 The Mechanisms of Activation of Substances by Minimal Catalyst Water and Application in Keeping Foods Fresh
The Mechanisms of Activation of Substances by Minimal Catalyst Water and Application in Keeping Foods Fresh
Sugihara S1*, Suzuki C2, Hatanaka K3
1 Kanagawa University, Yokohama, 221-8686Japan,
1 Sugihara Institute of Technology, Yokohama, 236-0046, Japan
2 Japan Atomic Energy Agency, Japan, Nuclear Science and Engineering Directorate, Japan Atomic Energy Agency, Ibaraki-ken, 319-1195, Japan
3 MCM Co. Ltd, Uehonmachi, Tennoji Ku, Osaka 543-0001, Japan
* Correspondence: E-mail: natsuyama41@gmail.com
Key Words: minimal catalyst water, activated nitrogen, terahertz, fresh foods, respiration
Received August 1st, 2010; Revised January, 12th 2011; Accepted March 15th, 2011; Published October 7th, 2011; Available online October 10th, 2011
Summary
When hydrogen bonds between water molecules are broken, the molecules can emit electromagnetic waves in the region from the near infrared to the terahertz-wave region (1–12 THz) that can activate other substances. By using the total energy spin DV-Xα program, we calculated the total energies of dinitrogen in its activated and nonactivated states by considering the spin of the molecule as a function of the length of the N–N bond. These calculations showed that, for certain bond lengths, excited nitrogen can exist in meta-stable forms that reduce energy slowly. Excited nitrogen should emit radiation below the frequency of infrared rays, and is stabilized on lowering of the energy, as the energy can changes from 3 eV to 1.5 eV, which corresponds to that of infrared rays. We discuss the mechanism whereby polyethylene films that have been activated by minimal catalyst water excite nitrogen in the air; this, in turn, keeps foodstuffs fresh by protecting them from attack by oxygen.
Article Outline
Introduction
Cavalleri et al. (2002) reported the X-ray absorption spectra of water and ice in terms of excited electrons, that is to say, their dynamic bonding characteristics. We will show how changes in the Xα spectrum for various patterns of coordination of water can be connected to changes in oxygen sp hybridization and to the molecular localization of various internal O–H bonds of the probed molecule. We focus on the N–N bond in dinitrogen (N2) and its relationship with activated water, some aspects of which we have discussed in a previous report (Sugihara and Hatanaka, 2009). Our first results on the calculation of the electronic structure of two water molecules by using the discrete variational (DV) Xα method were presented at the 22nd DV-Xα symposium (Sugihara, 2009). When hydrogen bonds between water molecules are broken, the molecules can emit electromagnetic waves in the region from the infrared to the THz-wave region (1–12 THz) (Sugihara, 2009). In relation to the applications of activated water, we believe that it can excite nitrogen, and we have studied its action in reducing exhaust gas production by cars; we proposed that redox reactions may take place within the engine compartment with formation of chemicals (Sugihara, 2009).
We will also discuss the mechanism whereby polyethylene (PE) wrapping films that have been activated by exposure to minimal catalyst water (MICA) can excite nitrogen in the air (as we have demonstrated experimentally). Nitrogen excited by the MICA-treated PE film keeps vegetables, fruits, mushrooms, or meat fresh by protecting them from attack by oxygen. No coating is applied to the wrapping material, and nothing is added to the film material. The MICA treatment merely provides energy to the wrapping material. Our calculations for the nitrogen molecule show that excited nitrogen, which has a slightly raised energy, can play the role of a reductant (and hence of an antioxidant).
Materials and Methods
The DV-Xα method was used to calculate the total energy of N2 and its excited form. The spin of the molecule was considered as a function of the length of the N–N bond by using the Total Energy Spin DV-Xα (TESDA) program developed by Nakagawa (2002, 2003). The value of the well potential α was 0.7 and the number of sample points was 20,000; the basis functions were 1s–4p.
In the experimental study, mushrooms were kept inside activated and nonactivated samples of 0.02-mm-thick PE wrapping film for five days. Levels of CO2 and O2 inside the films were measured by using a gas sensor (PBI Dansensor, Ringsted, Denmark), and the Fourier-transform infrared (FTIR) spectra of the activated and nonactivated films were recorded on an FT-IR-6100 type A spectrometer (JSACO, Tokyo).
