 The Study of Thyrotropin-Releasing Hormone Effect in a Wide Concentration Range on the Aquifer System by IR-Spectroscopy Method
The Study of Thyrotropin-Releasing Hormone Effect in a Wide Concentration Range on the Aquifer System by IR-Spectroscopy Method
Zhernovkov, VE1; Roshchina, IA2; Zubareva, GM2; Shmatov, GP3; Lokshin, BV4; Palmina, NP1,*
Key Words: thyrotropin-releasing hormone, super low doses, water structure, IR-spectroscopy
Received 8 December 2009; revised 25 March; accepted 11 April. Published 30 April 2010; available online 30 April 2010.
Summary
The effect of thyrotropin-releasing hormone (TRH) in a broad concentration range (10-20 to 10-2 М) on water transmittance index was studied by the method of infrared spectroscopy in the middle-IR range. It is indicated that high TRH concentrations induce the highest impact on transmittance, and a statistically significant maximum at TRH concentration of 10-16 M is observed. Hence, high concentrations cause no effect on IR-spectrum fluctuations, whereas the TRH low concentration range (about 10-16 M) is characterized by a strong dispersion increase (almost twice as much as the control) for the mean transmittance of IR radiation. It is concluded that different TRH concentrations, including super low ones, change the aquifer system state causing formation of new types of clusters, whose impacts on transmittances are rather high.
Article Outline
Introduction
To date, plenty of information on paradoxical impacts of biologically active substances (BAS), e.g., hormones, peptides, pesticides, poisons, antioxidants and other agents, in ultra low doses (ULD, 10-22 – 10-12 М) on the living systems of various complication degrees, from enzymes and membranes to entire organisms and populations, is accumulated in the literature (Ashmarin et al., 1992; Burlakova et al., 2004; Calabrese and Baldin, 2002; Khuda-Buksh, 2003; Milazzo et al., 2006; Mohr et al., 2003; Montfort, 2000; Mori et al., 1996; Morimoto et al., 1993; Pal’mina et al., 1994, 1995; Puchalsky and Smarina, 2001; Voronina and Molodavkin, 1999; Zhernovkov et al., 2003, 2005; Zhernovkov and Pal’mina, 2007, 2009; Yamskova et al., 1999).
The features of these effects (Ashmarin et al., 1992; Burlakova et al., 2004; Calabrese and Baldwin, 2002) are a nonlinear type of dose dependence, which has several extremes separated by the so-called ‘dead zones,’ in which agents are inactive or demonstrate minimal activity. The effect of BAS in ULD changes the sensitivity of different objects (cells, membranes, organisms) as a result of the impact of disturbing agents (irradiation, toxical substances).
It may be possible to stabilize the effect of BAS in ULD if a cell or an organism contains a particular substance in a concentration that is several orders of magnitude higher than the ligand-receptor complex dissociation constant. The effect of BAS on a receptor in a concentration that is several orders of magnitude lower than the ligand-receptor complex dissociation constant (Burlakova et al., 2004; Pal’mina et al., 1994; 1995; Zhernovkov and Pal’mina, 2007) may destabalize ULD.
BAS effect stratification—with its concentration decrease preserving the main effect and elimination of the side properties (Voronina et al., 1999)—has been determined. The studies of Ashmarin (Ashmarin et al., 1992, 2003, 2005) and Chepurnov SA (Chepurnov et al., 2002, 2005), and our own studies (Zhernovkov et al., 2003, 2005; Zhernovkov and Pal’mina 2007, 2009) have demonstrated that TRH—the hormone regulating the functional state of many organisms’ systems in a wide concentration range—is also ULD-active. In particular, we are presently concerned with its effect on a wide concentration range, including ultra low concentrations (10-18 – 10-4 М) of spontaneous contractive activity of lymphatic vessels.
The antispasmodic brain defense in epileptic fits—in animals and humans—has been demonstrated (Ashmarin et al., 1992, 2003; Chepurnov et al., 2002, 2005). As determined previously by our studies of invitro experiments (Zhernovkov et al., 2003, 2005; Zhernovkov and Pal’mina, 2007, 2009), TRH induces concentration-dependent changes in structural parameters of the lipid component of plasmatic and microsomal membranes: microviscosity, order parameter, and more statistically significant changes (the effect up to 30%) have been observed for super low concentrations (doses) rather than for standard physiological ones. The changes in microviscosity of membranes are accompanied by shifts in temperature of appropriate structural transitions and their activation energies.
