 Evaluation of Hen Egg White as a Model of Cytoplasmic Pumping Mechanisms
Evaluation of Hen Egg White as a Model of Cytoplasmic Pumping Mechanisms
Cameron, I1,*; Fullerton, G2
Key Words: Hen egg white, albumen structure, gel-sol, proton NMR, polarization microscopy, water
Received 16 May 2010; revised 29 July; accepted 14 August. Published 27 August 2010; available online 27 August 2010
Summary
The most widely taught explanation of non-homogeneous distributions of cellular metabolites and ions relies on molecular pumps residing in cellular membranes. This study uses fresh hen egg white to demonstrate the capacity of hen egg white to manipulate rheological properties that correlate with co-solute distribution changes previously attributed solely to active (energy consuming) membrane pumps. When egg white was placed on a sieve a thin sol albumen fraction flowed through the sieve and a thick gel albumen fraction was obtained from the sieve surface. The thick gel, but not the thin albumen sol fraction, excludes a low molecular weight dye, methylene blue. The two fractions were examined by polarization microscopy and their water proton NMR T1 and T2 relaxation times were measured. Thick gel was composed of elongated birefringent domains rich in microscopic particles. The thin albumen sol had no such domains, fewer particles and does not exclude dye. Gel agitation fragments the gel domains and frees their particles concomitant with conversion to a sol, and with loss of the dye exclusion property. The gel to sol conversion caused shortening of the NMR T1 relaxation time consistent with increased freedom of motion of water attached to proteins previously entrapped in the less mobile gel. Thus water in the gel differs from bulk water in both motional and dye exclusion properties.
Article Outline
- Introduction
- Methods and Materials
- Results
- Discussion
- Summary and Conlusion
- References
- Discussion with Reviewers
Introduction
Hen egg white provides a readily available source of biological gel and sol state material to test the capacity of protein interactions to change rheological properties that correlate with non-homogenous concentration of particles, proteins, co-solute metabolites and ions. Hen egg white is easily separated into gel and sol fractions by a sieve method. The fraction remaining on the sieve surface is referred to as the thick-gel fraction (Cameron 2010) while the fraction passing through is the thin sol fraction. Cameron’s report also showed the thick gel fraction has a number of physical properties, similar to those ascribed to the cytoplasm of cells (Fels et al. 2009).
What makes egg white form a thick hydrogel? According to the USDA database fresh whole hen egg white consists of: 88% water, 11% protein, 0.16% lipid and 0.7% carbohydrates. The protein fractions are: ovoalbumen 54%, ovotransferrin 12%, ovomucoid 11%, globulins 9%, lysozyme 3.4%, ovomucin 4.2%, others 7%. Of these protein types ovomucin is the type thought responsible for the gel-like properties of thick egg albumen (Brooks and Hale 1959, 1961, Rabouille et al. 1990, Hiidenhovi et al. 2002, 2007, Laghi et al. 2005, Robinson and Monsey 1971, 1972a,b). Crude ovomucin is composed of at least two subunits: α-ovomucin and β-ovomucin both of which are high molecular weight glycoproteins. The carbohydrate content is 22.9% in thick egg white (Hiidenhovi et al. 2002). Analysis of the ovomucin molecule, purified from egg white for electron microscopic observation reveals ovomucin to be a linear molecule that can form a linear polymer chain up to 2000 or more nm long. The ends of such long polymers are apparently linked via disulphide bridges (Rabouille et al. 1990). This super long chain of polymers is proposed by Rabouille 1990 to be organized as a fibrillar network or hydrogel with transient weak linkages that could confer non-Newtonian and viscoelastic properties to the thick egg white gel.
Assessment of differences in the amounts of ovomucin in thick vs. thin albumen reveals thick to have 1.6 times more total ovomucin and 4 times more β-ovomucin than thin (Rabouille et al. 1989). Rabouille et al. also observed that sonication caused concomitant loss of its gel fibrillar nature and of non-Newtonian properties of both thick egg-white and synthetic ovomucin gels. It seems likely that other protein types may interact with the fibrillar network of ovomucin to produce the thick egg-white hydrogel. One note of caution to the above description concerns the Rabouille et al. (1990) ovomucin purification procedure. The concern is with the first step in purification of ovomucin by a 1 to 10 fold dilution with distilled water. Such a dilution can and does cause unfolding and linearization of some folded (globular) proteins. Thus one can question if the linear molecular structure observed by electron microscopy is or is not the same linear structure thought responsible for the thick albumen hydrogel? It is clear, however, that the fundamental mechanism involves direct protein-protein associations that cause non-homogenous protein distributions that could cause redistributions of salts, metabolites and a small test molecule such as methylene blue.
Among the properties of thick egg white are the capacities to exclude vital dyes, respond osmotically and transform from a gel to a non-dye excluding sol under the influence of physical stress and to transform back to a dye excluding gel upon rest (Cameron 2010). Based on the above observations one can logically ask what changes occur when the gel is transformed to a sol? The present report examines two aspects of the hen egg white gel and sol state. One aspect deals with the morphological structure using polarization microscopy and the second aspect deals with water motion using proton NMR relaxation time measures.