Results and Discussion
Calculation Results
The results of our calculations are shown in Figure 1, in which the total energy (eV) is plotted against the N–N distance (Å). When one electron is excited, the total energy level and the N–N distance change, and N(0)-N(0) and N(a↓)–N(a↑) are the basis state. Figure 1 shows the calculation of the total energy corresponding to the N-N distance when one electron is excited and the importance here is to describe a relationship between the distance of N-N and total energy.
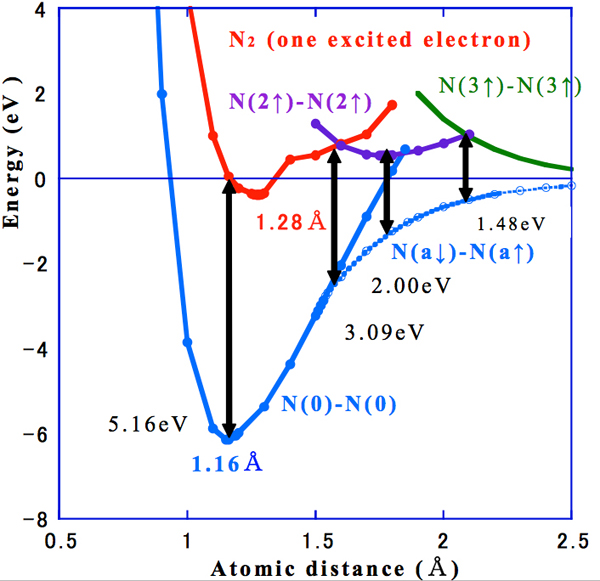
• Interatomic distance 1.28 Å: 5.16 eV [N(0)-N(0) and N2* (one excited electron)]
• Interatomic distance 1.60 Å: 3.09 eV [N(a↓)–N(a↑) and N(2↑)–N(2↑)]. N2 curve (in red) crosses with N(2↑)–N(2↑) purple color.
• Interatomic distance 1.75 Å: 2.00 eV [N(a↓)–N(a↑) and N(2↑)–N(2↑)]. Minimum point of N(2↑)–N(2↑).
• Interatomic distance 2.10 Å: 1.48 eV [N(a↓)–N(a↑) and N(3↑)–N(3↑)]. N(2↑)–N(2↑) crosses with N(3↑)–N(3↑) in green color.
Figure 1: Plots of the total energy versus atomic distance showing how the emitted energy changes with the N–N distance (the arrows indicate the energy differences)
The energy level of the conduction (unoccupied) level becomes lower by an amount that depends on the change in the N–N distance, and the potential of the free electron changes as a result. The value a in N(a↓)–N(a↑) describes a continuous change of spin from 0 to 3. We identified three meta-stable states at various N–N distances. The energy of the nonmagnetic ground state N(0)–N(0) is a minimum at a bond distance of 1.16 Å, which is 7% more than the experimental value (1.0998 Å) (Kagakubenran:edited by Nihonkagakkai,1993), and the difference of the energy between N(0)–N(0) and N2* (with one excited electron) is 5.16 eV. At a larger distance, the curve for one-electron-excited N2 crosses that of the N(2↑)–N(2↑) at 1.60 Å, where the difference of the ground state is 3.09 eV. The state N(2↑)–N(2↑) has a minimum energy at a bond distance of 1.75 Å as a result of lowering of the energy; the difference between the minimum state of N(2↑)–N(2↑) and N(a↓)–N(a↑) is then 2.0 eV. Finally, the curve for N(2↑)–N(2↑) shows a larger bond distance and crosses the curve N(3↑)–N(3↑) at 2.1 Å, where the difference of the energy is 1.48 eV. The N(3↑)–N(3↑) state can exist around 2.5 Å. The cross points are just one step to change the electron configuration, and does not mean that the emission take place at the cross point, and we can not clarify why and at which stages the emissions occur in scientifically at this moment. However, the latest information about hydrogen bond appears in Water J.2, 2010(Scott & Vanderkooi). The authors discuss the dependence of the hydrogen bond energy on the O-O distance and hydrogen bond angle. The bond energy of 7.9 kcal/mol is calculated to be 0.03eV.
