 Synthesis and Characterization of PVA/AAC Foam and their Application with Alum for Reduction of Turbidity, Calcium and Magnesium Ions, and Microbial Count in the Nile River
Synthesis and Characterization of PVA/AAC Foam and their Application with Alum for Reduction of Turbidity, Calcium and Magnesium Ions, and Microbial Count in the Nile River
El-Toony, MM1*, El-Kelesh, NA1, Abdel-Shafy, HI2
1 National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt
2 Water Research, Technology and Pollution Control Department, National Research Center, Cairo, Egypt
* Correspondence: Tel:20101318501, Fax:+202 22 944 803; Email:Toonyoptrade@yahoo.com
Key Words: Hydrogels, Foam, Gamma radiation, Alum, Nile water, Treatment, Bacteria, Fungi, Turbidity
Received December 16, 2010. Accepted January 20, 2011. Published July 31, 2011. Available online July, 31 2011.
Summary
Radiation techniques have proven particularly suitable for producing hydrogels for use in treating wastewater. We have used different ratios of polyvinyl alcohol and acrylic acid to form hydrogels with different doses of gamma radiation. We foamed the hydrogel to increase the size and number of pores. We characterized the foam using Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), and scanning electron microscopy (SEM). Foaming increased the water uptake level from 320 percent to 13.3 x 104 percent. We conducted this work with surface water samples from the Nile River (in Cairo). This water was tested before and after treatment using the jar test method. Some parameters were studied, such as contact time and different ratios of foam/alum. The results of our investigations included decreasing turbidity to 32.5 percent, calcium to 16.5 percent, and magnesium to 24.2 percent from the original concentrations. Further, we reduced the counts of bacteria and fungi colonies/ml (CFU/ml) to 88.9 percent and 99.4 percent, respectively, after one day of treatment, while both sets nearly disappeared after seven days.
Article Outline
- Introduction
- Materials and Methods
- Results and Discussion
- Conclusion
- References
- Discussion with Reviewers
1. Introduction
According to a 2007 World Health Organization (WHO) report, 1.1 billion people lack access to an improved drinking water supply, 88 percent of the 4 billion annual cases of diarrheal disease are attributed to unsafe water and inadequate sanitation and hygiene, and 1.8 million people die from diarrheal diseases each year. The WHO estimates that 94 percent of these diarrhea cases are preventable through modifications to the environment, including access to safe water (World Health Organization, 2007). The water purification process may reduce the concentration of particulate matter, including suspended particles, parasites, bacteria, algae, viruses, fungi, and a range of dissolved and particulate materials. Flocs are operationally defined as the aggregated suspended sediments composed of inorganic and organic components, as well as living organisms (Cantwell and Burgess, 2001; Droppo, 2001; Scendel et al., 2004); flocs form spontaneously because of the attraction between negative face and positive edge charge. Alum, the most widely used coagulant in water treatment (Hammer and Hammer, 1996; Mason et al., 2004), serves a dual role: (a) acting as a coagulant for suspended solids removal (Welch and Cooke, 1999) and (b) reducing or neutralizing the electrostatic forces between negatively charged particles in water, which leads to aggregation and settling (Reynolds and Richards, 1995). Alum, shown to be nontoxic, has a pH in the range of 5.5 to 9. At these pH values, the aluminum concentration is not expected to exceed 50 μgL-1, since aluminum hydroxide is highly insoluble in this range (Sojka et al., 1998). Previous research found that treating alkaline waters with alum should not chronically or acutely affect biota.
Wastewater treatment frequently involves the application of polymers to enhance coagulation and settling. The polymers aid the coagulant by chemically bridging reactive groups and increasing floc size (Reynolds and Richards, 1995). Polymers can vary in charge type (cationic, anionic, or neutral) and come in a range of charge densities and molecular weights. Polyacrylamide, a synthetic polymer, has been used in soil applications to reduce erosion (Zhang and Miller, 1996), increase infiltration (Green et al., 2000), promote flocculation (Laird, 1997), and enhance salt removal (Aly and Letey, 1990; Malik et al., 1991). The charge of the polymer and the type of suspended solids in the wastewater influence the degree of flocculation and the settling rate. Cationic polymers directly adsorb to the negative surfaces of the clay particles; anionic polymers form flocs by attaching to the clay surfaces through the van der Waal forces (Sakairi et al., 1998). The nature of the suspended solids in the water requires assessment to find the most effective polymer materials and methods.