Methods and Material
Specimen preparation for microscopy
The hen eggs used in this study were purchased from a grocery store and were reported to have been stored at 40o C for between 2 to 4 days after being laid. The specimen preparation procedure was to collect thick and thin albumen fraction using the sieve filter separation method as illustrated in Figure 1 (Cameron 2010). When 2 ml of 0.1% methylene blue dye was added to the whole egg white from an egg, prior to placement on the sieve, the thin sol albumen fraction was blue while the gel fraction remained almost free of blue color (Figure 2).

Figure 1: Photograph of the under surfaces of the egg white sieve taken during separation of thin sol and thick egg white albumen fractions. Thin drops through the sieve and thick is removed from the upper sieve surface.

Figure 2: Photograph of thin and thick egg white fractions. Methylene blue dye was added to whole egg white prior to being added to the sieve surface. The thin fraction that passed through the sieve is blue (right tube) and the thick fraction from the sieve surface is almost free of blue color (left tube).
For microscopy the thick albumen fraction on the surface of the filter was gently placed into a 50 ml beaker. The beaker contents were tipped to the beaker pouring groove so that a blob of thick albumen gel hung over the lip of the groove. A microscopic slide was positioned just under the protruding albumen blob and scissors were used to cut off a small drop of the blob that touched the horizontal surface of the microscope slide. A large number one coverslip was placed gently on top of the small drop and capillary action caused the gel drop to flatten and to form a thin layer of albumen between the microscopic slide and the cover slip.
Proton NMR relaxation measurement procedure
Pulsed proton MR relaxation times were measured with a Praxis Model II, with a permanent 0.25-T magnet, sample coil, and radiofrequency (RF) pulser tuned to 10.7 MHz. This machine is interfaced to a microcomputer for fast data acquisition and has built-in analytical software. The T1 or spin-lattice relaxation time was measured using the saturation recovery sequence (SR) 900 τ 900. The resultant free induction decay (FID) curves included 30 measurements with a sequence of increasing interpulse delay times. All 30 were fit by means of single-component exponential least-squares regression analysis. The T2 or spin-spin relaxation time was measured by using the Hahn spin-echo method, 900 τ 1800 with 30 increasing values of τ.
Results
Visualization of the thick and thin hen egg white albumen gel and sol state structure by polarization microscopy
Viewing a sample of the thick albumen fraction slide preparation under bright field light conditions using both a 3.2 and 10 X objective lens and a 10 X ocular lens, revealed no structure. However, reviewing the sample specimen field between cross polarizing filtered light at 32 X and 100 X magnification revealed large weakly birefringent elongated domains containing bright birefringent particles. The photograph in Figure 3 illustrates the birefringent domains. Figure 3 shows three parallel elongated domains (arrows) with small particles and areas with fewer particles between the domains. The arrows in Figure 3 are 20 μm in length. Three large bright objects are also seen in one of the large domains.
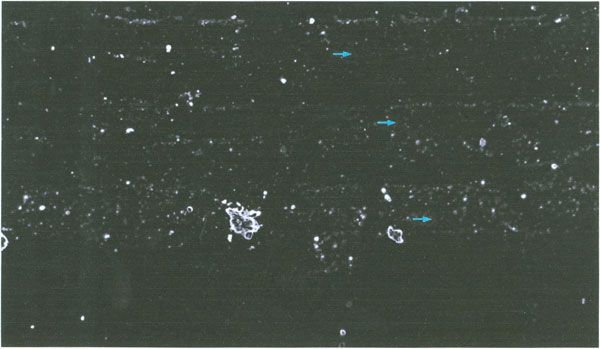
Figure 3: Polarization microscopic image of the thick albumen gel fraction (arrows are 20μm in length). Three parallel elongated weakly birefingent domains with numerous particles are seen. The intervening non-domain areas have fewer particles than the domain.
The size and numbers of particles was determined using an ocular grid calibrated with a stage micrometer. The depth of the sample was determined using the depth of the focus scale on the focus level dial. The focus level scale was calibrated using histological slide sections of known thickness. To determine thickness of the specimen the distance between where particles were in focus at the highest and the lowest focal level was measured. The number of small particles in a 1 mm2 area was scored in 3 eggs. As illustrated in the photograph (Figure 3) the small particles varied in diameter from about 0.5 to about 5 micrometer. The particles in a domain were not observed to move about or to demonstrate Brownian motion. The number of particles per mm2 times the sample depth was used to determine the number of particles per mm3. The numerical values are reported in Table 1.