It must be emphasized that, despite plenty of data on the BAS action including the TRH impact on biological processes, the mechanism of their action in doses below 10-12 М is not yet fully clear. Since biochemical processes in the organism proceed in the aqueous medium, it has been suggested that structural changes of intra- and extra-cellular water, which occur under the effect of BAS, are significant in these processes (Grigor’ev et al., 2003, 2003a; Lobyshev, 2003, 2005; Pollack, 2001, 2001a; Voeikov, 1999, 2005, 2009; Yamskova et al., 1999; Zubareva et al., 2003, 2003a, 2003b). Some authors suggest that as a substance dissolves in water, reactive oxygen species (ROS) are formed, and these species, rather than a biologically active substance, affect biological objects (Voeikov, 1999, 2006; Voeikov et al., 2006). Consider that long-living structural formations already exist in pure water, and they may be considered the primary target for low concentrations of dissolved substances (Lobyshev et al., 2003, 2005). The appropriate variation in water properties causes a change in biomembrane properties and, consequently, this leads to a change in functional activity of the cell.
An expanded set of investigations of diluted BAS aqueous solution properties using IR-spectroscopy methods was carried out (Yamskova et al., 1999; Zubareva et al., 2003, 2003a, 2003b). The authors of these works exercise various judgments on BAS action mechanism in ULD, but they agree with this statement: the substances studied change the structure of water as well as the multimodality of the effects, depending on the concentration of the dissolved substance being observed. To check on this hypothesis we have recorded IR spectra of TRH aqueous solutions using an IKAR spectrometer.
Materials and Methods
In this work, TRH of Sigma Company was used; TRH solutions were produced by consecutive dissolution of the initial mixture (10-1 M) by an order of magnitude using bidistilled water.
The spectrum of thyrotropin-releasing hormone was measured on an IR Fourier spectrometer Magna IR 750 (Nikolet Company) in the range of 4000-500 cm-1, in a tablet with KBr.
Transmittances and their dispersion for TRH solutions in some wide (50-100 cm-1) ranges in the middle part of the infrared spectrum were determined using IKAR, a new programmed hardware set designed in Tver Medical Academy. This set allows quick quantitative determination of transmittance fluctuations in nine ranges of the IR spectrum. The record rate is 1 measurement by 9 channels per second. Transmittance values were recorded for the following wavelength ranges: 3500-3200 cm-1 (channel 1), 3085-2832 cm-1 (channel 2), 2120-1880 cm-1 (channel 3), 1710-1610 cm-1 (channel 4), 1600-1535 cm-1 (channel 5), 1543-1425 cm-1 (channel 6), 1430-1210 cm-1-1 (channel 8), 1067-963 cm-1 (channel 9). The range width was defined by optical parameters of the appropriate interference filter. The measurement was carried out in cuvettes from КRS-5 20 μm thick. For IKAR system, the measurement error of transmittance is below ±0.3%. For specific transmission of infrared radiation in each range, the transmittance (Ktr)×100 (r.u.) was used. The detailed description of the unit is presented in the patent (Kargapolov et al., 1999).
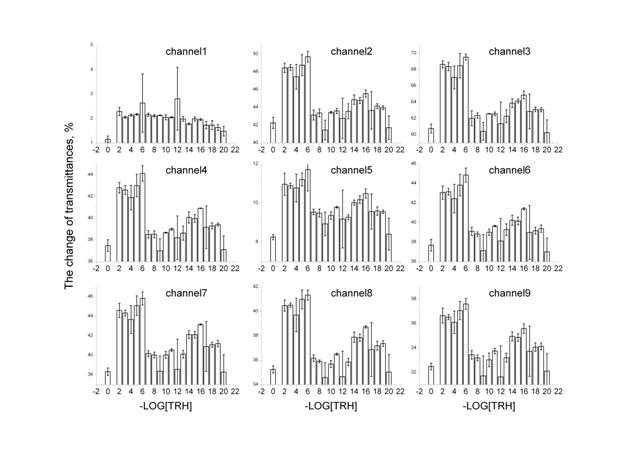
Figure 1: The change of transmittances for 9 channels: 3500-3200 cm-1 (channel 1), 3085-2832 cm-1 (channel 2), 2120-1880 cm-1 (channel 3), 1710-1610 cm-1 (channel 4), 1600-1535 cm-1 (channel 5), 1543-1425 cm-1 (channel 6), 1430-1210 cm-1 (channel 7), 1127-1057 cm-1 (channel 8), 1067-963 cm-1 (channel 9).