The present study sought to treat River Nile wastewater samples to fit the water for human consumption. We prepared and physicochemically investigated acrylic acid/polyvinyl alcohol foams and examined their ability to aid coagulation and sorption of such pollutants as calcium and magnesium ions, bacteria, and fungi. We also investigated the impacts of several parameters, such as temperature, contact time, and foam/alum ratios.
2. Materials and Methods
2.1. Site and sampling description
We collected surface water samples from the Nile River, at Maspero in Cairo, Egypt, on August 1, 2008.
2.2. Materials
We used commercial grades of polyvinyl alcohol (PVA) and acrylic acid (AAc) to prepare the copolymer. We used bi-distilled water to dissolve PVA (10 percent) and to foam the prepared hydrogels. Commercial hydrogen gas was used to foam the hydrogel. We obtained all chemicals and the gas from OPT. Co. (Ortho Para Trade Company, Cairo, Egypt) and used them without further purification.
2.3. Preparation of Hydrogel
We mixed 1g of 10 percent PVA with AAc in different ratios. We tested compositions of 0.05, 0.09, 0.17, 0.23 and 0.33ml of AAc to PVA were applied, whereas copolymer hydrogels with smaller ratios showed weak texture. We copolymerized the mixture using gamma irradiation at different doses, including 0.5, 1, 1.5, 2 and 2.5 Kilo Gray (KGy), and an irradiation dose rate 1.7 Gy/s.
2.4. Foaming the Hydrogel
We performed the experiment in a fuming cup hood, and we placed the swollen hydrogel in a 15 cm Petri dish filled with water. Then, we carefully heated the Petri dish to 65 ºC during moderate bubbling of hydrogen under the water surface for at least 30 minutes.
2.5. Water Uptake Studies
We studied the water uptake/swelling behavior of different ratios of hydrogels and foam in water as a function of different doses (KGy) and different compositions. We also considered other parameters, such as contact time and pH. Swollen polymer was wiped off with tissue paper and then weighed immediately to determine the swelling percentage or water uptake percentage, which was calculated as:
2.6. Jar Test Method
We performed all laboratory scale jar tests in a 2 L jar apparatus (PB-700; Phippe & Bird, Richmond, VA). During preliminary studies, we mixed the jar contents and let the floc settle for a predetermined amount of time. Since the flocs settled quickly, the effectiveness of each treatment depended on the length of settling time and foam/alum added. We filled the jars with the water samples to the 2 L mark (14.5 cm water depth) and collected an initial sample using a pipette at a depth of approximately 3.5 cm while mixing the water at 300 RPM. We collected samples for turbidity, TDS, pH, calcium, magnesium, and colony counting for bacteria and fungi at different times.
2.7. Calcium, Magnesium and Microbial Sorption
We studied ion sorption as a function of the foam structure and of environmental factors, like time and foam/water ratios. We analyzed the collected 10 ml samples by atomic absorption analyzer (Unicam, Solaar 929, England) to measure calcium and magnesium. We used sterile test tubes for sampling treated water for microbiological investigations. We defined the relationships used to express sorption behavior as:
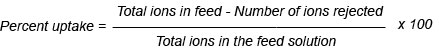
2.8. Scientific Tests
We used FTIR (Mattson 1000, PYE-Unicam, England) to analyze the functional groups of the prepared hydrogels. Investigation and magnification of the polymer surface was carried out by SEM (JEOL-JSM-5400, Japan). We used the Shimadzu TGA -50 (Japan) to characterize the thermal stability of the copolymer hydrogel. We performed gamma irradiation using 60Co gamma rays with a cylinder irradiation chamber. We performed all irradiations at ambient temperature (about 45 ºC at the chamber) and a dose rate of about 1.22 Gy/Sec.