We can find such a state in N2(B3 Πg) → N2(A3 Σu+) from 1.2 to 2.2 Å (Wright & Winkler (1968), and Tanaka et.al. (1964)) which is still relatively high energy compared with our meta-stable excited nitrogen. In particular, various kinds of potential energy curves of low-lying N2 state vs nuclear separation (around 1Å to 2.2 Å) have been reported here. So we have postulated that their levels are identified in the far-infrared and terahertz levels. Furthermore, we measured the Raman spectra at 11,000-12,800 cm-1 (corresponding to around 1.5eV), which is a higher energy than that of the hydrogen bond of water, and we could find no difference between the Raman spectrum of activated water and that of normal water, although the less energy (far infrared though terahertz) of the Raman wave numbers may relate to the rotational vibration.
Thus we postulate that the emission below infrared rays may occur due to momentum and spin of electrons rather than transition of the state, and that the THz wave is generated by the parametric oscillation due to the nonlinearity arising from both electronic and rotational contributions of the water; ω3(infrared )=ω1(far infrared)+ω2 (THz). There is no origin to excite N2 electronically, but the origins to excite N2 is the activated substance which has an energy of far-infrared through terahertz and is contacting to nitrogen (polyethylene film in our discussion).
For certain bond lengths that lose energy slowly, and that it could then undergo expansions and contractions in its bond length. Excited nitrogen should emit low-energy radiation (below the frequency of infrared radiation), although we could not detect it experimentally. It is generally said that the basis state of potential energy moves according to the coordinate and another minimum state when excited. Furthermore, N-N can exist and becomes equilibrium. In the excited state, however, the equilibrium position of the nucleus differs from that of the basis due to the different distribution of electron. That is why the energy curves vary corresponding to the distance of N-N. The movement of the nucleus takes a time more than 10-15 s from R to R’, and electrons are also itinerant within the vibrating molecule like 10-14 ~ 10-12 s (which corresponding to 100 ~ 1 THz ).
The X-ray absorption near-edge structure (XANES) spectrum of N2 has been reported (Nakamatsu et al., 1993) by using the basis set for the level up to 20eV, and a strong peak appears at an energy level above that of the vacuum level around the absorption edge in the XANES. It has been suggested by Nakamatsu et al. (1993) that the excited electron is scattered by the potential energy and that a quasi-stable energy level exists. Therefore, as our calculation also shows, the excited electron may participate in a certain meta-stable state. The absorption edge seems to appear at around 5 eV in the N K X-ray absorption spectrum of N2 (Bianconi et al., 1978). According to our calculations, when one electron is excited, the lowest energy level at a bond distance of 1.28 Å corresponds to the difference of the energy of 5.16 eV from the ground state. Because the difference of the energy roughly describes the absorption edge, this result coincides with our calculation of the dependence of the energy level on the N–N distance. Furthermore, the theories considered are postulated for a dissociation energy of ground-state molecular nitrogen of 9.76 eV, at which Wright and Winkler (1968) state that the corresponding high association energy for ground-state nitrogen atoms makes it unnecessary to postulate any recombination of electronically excited atoms, and the primary process may be the recombination of N(4S) atoms along a 5Σg+ potential energy curve.
Excited nitrogen is generally produced by an electric discharge through nitrogen, and many methods have been reviewed in the previous book written by Wright and Winkler (1968). However, these “forms of active nitrogen” have a high energy (up to 9.764eV), which is referred to as the dissociation energy of the ground state of nitrogen, with a corresponding uncertainty in the heat of formation of the N(4S) atom. Our “active nitrogen” has less energy than 1.5eV. The HOMO–LUMO gap has been reported to be about 8.5eV (Ellis, DE(1976)), by using molecular cluster theory for chemisorption of the first row atoms on nickel (100) surfaces. This is close to our result for the ground state. The changes in the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) with changes in the N–N distance are plotted in Figure 2. As the N–N distance increases and the potential of the free electron changes, the energy of the conduction level (LUMO) increases and that of the HOMO decreases, approaching that of the LUMO. Therefore, excited nitrogen can play the role of a reductant for fresh materials wrapped in activated PE film.