At the first stage of the experiment, the values of transmittances and their dispersions for pure water (etalon) and then for the TRH solution under study were recorded. Further on, in the evaluation environment of MATLAB integrated system of calculations, the linear discriminative analysis was performed. It is noted that in every range of wavelengths normal distribution of fluctuations was observed. This gave an opportunity of quantitative characterization of the water state in the presence of various TRH concentrations by nine dispersions determined at various frequencies of the spectrum using the Mahalanobis criterion (the Mahalanobis distance). This criterion characterizes the distance between the centers of two compared groups (the etalon and the sample) in the multidimensional space with regard to their paired correlations between cognominal matrix columns of spectral characteristics. Contrary to Euclidean and other metrics, this metric is associated with correlations of infrared indices via the dispersion matrix. When the correlation between variables is zero, the Mahalanobis distance is equivalent to the Euclidean distance. Theoretically, the Mahalanobis criterion is reasoned well and qualitatively estimates the difference between the sample and the etalon (Maesschalck et al., 2000). The mathematical expression is the following:
![]()
where Yk,n is the matrix of spectral characteristics of the sample; Xm,n is the matrix of spectral characteristics of the etalon;
![]()
is the vector of mean arithmetical columns of the matrix X; S-1 is the inverse matrix to general intraclass dispersion-covariance matrix S:
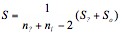
where S? = (n? – 1)cover(X) is the covariance matrix of the etalon; is the covariance matrix of the analyzed sample; net, ns are sampling sizes of the etalon and the sample. Calculations and charting were performed in MATLAB and Origin software media.
Results and Discussion
Figure 1 shows changes in transmittance depending on the TRH concentration for 9 channels (various ranges of IR-spectrum). Apparently, the effect dependence on the TRH dose for all channels, in general, with the exception of 1, is analogously nonlinear: more or less for each channel two maximums in the range of low (10-12 – 10-20 М) and high (10-2 – 10-6 М) TRH concentrations are expressed. It should be emphasized that high concentrations induce the highest impact on transmittance. However, at the same time, a statistically significant maximum, comparable by value with the maximum in the high dose range, at the TRH concentration of 10-16 M is also observed.
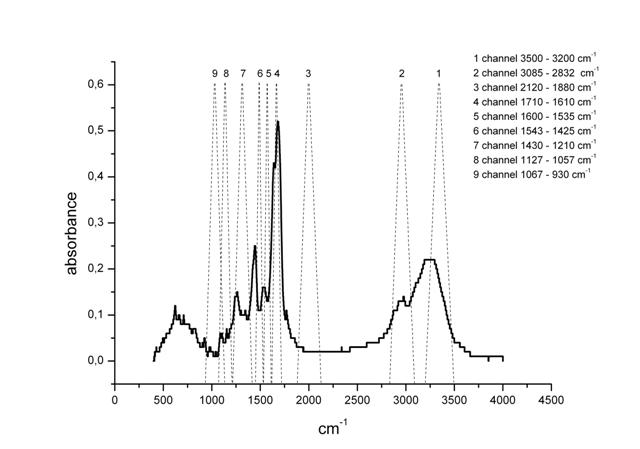
Figure 2: The spectrum of TRH in the middle range of IR-spectrum. Dry substance.
It is suggested that the changes observed in the IR-spectrum of the TRH aqueous solutions may be stipulated by the absorption of the substance itself. To check the correctness of this suggestion, we have measured the IR-spectrum of dry TRH samples in the range of 4500 – 500 cm-1. As far as we know, previous spectral TRH characteristics in this wave range have not been determined. As is shown in Figure 2, this spectrum has several maximums explained according to (Bellami, 1963) valence (ν) and deformational (δ) oscillations of the following groups: wide absorption bands ν(NH) at 3271 cm-1; ν(СН) at 2977 cm-1; ν(С=О) at 1683 cm-1 (amide I band); δ(NH) at 1539 cm-1 (amide II band); and δ(СН) at 1445 cm-1.
In Figure 2, IR-spectrum of TRH showed nine ranges or nine channels, for which measurements by IKAR unit were performed. Some of these channels coincide with the absorption maximums in the TRH spectrum. However, analysis of the data on the absorption index of anhydrous pure TRH using Magna-IR 750 spectrometer of Nikolet Company demonstrates that TRH does not absorb in the frequency ranges of 900-1100 and 1800-2800 cm-1 that corresponds to the third (2120-1880 cm-1) and the ninth (1067-963 cm-1) channels of IKAR spectrometer. It is suggested that, for the purpose of elimination of the substance’s influence on the effect, it is desirable to consider data on these wavelengths. As observed in Figure 1, the transmittance behavior is analogous for both channels, but for the third channel the changes are greater. This range of wavelengths will be discussed in greater detail.