3. Results and Discussion
3.1. Characterizations of the Hydrogel and Foam
3.1.1. FTIR Characterization
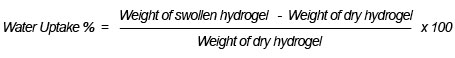
We obtained evidence of copolymerization and network formation by characterizing the synthesized hydrogel and foam. In the FTIR spectrum shown in Figure 1, the peak at 3400 cm-1 is due to the O-H stretching vibration band. Some broadness in OH, due to intermolecular hydrogen bonding between the hydroxyl groups, participated along the chains of the copolymer network structure. The stretching vibration band appearing at 2500 cm-1 was for anhydride structure, resulting in an interaction between two carboxylic groups participating in two parallel chains of the copolymer structure. Also, the presence of the intense characteristic band at 1560 cm-1 occurred due to C=O asymmetric stretching in the carboxylate anion, which was reconfirmed by the symmetric stretching mode of the carboxylate anion causing another sharp peak at 1434 cm-1. The band appearing around 1730 cm-1 constitutes the evidence of the presence of the carbonyl group C=O in some ester formations, which resulted from the interaction between the COOH group of acrylic acid and the OH group of polyvinyl alcohol. In addition, increasing the intensity of the stretching vibration band of the C-C group at 1450 cm-1 provides evidence of a crosslinking copolymerization reaction due to free radical copolymerization. We saw these later characteristic peaks with both foam (Figure1-b) and hydrogel (Figure1-a), which provides evidence that no chemical effect occurred through the foaming of the hydrogel; the foam’s peak had greater intensity than the hydrogel, as Figure 1 shows, which may be due to the hydrogen actively interacting with the polymer net matrix (El-Toony, 2007).
Figure 1: FTIR spectra of PVA/AAc at 0.17% acrylic acid and 1 KGy irradiation dose; a) hydrogel, b) foam.
3.1.2. Thermogravimetric Analysis (TGA)
As a result, the weights of the polymer specimens decreased. The determination of this loss in weight is the principle of different variant of thermogravimetric analysis (TGA). The temperature at which the polymers lose weight is called the thermal resistance of polymer (Korshaki, 1997; Wang et al., 2003). The initial weight loss occurred between 180 ºC to 190 ºC. These temperatures represent the difference between foam and hydrogel due to the weight loss of volatile compounds, as can be seen in Figure 2. After that, the second step shown in the thermogram was accompanied by some deformation of the hydrogel backbone skeleton structure. In this step, the hydrogel lost significant weight at temperatures ranging from 190 to 420 ºC; the foam showed that the weight loss in the second step occurred in two stages: the first stage occurred from 190 to 280 ºC and the second stage from 260 to 380 ºC. Clearly, the foam composite structure was more stable thermally than the hydrogel copolymer.
Figure 2: Thermogravimetric analysis of PVA/AAc at 0.17% acrylic acid and 1 KGy irradiation dose; a) hydrogel, b) foam.
3.1.3. Scanning Electron Microscope (SEM) Investigation
We saw obvious spatially scattered gaps due to the foaming of the hydrogel. Big pores, in-between spaces, and long arms have been shown to differentiate between the foam and hydrogen (Sugino et al., 2008). Therefore, the foam material showed higher pore content on its surface than the hydrogel, proving that foam has a greater capacity for adsorption than the hydrogel copolymer. We observed a distribution of light zones over dark ones in a regular manner while whitish to grayish colors also appeared, which may be due to complete homogenization of the synthesized foam, while in the case of hydrogel regular distribution of white spots irregularly distributed over a dark matrix showed less homogeneity.
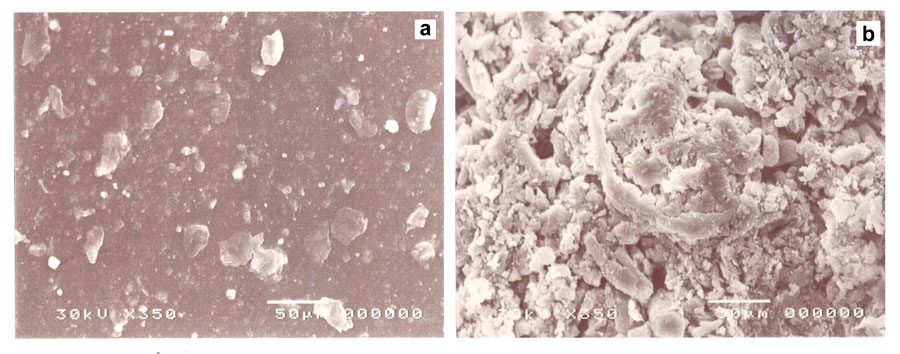
Figure 3: SEM of PVA/AAc copolymer; a) hydrogel, b) foam.
3.2. Water Uptake Studies
Copolymerization of a hydrophilic monomer (acrylic acid) onto a hydrophilic polymer (polyvinyl alcohol) leads to a hydrophilic copolymer. We studied the water uptake of hydrogels and foam as a function of composition, gamma irradiation doses, various contact times, and the water’s pH; we assigned 1 KGy as the optimal dose for water uptake.