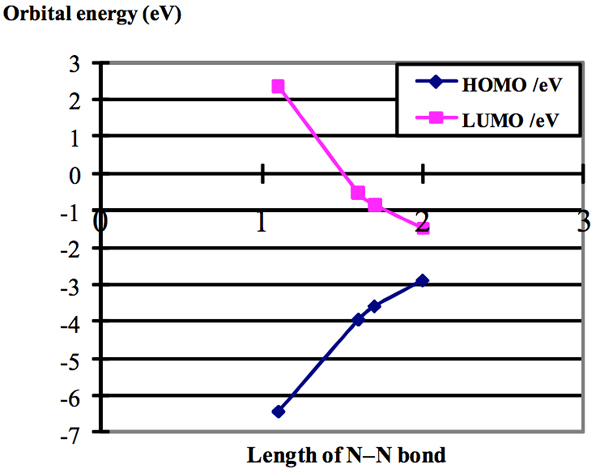
Figure 2: Plots of the orbital energy of the HOMO and LUMO versus the N–N distance (Å).
The Effects of Excited N2 on Wrapping Film
In our MICA system, activated water emits electromagnetic waves in the infrared-through-terahertz region, causing the nitrogen in the air to become excited, as we have discussed above. Car exhaust gases reduction and saving of fuel consumption have been interpreted by the function of the excited nitrogen (Sugihara and Hatanaka 2009). Furthermore, excited nitrogen (N2*) attacks hydroxy bonds in water when hydrogen bonds are disrupted, and the energy of free electrons can be changed by this attack, as shown in Figure 3. Tamenori et al. (2009) explained an excitation of the 3pπ(CH3) orbital in acetone, which can be interpreted in terms of a change in the molecular orbital caused by hydrogen-bonding interactions in acetone clusters probed by near-edge X-ray absorption fine-structure spectroscopy (NEXAFS) in the carbon and oxygen K-edge regions. We will now examine the relationship between the energy and the bond strength during the chemical reaction. Because the excited nitrogen N*=N will attack an O—H+ bond, the usual strength of which is 4.8 eV, its energy must be less than 4.8 eV (although the precise value is not known) as a result of the existence of the free electron. The excited dinitrogen can then form a temporary O—H+—N*=N bond. The strengths of these series of bonds are close to one another: <4.8 eV (O—H), <3.9 eV (N—H) and <4.2 eV (N*=N). These close values are similar to those in the amino groups (–H2N) of an amino acid, which can form a peptide link with the carboxylic acid group in another molecule of amino acid.

Figure 3: Model for the attack by –N*=N on an O–H+ bond (dotted line: hydrogen bond). The values of energy of the covalent bond and the bond lengths are taken from Emsley (1998). The energy of the covalent bond is indicated by converting from kJ/mol into eV.
As shown in Figure 4, when the gas inside the film was analyzed, the activated PE film showed normal respiration conditions, whereas the untreated one emitted CO2 only. Thus, activated PE film keeps vegetables, fruits, mushrooms, and meat fresh by protecting them from attack by oxygen. We now have evidential results of meats, vegetables and fruits in the wrapped film in the market, too. One meat example is shown in Figure 6.
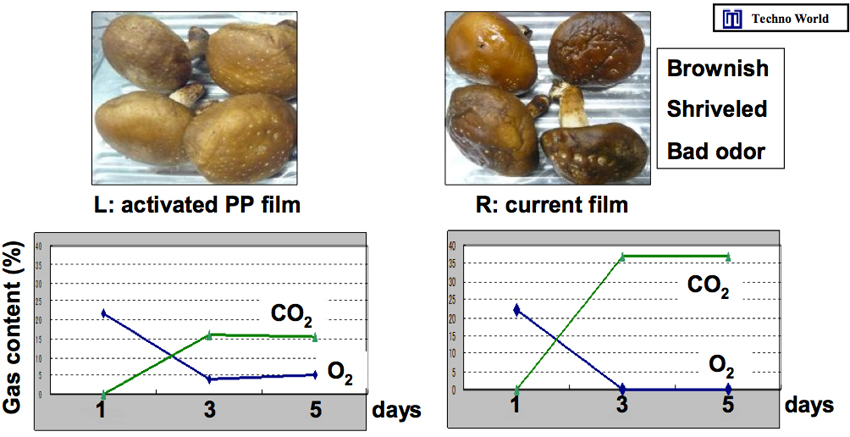
Figure 4: The difference in the freshness of mushroom kept inside an activated PE film and an untreated one, and the analyses of the gases inside the films. The activated PE film showed normal respiration, whereas the untreated film contained CO2 only and almost no dioxygen.