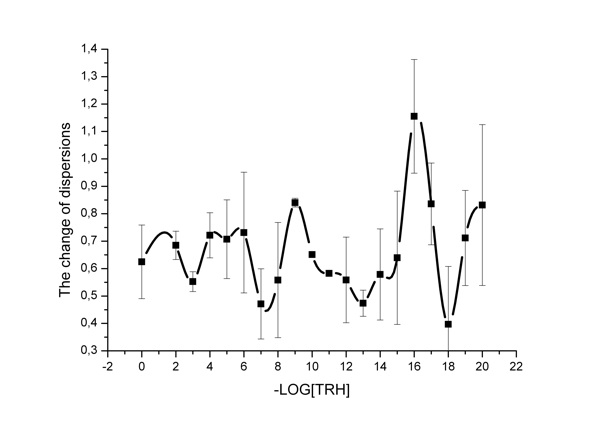
Figure 3: The change of dispersions for channel 3.
In fact, the data on the third channel (Figure 1, channel 3) reproduces a general picture of changes in transmittances observed for the rest of the channels. As is seen in Figure 1, high TRH concentrations induce the highest impact on transmittance. At the same time, a statistically significant maximum—at TRH concentration of 10-16 M with transmittances comparable with these for high TRH doses—is also observed. This super low TRH concentration is so low that it is impossible to explain the effects observed by the presence of the substance itself in solution. Therefore, it may be concluded that the effect observed is stipulated by a change in the structure of water induced by TRH.
Such an increase of transmittances at high dissolution degrees may be caused by differences in the processes of cluster formation in water. There are plenty of data in the literature on the existence of microdomains or clusters in water, the state of which depends on temperature and the presence of additives (Hribar et al., 2002; Keutsch et al., 2001). For the purpose of water dynamic parameters estimation, we analyzed dispersions of IR-spectrum transmittances.
Figure 3 shows data on the ratios of transmittance dispersions in the third channel, and the dependence obtained is of a cyclic wave-like type. High concentrations cause no effect on IR spectrum fluctuations, whereas TRH low concentration range (namely, 10-16 M) is characterized by strong changes in the fluctuation of the spectra of aqueous solutions. Comparative analysis of transmittance dispersions by the Fischer criterion shows significant difference—the dispersion ratio equals 2.46, which is higher than the table value of the Fischer criterion F(0.05, 50, 50) = 1.60. Therefore, the dispersions are significantly different in the third channel range (2120-1880 cm-1). Similar data were obtained for channel 9.Thus, we recorded rather strong changes of both static and dynamic parameters of the aquifer system at the frequency of channel 3 of 2120-1880 cm-1, outlined primarily against the rest of the frequencies.
As mentioned above, for the purpose of complex assessment of the effect of one substance or another on water, in the literature (Zubareva et al., 2003a, 2003b, 2003c) the Mahalanobis criterion was used, the changes of which with TRH concentration are shown in Figure 4. It should be noted that the Mahalanobis criterion is significantly affected by average transmittances and their dispersion. The curve (Figure 4) has three maximums at dilution of the initial TRH solution to concentration of 10-2, 10-6 and 10-16 М.
Thus, different TRH concentrations—both super low and high ones—cause similar, though quantitatively unequal, changes in the aquifer system state. In the authors’ point of view, similar changes of total characteristics of the aquifer system obtained under the effect of other agents (Yamskova et al., 1999; Grigoriev et al., 2003a, 2003b). It is crucial to consider structural heterogeneity of water, its perceptivity to low impacts, its ability to form associates and clusters sized up to 10-9 M (Ponomarev and Fesenko, 2000; Ponomarev et al., 2001), and microzones having physical properties different from voluminous water. Similar changes of the light scattering were recorded in other works (Yamskova et al., 1999) in which the oscillation type of light scattering depending on concentrations of low-molecular protein solutions.