Foaming of the hydrogel caused a dramatic 4,000-fold change in water uptake. We may attribute this a larger net surface area of the foam’s water-capturing matrix, caused by increases in pore number, pore size, and the dimensions of the constructed foam.
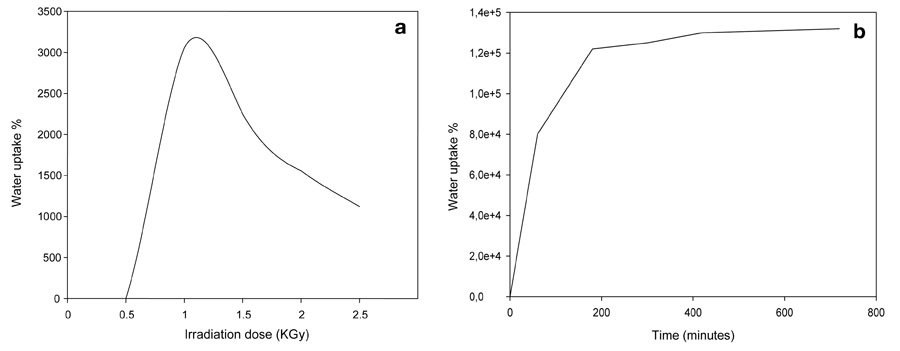
Figure 4: Water uptake of PVA/AAc copolymer at 0.17% acrylic acid; a) irradiation dose on hydrogel, b) contact time on foam.
3.3. Turbidity
The coagulation process traditionally occurs by adding aluminum ions (Hering et al., 1996). In this process, fine particles in water first aggregate into coagulates, since adding aluminum ions strongly reduces the absolute values of the particles’ zeta potentials. Metal ions, organic particles, and microbial cells, etc. then coagulate, thus concentrating in a floc. The aggregative stability of colloidal suspensions is due to the existence of a potential energy barrier preventing the particles from coming into proximity. Research into this stability yielded the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory (Verwey and Overbeek, 1948), which asserts that the potential energy barrier arises as a result of the interacting energies of the electrical double layer and the van der Waals attraction. In a suspension of heterogeneous particles, we can express the potential energy of the electrical double layer interaction between two heterogeneous spheres (VR) (Wang, 1991) as:
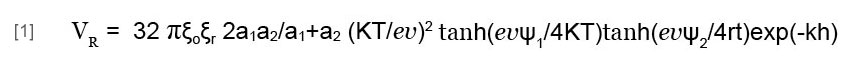
where a1 and a2 are the radii of particles 1 and 2, respectively; ξo is the vacuum dielectric permittivity; ξr is the relative dielectric permittivity of the medium; k is the Boltzmann constant; T is the absolute temperature; e is the elementary charge; v is the ionic valence of the electrolyte; ψ1 and ψ2 are the outer Helmholtz plane (OHP) potentials or zeta potentials of particles 1 and 2, respectively; k is the Debye reciprocal length; and h is the shortest separation between the two particles.
The potential energy of the van der Waals interaction between two heterogeneous particles (VA) is expressed by:
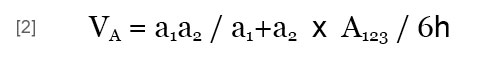
where A123 is the Hamaker constant of particles 1 and 2 in medium 3, which may be obtained by (Israelachvili, 1992):
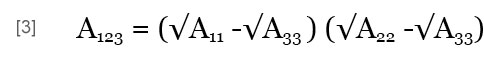
where A11, A22 and A33 are the Hamaker constants of particles 1 and 2 and of medium 3 in a vacuum.
In this work, the Hamaker constants of the floc and foam/alum are larger than water; i.e., A11 > A33 and A22 < A33. So, A123 < 0 ; thus, VA < 0 according to Eqs. [2] and [3], indicating an attractive interaction of van der Waals between the two heterogeneous particles. In the range of pH = 4.9 to 9.3 at 28 ºC, ψ1 < 0 (Floc), and tanh (ev ψ1 / 4kt) < 0, and tanh (ev ψ2 / 4kt) > 0; thus, VR < 0 according to Eq. [1]. Hence, the electrical double layer within the interaction can be active between the two heterogeneous particles, floc and foam/alum applied. Therefore, in this pH range, the total potential energy of interaction established between the floc and the foam/alum particles was attractive at every distance. In other words, no potential energy barrier existed between the two particles (Song et al., 2005), and a strong coagulation should take place.