Let us consider the mechanism that is responsible for this phenomenon.
FTIR spectroscopy of the film in the terahertz region (Figure 5) identified differences between the activated and untreated PE films. The spectra shows the transparency in the arbitrary units (not 100%) on the vertical axis. If we measure the range from 15 to 165 THz, we could not recognize subtle differences with the full-scale axis. That is why we select such a relative scale. We assume that N2 can absorb radiation in the range 0.6–5 THz and that it resonates with electromagnetic waves in the THz region outside the range 0.6 to 5 THz, but N2 can also absorb more than those, depending on the N–N distance when accesses to other substance. PE film generally transmits electromagnetic waves in the terahertz region, but the activated PE film absorbed more terahertz energy than did the untreated one and, in particular, transmitted less radiation in the region above 5 THz. The PE, in the form of plastic pellets, is therefore provided with energy during the activation process, and the organic material is changed so that it possesses an energy. Actually, the activated PE film has continued to have the same property with the FTIR examination after three years or so, as well as in our previous experience of the activated water. This is evidenced by the fact that water with broken hydrogen bonds was predicted to show a stable existence by means of DV-Xα calculations, as described in our previous report (WATER, 2009).
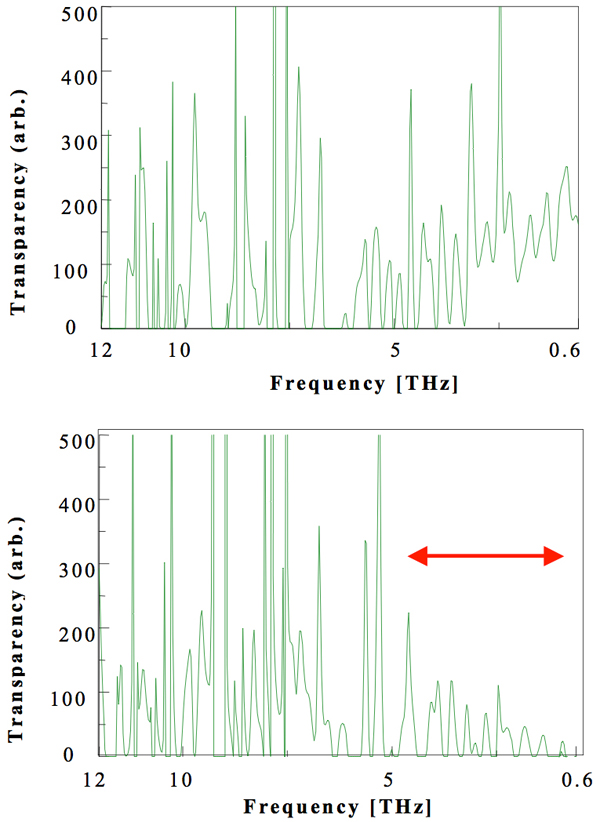
Figure 5: FT-IR spectra in the THz region of the untreated polyethylene film (thickness 0.02 mm) (upper spectrum) and activated polyethylene film (lower spectrum). The untreated polyethylene film transmits less electromagnetic energy in the terahertz region.
Figure 6 shows another demonstrations of different kinds of meats; upper and lower, pork and beef, respectively: a) meats wrapped in activated film, and b) in control film. Pork and beef were kept for 4 days and 3 days, at room temperature, respectively. The meat wrapped in activated film become tender, juicy and had less dripping compared with those in control film.
As a result of this, nitrogen gas inside the film can be excited or, in other words, energized, keeping foodstuffs fresh.