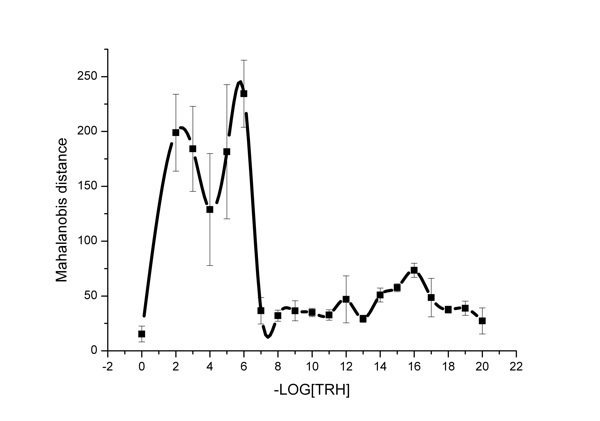
Figure 4: The influence of TRH solutions on fluctuations of IR-spectra of water. Quantitative assessment by the Mahalanobis criterion is given.
We obtained nonlinear dependencies of TRH doses for both the integral parameter of the Mahalanobis criterion and some wavelengths in the IR-spectrum using an IKAR unit. Maximums of transmittances in the range of high and super low TRH doses were recorded. It might be concluded that both high concentrations and super low doses of TRH affect the water structure and, consequently, modify its biological effect. However, as we demonstrated before, the variation mechanism of the physicochemical properties of biological membranes in vitro is unequal for different TRH doses. This is nonspecific incorporation into the membrane in the high concentration range (10-4 – 10-6 М) and ligand-receptor interaction in the range of low doses (10-9 – 10-10 М) (Zhernovkov and Palmina, 2007).
If we turn our attention to the fact that the highest activity of the aquifer system (or increased fluctuations of transmittances) associated with formation and decay of aqueous associates have been determined for ultra low TRH doses only (10-16 М), we can see that, apparently, the effect of structural changes in water induced by TRH influences the effect in this concentration range. The totality of data obtained leads us to conclude that, apparently, each of the mechanisms considered makes its own contribution to the TRH effect on the membranes. But only one of the mechanisms dominates in each concentration range.
For example, the mediate influence via the water structure is the most clearly displayed in the ULD range of TRH (10-16 М) that is confirmed by the exact correspondence of change in the dispersion ratio (Figure 3) as well as in the microviscosity of the lipid component of the membranes which we obtained in previous investigations (Zhernovkov et al., 2003, 2005; Zhernovkov and Palmina, 2007, 2009). Thus, it may be concluded that under experimental conditions different TRH concentrations change the state of heterogeneous aquifer systems, apparently causing formation of new types of clusters or their rearrangement. The impacts on transmittances are rather high. In the case of TRH in ULD injection, a significant increase of fluctuations of these parameters indicates instability and, apparently, the initial non-equilibrium nature of the processes proceeding in water (Lobyshev et al., 2005).
Water is the active dynamic medium sensitive to external impacts. The external factors of low intensity are biologically active substances in ULD, or low physical impacts may affect these processes. By all appearances, it is the latter. These changes in the water structure may affect the functioning of substances dissolved in it, proteins and enzymes in particular, as well as the state of cellular membranes and regulatory systems localized in it. This work results in obtaining experimental data allowing, on the example of TRH peptide, to conclude with certainty about possible mechanisms of BAS in ULD based on the effect of the low factors of impact on the aquifer system.
References
Ashmarin, IP; Asanova, LM; Abbasova, KP; Chepurnova, NE; Kosova, GV; Chepurnov, SA; Inyushkin, AN; Goncharov, OB (2003). Neuropeptide thyroliberin in ultra low doses – anticonvulsant defense of brain, Radiats Biol Radioecol. (Russian) 43: 324-327. Radiazionnaya biologiya. Radioecologiya (Moscow) 43: 324-328.
Ashmarin, IP; Karazeeva, EP; Lelekova, TV (2005). Effectiveness of ultrasmall doses of endogenous bioregulators and immunoactive compounds. Zh Mikrobiol Epidemiol Immunobiol. (Moscow) 3: 109-16.
Ashmarin, IP; Lelekova, TV; Sanzhieva, Lts (1992). The effectiveness of ultralow doses and consentrations of biologically active compounds. Izv Akad Nauk SSSR Biol 4: 531-536.
Bellami, L (1963). Infrared spectrum of complicated molecules. Chemistry (Moscow): 590.
Burlakova, EB; Konradov, AA; Maltseva, EL (2004). Effect of extremely weak chemical and physical stimuli on biological systems. Biophysica (Moscow) 49: 522-534.