This mechanism forms small flocs, as shown in Table 1 (a and b).
Table 1: a) Effect of alum/foam (PVA/Acc) on TDS, turbidity and pH on Nile water samples at 28 oC temperature after 30 minutes of shaking. b) Effect of time (hour) on TDS, turbidity and pH on Nile water samples at 28 oC temperature treated with 20 mg foam:26 mg alum.
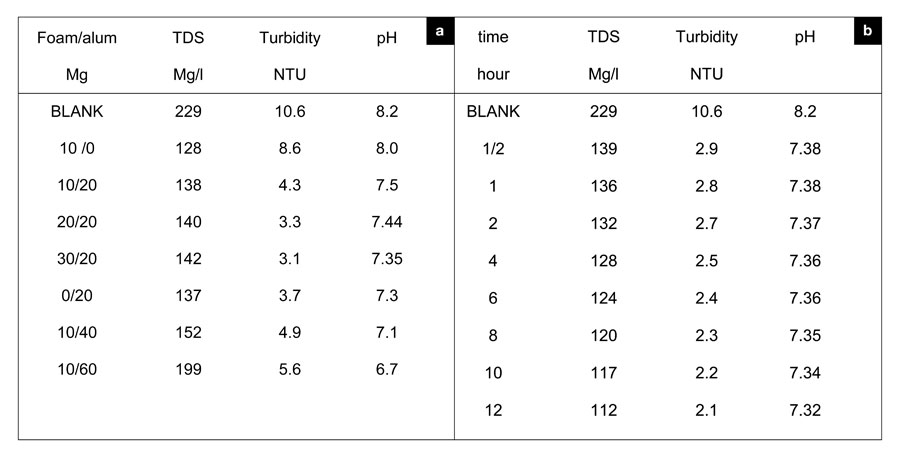
We used jar tests to measure turbidity reductions to determine the removal efficiency of sediment in the Nile. Subsequent increases in stirring speeds increased turbidity, although turbidity levels generally remained lower than they were during settling. It is important that this floc remain settled, so as to not resuspend sediment load in the Nile. Relative to the control, adding alum actually increased the turbidity during the settling period at 300 RPM. The slightly higher turbidity is explained as the formation of the colloidal Al(OH)3. Although this floc increased turbidity at high speed, alum reduced the turbidity to that seen in the foam water with no stirring. The mixing speed, or power input, can be described by the G value (velocity gradient):
![]()
where W is the power imparted to the water per unit volume of the basin and μ is the absolute viscosity of the water (Reynolds and Richards, 1995). The value of W depends on the container, paddle geometry, and paddle speed. The G-value is the common variable for comparing mixing between the jar tests and larger-scale flows, like furrows or drains. The corresponding G-values associated with paddle speeds of 25 and 50 RPM are 18 and 45 s-1 (at 22 ºC), respectively. These paddle speeds resulted in efficient collisions and effective bridging interactions for floc formation between the suspended solids. Any G-value above these limits destabilized the floc (Young et al., 2000). The alum treatment, however, required slower mixing speeds (<5 RPM) for proper floc settling (G<10 s-1).
Although alum and foam can reduce turbidity at different G-values, mutual implementation could improve turbidity removal compared to the application of either treatment alone, as shown in Table 1-a . Table 1-b lists the effective times corresponding to applied ratios at which we obtained maximum turbidity removal. Other researchers have demonstrated that higher molecular polymers enhance flocculation by better bridging between particles (Verwey and Overbeek, 1948). Also, many authors claim that polyvalent cations, like calcium, magnesium, and aluminum, encourage cation bridging between polymer and clay (Peng and Di, 1994).
In our work, we kept the pH in the range of 7 to 8.5 throughout the treatment process; we also kept the temperature between 30 and 33 ºC.
3.4. Microbial Populations (Bacterial, Fungi and Molds)
The composition and structure of the water-sediment interface show sensitivity to environmental conditions, largely to biological components (e.g., bacteria and fungi). Moreover, the density and diversity of meiofauna decrease with the decreasing oxygen penetration caused by eutrophication of the overlying waters (La Rosa et al., 2001). This may explain the elevated population estimates below fish farms in comparison with points away from aquaculture sites (Herwig et al., 1997). The high concentration of bacteria within flocs reflects the importance of microbial activity in the transport and loss of aquaculture wastes, the adhesion of these suspended particles (Tlusty et al., 2000; Austen et al., 2002).
