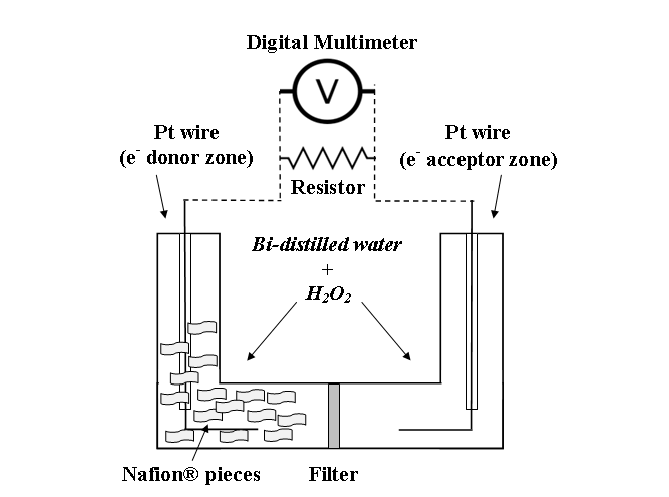
A DC power on the order of tenths of nW, lasting for many hours, was measured through a resistor connected to twin Pt electrodes immersed into bi-distilled water, after the addition of some pieces of Nafion® in one semi-cell and of a very small amount of hydrogen peroxide (H2O2) — as a source of oxygen — in both semi-cells.
In our system two platinum electrodes are immersed in two identical chambers of a glass U-tube, one containing 91% ethyl alcohol and the other containing bi-distilled water; the two chambers being separated by a platinum foil.
A DC power on the order of nW was measured for hours through a resistor connected to the twin Pt electrodes.
| Author(s) | Year | Chamber | Solution |
| I. Germano et al. | 2012 | Cylinder | water with K2CO3 + nafion+H2O2 |
| A DC power on the order of hundredths of nW was measured for days. | |||
| II. Germano et al. | 2013 | L-shaped cell Divider: porous Filter | Bi-distilled water and H2O2 in both sides + nafion in one |
| A DC power on the order of tenths of nW, lasting for many hours, was generated. | |||
| III. P. Bandyopadhyay et al. | 2017 | U-shaped cell Divider: Pt foil | Bi-distilled water in one side, 91% ethyl alcohol on the other side |
| A DC power on the order of nW was measured for hours. | |||
|
The advantages of our system over those of Germano et.al., (2012, 2013) are: a. No external agent was needed in the present system. b. Alcohol is a natural product, which does not release any compounds. Nafion instead releases small amount of fiour and sulphate ions. c. Our system elucidates an aspect of homeopathic liquids (remedies), i.e., succussions of an alcohol-water mixture affect the number of its quasi-free electrons. Thus, our experimental findings have implications beyond those of power generation. |
|||