Chepurnov, SA; Chepurnova, NE; Abbasova, KR; Goncharov, OV (2002). Neuropeptide thyroliberin – an endogenous anticonvulsant in the brain. Usp Fiziol Nauk (Russian) 33: 29-39.
Chepurnov, SA; Chepurnova, NE; Redkozubova, OM; Saakian, SA (2005). Status epilepticus-the new mechanisms and lines of inhibition (the lithium – pilocarpine model). Usp Fiziol Nauk (Russian) 36: 68-84.
Colabrese, EJ; Baldin, LA (2002). Application of hormesis in toxicology, risk assessment and chemotherapeutics. TRENDS in Pharmacological Sciences 23: 331-337.
Grigor’ev, EI; Khavinson, VKh; Malinin, VV; Grigor’ev, AE; Kochnev, IN; Kudriavtseva, TA (2003a). The study of the role of the water medium in the mechanism of action of peptides in low and ultra low doses. Radiats Biol Radioecol. (Moscow) 43: 358-362.
Grigor’ev, EI; Khavinson, VKh; Malinin, VV; Grigor’ev, AE; Kudryavtseva, TA (2003b). Role of aqueous medium in mechanisms underlying the influence of immunoactive peptides in ultralow doses. Bull Exp Biol Med 136: 150-154.
Hribar, B; Southall, NT; Vlachy, V; Dill, KA (2002). How ions affect the structure of water. J Am Chem Soc 124: 12302– 12311.
Kargapolov, AV; Pligin, AM; Zubareva, GM; Shmatov, GP (1999) Patent N2137126 from 10.09.
Keutsch, FN; Saykally, RJ (2001). Water Clusters: Untangling the mysteries of the liquid, one molecule at a time. PNAS 98: 10533-10540.
Khuda-Buksh, AR (2003). Towards understanding molecular mechanisms of action of homeopathic drugs: an overview. Mol Cell Biochem: 253- 245.
Lobyshev, VI; Soloveĭ, AB; Bul’enkov, NA (2003). Computer module design of the parametric structure of water. Biofizika (Moscow) 48: 1011-1021
Lobyshev, VI; Tomkevich, MS; Petrushanko, IIu (2005). An experimental study of potentiated aqueous solutions. Biofizika (Moscow) 50: 464-469.
Maesschalck, RDe; Jouan-Rimbaud, D; Massart, DL (2000). The Mahalonobis distance. Chemometrics and Intelligent Laboratory Systems 50: 1–18.
Milazzo, S; Russeli, N; Ernst, E (2006). Efficacy of homeopathic therapy in cancer treatment. Eur J Cancer 46: 282-289.
Mohr, P; Weichenthal, M; Hauschild, A (2003). Adjuvant therapy in melanoma. Onkologie 26: 227-233.
Montfort, H (2000). A new homeopathic approach to neoplasmic diseases: from cell destruction to carcinogen-induced apoptosis. Br Homeopath J 89: 78-83.
Morimoto, H; Yonehara, S; Bonavida, B (1993). Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anty-Fas antibody and drug of toxins. Cancer Res 53: 2591-2596.
Mori, S; Muracami-Mori, K; Jewett, A; Nakamura, S; Bonavida, B (1996). Resistance of AIDS-associated Kaposi’s sarcoma cells to Fas-mediated apoptosis. Cancer Res 56: 1874-1879.
Pal’mina, NP; Mal’tseva, EL; Burlakova, EB (1995). Protein kinase C- peroxyl-lipid-dependent enzyme. Phys Reports 14: 1753-1767.
Pal’mina, NP; Kurnakova, NV; Mal’tseva, EL; Burlakova, EB (1994). Effect of α-tocopherol in a wide concentration range (10-2-10-16M) on protein kinase C activity. Biochimiya (Russian) 59: 193-200.
Pollack, GH (2001). Cells, Gels and Engines of Life. Ebner and Sons: Seattle, USA.
Pollack, GH (2001). Is the Cell a Gell – and Why Does It Matter? J Cell Phys 51: 649-660.
Ponomarev, OA; Fesenko, EE (2000). The properties of liquid water in electric and magnetic fields. Biofizika (Moscow) 45: 389-398.
Ponomarev, OA; Zakir’ianov, FK; Terpugov, EL; Fesenko, EE (2001). Absorption of infrared radiation by a thin water layer. Biofizika (Moscow) 46: 402-407.
Puchalsky, AL; Shmsrina, GV (2001). Stimulatory and protective effects of alkylating agents applied in ultra-low concentrations. Pharmacology 62: 129-132.