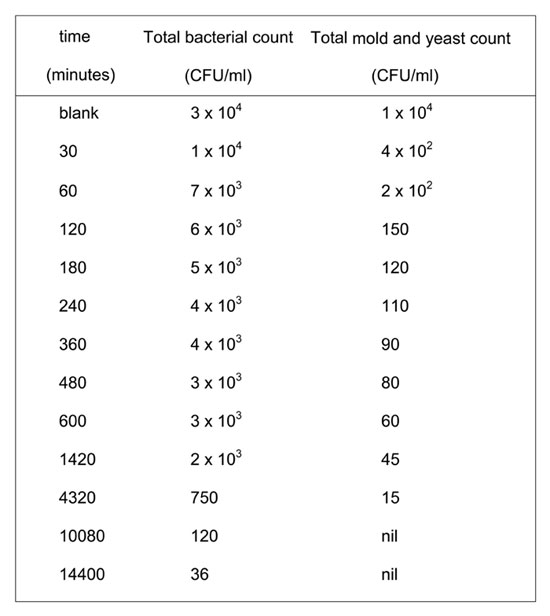
Both alum and foam play important roles in eliminating the water pollution caused by microbial populations; they act by adsorbing charged bacterial cells to negatively charged polymers or positively charged trivalent aluminum. Floc formation via the alum/foam system also removed the microbial populations. Time is a critical factor in the removal of fungi and bacteria. Fungi showed remarkably more sensitivity towards the implanted system of treatment, as shown in Table 2. We also found that seven days suffices to completely remove fungi, while it takes ten days to reduce bacteria populations below 50 CFU/ml.
Table 2: Effect of contact time (hours) on the reduction of bacterial and molds colonies count in the treatment of Nile water samples with 20 mg foam and 26 mg alum.
3.5. Calcium and Magnesium Ions
Researchers have noticed different trends for calcium and magnesium (Mg) within the sediment. The flocs contain much higher levels of available Mg than of other metals found. This most likely results from the abundance of diatoms commonly found in flocs (Droppo, 2001). Calcium, however, presents a different scenario; two studies (Sugiura et al., 1998; Storebakken et al., 2000) assessed the apparent absorption of various elements from fish feed. Both studies found a possible negative adsorption of calcium caused by fecal excretion.
The removal of calcium and magnesium depends on the pore size of the membrane filter disks used for coagulation (Han et al., 2003), since coagulates smaller than the pore size can pass through the filter and remain in the bulk water. We created a closed system in a container to perform chelation using two arms of the carboxylic group of acrylic acid and the hydroxyl group of polyvinyl alcohol. Using foam with a large surface area and wide pore sizes increased the likelihood of adsorption. Time is a dependent factor for controlling the sorption of calcium and magnesium; increasing the contact time increased the removal percentage of each metal ion, as shown by Figure 5. Magnesium showed a greater removal percentage, perhaps due to its smaller ionic radius (72 Pm; 1 Å = 100 pm), than calcium, which has a larger radius (100 Pm). A foam/alum system also reduced TDS, which resulted from the settlement of charged particles (organic and inorganic). When stabilizing such floc by adding foam, we considered the adsorption of such charged particles. As Table 1a makes obvious, time played an important role in reducing such values.
4. Conclusion
We used gamma irradiation to prepare PVA/AAc hydrogel and performed hydrogen foaming to improve the physicochemical characteristics of the copolymer. We found that a hydrogel with 17 percent AAc and 1 KGy were factors that optimized the improvement of real Nile river water pollution.
To reduce TDS, turbidity, calcium, magnesium, and microbial populations at a constant pH in River Nile water samples, we can recommend using foam/alum at a ratio of 20 mg foam:26 mg alum. Twelve hours allows sufficient time to attain very good results and to achieve a reduction in the mass of alum added to treat the intake water (from 40 or 50 mg/l to 26 mg/l). This adsorption of excess aluminum by the applied foam creates water quite safe for human consumption. This system reduces the levels of chlorine needed to disinfect the water, limiting its adverse impact on health and its corrosive effect on the municipal potable water networks while keeping human health safe and protecting water pipes against corrosion. We found that ten days of treatment with this system completely eliminated fungi and bacteria, a much more safe method than chlorine added for disinfection purposes.