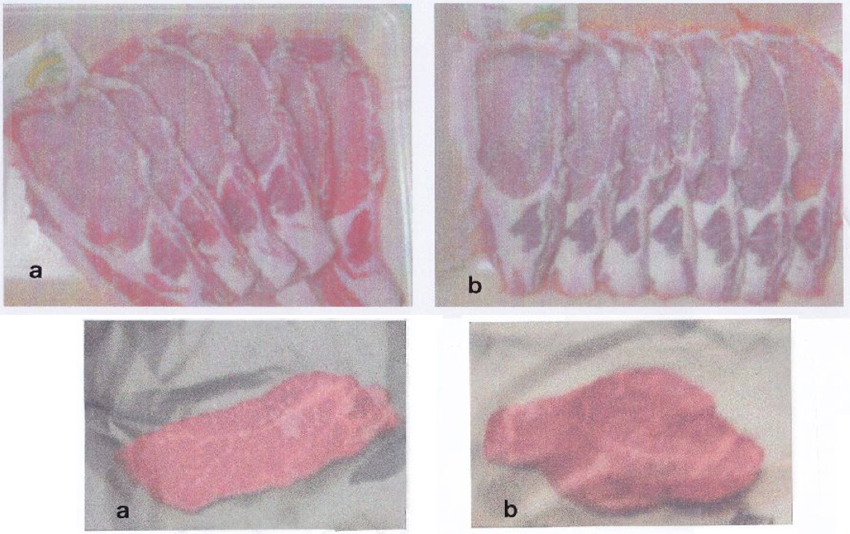
Figure 6: Wrapping film-effects on freshness of meats; a) activated film, b) control film. Upper: pork and lower: beef. Both meats wrapped in activated film are fresh, juicy and have less drip.
Conclusion
Calculations on dinitrogen (N2) were performed by means of the DV-Xα method by using the TESDA program. The calculations showed that dinitrogen can exist in three meta-stable states, each with a certain energy that changes depending on the N–N distance. Furthermore, we found that the activated PE film may absorb radiation in the region below 5 THz and is transparent in the region above 5 THz, an energy level that may result in excitation of dinitrogen. Then activated wrapping film containing the excited dinitrogen keeps foods inside the film fresh.
Acknowledgement
We thank Dr K. Nakagawa of Canon Inc. for use of the TESDA program and for fruitful discussion on the dinitrogen calculation. We also express our gratitude to Professor H. Adachi (Kyoto University) for use of the DV-Xα method.
References
Bianconi A; Petersen H; Brown F C; Bachrach RZ (1978). K-shell photoabsorption spectra of N2 and N2O using synchrotron radiation. Phys. Rev. A: At., Mol., Opt. Phys. 17: 1907–1911.
Cavalleri M; Ogasawara H; Pettersson LGM; Nilsson, A (2001). The interpretation of X-ray absorption spectra of water and ice. Chem. Phys. Lett. 364: 363–370.
Ellis DE (1976). Molecular cluster theory for chemisorption of the first row atoms on nickel (100) surfaces, Surf. Sci., 58, 497–510.
Emsley J, The Elements, 3rd edn, Clarendon Press, Oxford, 1998, 52, 142,148.
Nakagawa K (2002). Total energy calculation of molecules using DV-Xα molecular orbitals, Bull. Soc. Discrete Variational Xα 15: 121–125.
Nakagawa K (2003).Total energy calculation of molecules using spin- DV-Xα molecular orbitals, Bull. Soc. Discrete Variational Xα 16: 93–97.
Nakamatsu H; Mukoyama T; Adachi H (1993). Bull. Soc. Discrete Variational Xα 6: 83–86.
Scott, JN: Vanderkooi, JM(2010). A New Hydrogen Bond Angle/Distance Potential Energy Surface of the Quantum Water Dimer. WATER 2:14-28.
Sugihara S (2009). Analysis of water using DV-Xα method and innovative applications, Bull. Soc. Discrete Variational Xα 22: 284–291.
Sugihara S; Hatanaka K (2009). Photochemical removal of pollutants from air or automobile exhaust by minimal catalyst water. WATER 1: 92–98.
Tamenori Y; Takahashi O; Yamashita K; Yamaguchi T; Okada K; Tabayashi K; Gejo T; Honma K, Hydrogen bonding in acetone clusters probed by near-edge x-ray absorption fine structure spectroscopy in the carbon and oxygen K-edge regions. J. Chem. Phys. 131:174311 (9 pp).
Tanaka Y; Ogawa M; Jursa AS (1964). Forbidden Absorption-Band System of N2 in the Vacuum-Utraviolet Region. J. Chem. Phys. 40: 3690-3700.
Wright AN, Winkler CA (1968). Active Nitrogen, Academic Press, New York: pp.30 and pp. 140–152.