Voeikov, V (1999). The scientifc basis of the new biological paradigm. 21st Century Science & Technology 12, No.2: 18-33.
Voeikov, VL; Koldunov, VV; Kononov, DS (2001). Long-duration oscillations of chemi-luminescence during the amino-carbonyl reaction in aqueous solutions. Russ J Phys Chem 75: 1443-1448.
Voeikov, VL (2006). Reactive Oxygen Species (ROS): Pathogens or Sources of Vital Energy? Part 1. ROS in Normal and Pathologic Physiology of Living Systems. J Altern Complement Med 12: 111-118.
Voronina, TA; Molodavkin, GM (1999). Experimental analysis of phenozepam effect in ultra low doses. Rossiiskii himicheskii zchurnal (Moscow): XL111, 89-96.
Yamskova, VP; Yamskov, IA; Danilenko, AI; Klemenkova, ZS; Antipov, BG; Chernikov, FR; Gusinina, MM; Rybakova, EYu (1999). Experimental proves of physico-chemical mechanisms in biological effect of ultra low doses. Rossiiskii himicheskii zchurnal (Moscow): XL111: 34-39.
Zhernovkov, VE; Bogdanova, NG; Lelekova, TV; Palmina, NP (2003). The effect of thyroliberin in a wide concentration range on the lipid structural papameters of endoplasmic reticulum membranes. Radiazionnaya biologiya. Radioecologiya (Moscow) 43: 331-334.
Zhernovkov, VE; Bogdanova, NG; Palmina, NP (2005). Effects of ultra low concentrations of thyroliberin on structural parameters of membrane lipids of endoplasmic reticulum in vitro. Biological membranes (Moscow) 22: 388-395.
Zhernovkov VE, Palmina NP (2007). In vitro effects of thyroliberin on structural state of plasma membranes in mouse brain and liver. Bulletin of Experimental Biology and Medicine (Moscow) 144: 185-187.
Zhernovkov, VE; Pal’mina, NP (2009). Cell membrane structure and thyroliberin physiological activity. In: Biochemical Physics Research Trends., Varfolomeev, SD; Burlakova, EB; Popov, AA; Zaikov, GE (Eds). Nova Science Publishers, Inc., NY, USA: 11-18
Zubareva, GM; Kargapolov, AV; Iaguzhinskiĭ, LS (2003a). Effect of superlow quantities of hydrogen peroxide on water base of solutions. Biofizika (Moscow) 48: 581-584.
Zubareva, GM; Kargapolov, AV; Iaguzhinskiĭ, LS (2003b). Specific effect induced by subminute amounts of ascorbic acid on the fluctuations of transmission factor of water in the infrared spectral range. Dokl Biochem Biophys (Moscow) 388: 43-45.
Zubareva, GM; Kargapolov, AV; Iaguzhinskiĭ, LS (2003c). Fluctuations of pass band coefficients of water and water salt solutions in the spectral infrared region. Biofizika (Moscow) 48: 197-200.
Discussion With Reviewers
Igor A. Yamskov1: Now several papers concerning the changes in IR-spectrum of water solutions in the near IR-region (5200 – 14000 cm-1) have appeared. In this regard, I would like to ask what are you thinking about the possibility to find the effect of TRH on the water system in this area of IR-spectrum?
Palmina: We have known very well about the interesting data obtained by several authors concerning the changes in IR-spectrum of different water solutions in the near-IR region (5 200-14000 cm-1): Ken-Ichi Izutsu et al., J. Pharm. Sci. 2006; Akikazu Sakudo et al., J. Toxicol. Sci. 2007; J. Vet. Med. Sci., 2006; Symons M.C., Cell Mol Life Sci., 2004; Chen Y. et al., Appl. Spectrosc., 2009; Amerov A.K., Appl. Spectrosc., 2004; Murayama K., Ozaki Y., Biopolymers, 2002; Wu Y. et al., J. Phys. Chem., 2000. Some of them are dealing with diluted water solutions of proteins and DNA. TRH is water-soluble polypeptide, and there were hints about the possible change of the absorption index of water in the near-IR region (5200 – 14000 cm-1) having many spectral windows in the water spectrum, where IR absorption is very low and, moreover, measurements are performed in a rather thick layer of the solution. At the moment we have carried out these experiments, and obtained results will be presented in the next paper.
Yamskov: There are data about nonlinear concentration dependences for the size of nanoassociates BAC and the specific electrical conductivity nonlinear concentration–bioeffect dependences found for this agent earlier. What is your opinion concerning the possible contribution of such properties of diluted water solution in observed TRH effect?