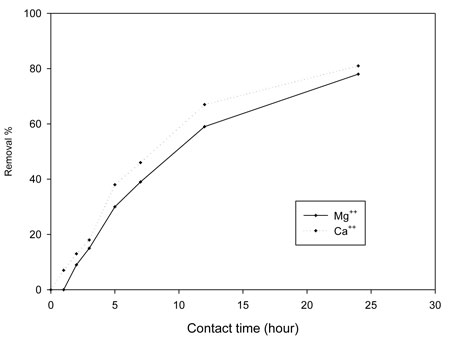
Figure 5: Effect of contact time (hours) of 26 mg alum:20 mg foam (PVA/AAc)/liter on the percentage of magnesium and calcium removed from the Nile.
Acknowledgements
We are grateful to the referees, and to Dr. Dessouki A.M., professor of radiation chemistry at the National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority (AEA), Egypt, for his constructive suggestions and guidance during preparation of this paper.
References
Aly S.M., and Letey J., 1990. Physical properties of sodium-treated soil as affected by two polymers., Soil Sci. Soc. Am. J., 54:501–504.
Austen M.C., Lambshead P.J.D., Hutchings P.A., Boucher B., Snelgrove P.V.R. Heip C., King G., Koike I. and Smith C., 2002. Biodiversity links above and below the marine sediment-water interface that may influence community stability. Biodiversity and Conservation, 11,113-136.
Cantwell M.G and Burgess R.M., 2001. Metal-colloidal partitioning in artificial interstitial waters of marine sediments: influence of salinity, pH and colloidal organic carbon concentration. Environment Toxicology and Chemistry, 20, 2420-2427.
Droppo I.G., 2001. Rethinking what constitutes suspended sediment. Hydrological processes 15, 1551-1564.
EL-Toony M.M., PH.D Thesis of, 2007. The Use of Ionization Radiation for the Modification of Some Polymeric Materials for Use in Practical Applications, Faculty of Science, El-Menofia University.
Green V.S., Scott D.E., Norton L.D., and Graveel J.G., 2000. Polyacrylamide molecular weight and charge effects on infiltration under si mulated rainfall. Soil Sci. Soc. Am. J., 64:1786–1791.
Hammer M.J. and Hammer M.J Jr., 1996. Water and wastewater technology. 3rd ed. Prentice Hall, Columbus, OH.
Han B., Zimbron J., Runnells T.R., Shen Z. and Wickramasinghe S.R., 2003. New arsenic standard spurs search for cost effective removal techniques. J. AWWA, 95 (10), 109–118.
Hering J.G.,.Chen P.Y, Wilkie J.A., Elimelech M. and Liang S., 1996. Arsenic removal by ferric chloride. J. AWWA, 88 (4), 155–167.
Herwig R.P., Gray J.P. and Weston D.P., 1997. Antibacterial resistant bacteria in surficial sediments near salmon netcage farms in Puget Sounds, Washington. Aquaculture, 149,139-146.
Israelachvili J.N., 1992. Intermolecular and Surface forces, second ed. Academic Press, Inc., New York.
Korshaki V.V., 1997. The chemical structure and thermal character of polymer, Keter press, 24.
Laird D.A.,1997. Bonding between polyacrylamide and clay mineral surfaces. Soil Sci., 162:826–832.
La Rosa T., Mirto S., Mazzola A. and Danovaro R., 2001. Differential responses of benthic microbes and meiofauna to fish-farm disturbance in coastal sediments. Environmental Pollution, 112, 427-434.
Malik M., Amrhein C., and Letey J., 1991. Polyacrylamide to improve water flow and salt removal in high shrink-swell soil. Soil Sci. Soc. Am. J., 55:1664–1667.
Mason L. B., Amrhein C., Goodson C.C., Matsumoto M.R. and Andersson M.A., 2005. J. of Environmental Quality, 34, 1998-2004.
Peng F.F., and Di P., 1994. Effect of multivalent salts, calcium and aluminum on the flocculation of kaolin suspension with anionic polyacrylamide. J. of Colloid Interface Science, 164:229–237.
Reynolds T.D. and Richards P.A., 1995. Unit operation and process in environmental engineering. 2nd ed. PWS Publ., Boston.
Sakairi N., Suzuki S., Ueno N., Han S.-M., Nishi N. and Tokura S., 1998. Biosynthesis of hetero-polysaccarides by Aerobacter xylinum-synthesis and characterization of metal-ion adsorptive properties of partially carboxymethylated cellulose, Carbohydrate. Polymer, 37, 409-414.
Scendel E. K., Nordström S. E. and Lavkulich L. M., 2004. Aquaculture Research, 35, 483-493.
Sojka R.E., Lentz R.D., and Westermann D.T., 1998. Water and erosion management with multiple applications of polyacrylamide in furrow irrigation. Soil Sci. Soc. Am. J., 62:1672–1680.
Song S., lobez-Valdivieso A., Hernandez-Campos D.J., Peng C., Monroy-Fernandez M. G.and Razo-Soto I., 2005. J. of Water Research.
Storebakken T., Shearer K.D. and Roem A.J. 2000. Growth, uptake and retention of nitrogen and phosphorus, and adsorption of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soil-protein concentrate as the main sources of protein. Aquaculture Nutrition, 6,103-1028.
Sugino A., Uetsuki K.,.Tsuru K, Hayakawa S., Osaka A. and Ohtsuki C., 2008. Surface topography designed to provide osteoconductivity to titanium after thermal oxidation. Mater Trans, 49:428-34.
Sugiura S.H., Dong F.M., Rathbone C.K., and Hardy R.W., 1998. Apparent protein digestibility and mineral availabilities in various feed ingredients for salmonid feeds. Aquaculture, 159,177-202.
Tlusty M.F., Snook K., Pepper V.A. and Anderson M.R., 2000. The potential for soluble and transport loss of particulate aquaculture wastes. Aquaculture Research, 31, 745-755.
Verwey E.J. and Overbeek J.G., 1948. Theory of the Stability of Lyophobic Colloid. Elsevier, Amsterdam.
Wang Q., 1991.Theoretical analysis of Brownian heterocoagulation of fine particles at secondary minimum. J. of Colloid Interface Science, 145, 305–313.
Wang X.X., Yan W., Hayaakawa S., Tsuru K.and Osaka A., 2003. Apatite deposition on thermally and anodically oxidized titanium surfaces in a simulated body fluid. Biomaterials, 24: 4631-7.
Welch E.B. and Cooke G.D., 1999. Effectiveness and longevity of phosphorus inactivation with alum, Lake Reservoir Manage, 15: 5-27.
World Health Organization, 2007. Combating Waterborn Diseases at the Household Level. Part 1.
Young S. S., Stanley, and Smith D.W., 2000. Effect of mixing on the kinetics of polymer-aided flocculation. J. Water Supply Resource Technology, 49:1–8.
Zhang X.C., and Miller W.P,1996. Polyacrylamide effect on infiltra Wiley and Sons, New York. tion and erosion in furrows. Soil Sci. Soc. Am. J. 60:866–872.
Discussion with Reviewers
Hosnia Abu-Zeid1: Why did you not study water samples from different depths in the same site, and in different seasons not only summer season?
M. M. El-Toony, Nabil A. El-Kelesh and Hussien I. Abdel-Shafy: As my work aims to synthesis of polyelectrolyte which could replace the chlorine adding and safe alum which add with extensive ratio to reduce turbidity to human consumption acceptable range. Characterization is also an important factor to confirm their availability for application on real water sample which has been taken in front of maspero which is enriched by pollution especially in summer season.
Abu-Zeid: Why the authors did not compare their results with those obtained with other authors?
El-Toony, El-Kelesh and Abdel-Shafy: I put a target which is reaching to that WHO acceptable ranges for human consumption water, and throughout my work I do my best to achieve these ranges. There are many works purified American rivers from turbidity sulfur compounds and other pollution they start from more pollution than that present in our river. Some authors used commercial polyelectrolyte which is less efficient than that mentioned in my work as it has less effect on microorganism, calcium and magnesium as well.
Abu-Zeid: There are many other sites on the Nile River across Cairo city needs study similarly to get valuable conclusion, why these sites were not taken into the considerations of the authors besides Maspero region?
El-Toony, El-Kelesh and Abdel-Shafy: I thank the auditor for such intelligent notice, that it must take in our consideration to be treated in the future work. You know that many efforts were offered to prepare the polyelectrolyte foam and fit the ratio to attain optimal results which could be met the WHO accepted range for men consumption.
Abu-Zeid: Which type of γ-gamma radiation source is used in this study?
El-Toony, El-Kelesh and Abdel-Shafy: Irradiations were carried out using 60Co gamma rays with a cylinder irradiation chamber. All irradiations were performed at ambient temperature (about 45 ºC at the chamber) and a dose rate of about 1.22 Gy/Sec. and I add them to the manuscript.
1 Physics Department, University College for Women, Ain Shams University, Cairo, Egypt