Palmina: It is a quite reasonable question because academician A.I. Konovalov with his colleagues—in the Arbuzov Institute of Organic and Physical Chemistry, Kazan Research Center, Russian Academy of Sciences—have for a long time studied the dependences for the size of nanoassociates and specific electrical conductivity of high diluted water solutions of biologically active substances (BAC) versus their concentration. They have found that these regularities, for many different BAC, have nonlinear character with several maxima. Recently they experimentally showed, using the plant regulator melaphene as an example, that approximately 20-nm nanoassociates are formed with participation of solvent structures in aqueous solutions of melaphene in the concentration range 10–20–10–4M.
The concentration dependences for the size of nanoassociates and the specific electrical conductivity are interrelated and resemble the nonmonotonic nonlinear concentration–bioeffect dependences earlier found for this agent. Thus, the electrical conductivity data may serve as indicators of formation and transformation of nanoassociates in aqueous solutions. Dr. Pollack, with co-workers, has developed the idea on the existence of near surface water layers in the vicinity of hydrophilic surfaces and particles (including the membranes), which differ from the deeper water layers in viscosity, dielectric permittivity, and electrical conductivity.
So, it is quite possible that these properties of water confirm our assumption made on the basis of experiments on IR spectroscopy of aqueous solutions of TRH. Changes in the concentration of biologically active compounds cause changes in the physicochemical properties of water. The last fact is apparently determined by the formation and rearrangement of nanoassociates in aqueous solutions of biologically active compounds, which are associates of hydrated ions or molecules of a compound and the molecules or associated structures of water.
With regard to our earlier data, it is obvious that the structure and properties of nanoassociates change as the concentration range of biologically active compounds changes, which is reflected in the expression of the overall effect exerted by a dissolved compound on cell membranes. Significant fluctuations detected after the addition of biologically active compounds at ultra-low concentrations for determination of the electrical conductivity of solutions and membrane parameters indicate the occurrence of instability and, respectively, the creation of non-steady-state conditions in the aqueous system, which, in turn, may affect the physicochemical and biochemical properties of membranes.
Vladimir Voeikov2: What particular method of serial dilution did you use? What was the volume of an aliquot that you took from the preceding dilution and what was the volume of water to which you added this aliquote? Was any kind of potentiation used?
Palmina: TRH solutions were produced by consecutive dissolution of the initial mixture (10-1 M) by an order of magnitude using bidistilled water: aliquot 100 µl was taken from the preceding dilution and 900 µl of bidistilled water was added to this aliquote. Then this mixture was shaken using the Vortex for 1 minute and used for measurements. Changes in Vortex speed did not influence the results.
Voeikov: For how long a period of time after the preparations of diluted samples of TRH did the characteristic pattern of dose-response curves presented in Figures 1, 3 and 4 persist?
Palmina: All samples were prepared just before measurements. 20 µl solution was placed into a special cuvette and multiple measurements of transmission coefficient were carried out during 5 seconds. For one cycle 50 measurements in each from 9 wavelength ranges of IR spectrum were recorded. Experiments were repeated 3 times. All statistical calculations were produced on the basis of 150 values for each concentration. The characteristic pattern of dose-response curves presented in Figures 1, 3, and 4 persisted for 2-3 days. The next estimation of dose-response dependences was carried out only after 30 and 60 days.
Voeikov: Did the effects reported in the paper depend upon a certain amount of time between the samples preparation and IR-spectra measurements? In other words, did you observe any kind of “ripening” of solutions that would indicate significant differences between different dilutions?
Palmina: We repeated the same kind of experiments with solutions which were stored at the temperature 8oC during 30 and 60 days. We have found that after 30 days maxima on the plots of the dependence of Mahalanobis value on concentrations decreased two-fold. Also, the first maximum was shifted to lower concentrations (10-7-10-9M), the second maximum was shifted to higher concentrations (10-11-10-13M). After 60 days, the maximum at ultra low concentration (10-16M) disappeared. So we draw the conclusion that it is reasonable to use TRH in biological experiments and clinical practice not later than 30 days after production. It is best to use it during the first three days. This conclusion was confirmed by the data obtained in the works of academician I.P.Ashmarin’s laboratory, which is devoted to antiepileptic properties of TRH.
1 Head of laboratory of physiologically active Polymers, A.N.Nesmeyanov Institute of Organoelement Compounds, professor.
2 Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia.