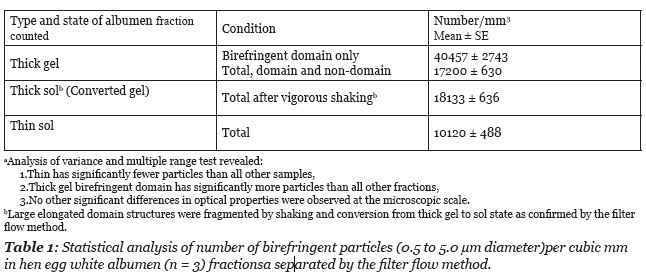
As illustrated in Figure 3, the number of particles is greater within a domain volume vs. the total field volume. The scoring of particles per domain field gave a mean value of 40,457 per mm3. This value is about 2.4 times higher than the mean number of particles per unit volume of the total thick albumen fraction (Table 1).
When a fresh thick albumen fraction gel was shaken in a closed NMR vial, the thick albumen became more fluid or sol-like and the shaking allowed this fraction to spontaneously pass through the pores of the separation sieve. When fresh thick albumen gel was vigorously shaken in a closed NMR vial prior to microscopic slide preparation, the elongated domains were fragmented into domains of even smaller size.
The preparation of the thick albumen fraction for microscopic observation required application of a cover slip, thus the albumen was subjected to a capillary force prior to microscopic observation. Given this fact, it was impossible to discern if the thick albumen might have had an even larger domain structure prior to the specimen preparation procedure. It is observed, however, that domain structures exist in elongated band arrangements (Figure 3).
In summary of the structural observations it was found that the fresh unagitated thick albumen gel has a series of elongated weakly birefringent bands of microscopic protein fibers, that fragment into domains of smaller size under the influence of mechanical agitation. The fragmentation of the domain structure is associated with a loss of the gel state as judged by flow through the sieve filter. Polarization microscopy of the fresh thin albumen fractions reveals fewer birefringent particles than the thick albumen (Table 1). The microscopic particles in the thin fractions are randomly dispersed with significantly lower concentration than in thick albumen (Table 1). No microscopic domain structures were observed in the thin albumen fraction. The concentration of particles in thin albumen ranged from 8900 to 13,100 per mm3 or about half the mean concentration in thick albumen and a quarter the concentration in the birefringent domains of the thick albumen gel (Table 1).
Proton NMR T1 and T2 relaxation times
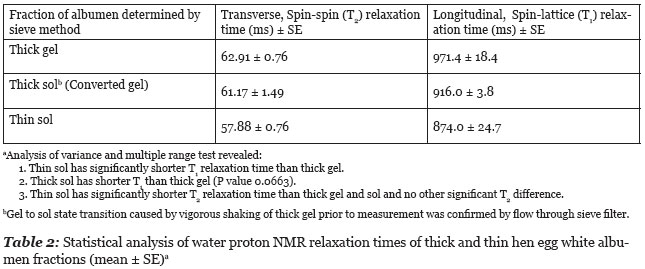
The results of longitudinal (T1) and transverse (T2) relaxation time study of thick and thin hen egg white albumen fractions are summarized in Table 2. A single T2 relaxation time gave a good fits to a monoexponential decay curve. The shaking of thick albumen gel caused transition from a gel state to a sol state as shown by flow through the sieve filter. This gel to sol phase transition did cause a marginally significant (P = 0.0663) shortening in the T1 relaxation time but did not cause a significant change in the T2 relaxation time.
Discussion
Structural and physical state of fresh thick and thin hen albumen fractions
Brooks and Hale (1959) reported that thick hen egg white, as seen with the naked eye, “to consist of a transparent phase separated by a series of translucent bands with their centers about 1 mm apart. Microscopically a band is seen to be a stratum of closely packed parallel fibers or sheets whereas few fibers can be seen between the bands.” They go on to assume that the thick albumen is held together by microscopic fibers and the translucent phase is a liquid resembling the thin albumen fraction.
When fresh thick gel was subjected to vigorous agitation the elongated domains observed by polarization microscopy were no longer observed but a few smaller domain fragments were still seen. Birefringent particles were more uniformly distributed in the field of view of the agitated thick albumen but the mean concentration was not significantly different between the sol and gel states (Table 1). It was also observed that the particles in the elongated gel domains were immobilized but were free to move in the sol. Fragmentation of large domains into smaller domains by vigorous agitation is associated with transformation to a sol state as determined by flow through the sieve filter.
The elastic and rigidity properties of both the translucent and transparent phase of the thick albumen have been studied (Brooks and Hale 1959). Elasticity was measured using displacement of minute nickel spheres inserted into the gel when subjected to forces applied with a magnetic field. Both gel phases demonstrated elastic behavior and rigidity. Rigidity was much greater in translucent than in transparent albumen. Also the nickel spheres in the translucent gel band demonstrated greater resistance when pulled perpendicular vs. parallel to the filament long axis. No directional effect was observed in thin albumen. Thus it was concluded that the thick albumen gel is not a simple homogenous entanglement network.
Based on the above descriptions thick albumen is best described as a two phase gel with elastic properties. One phase consists of linear domains enriched with relatively immobile bi-refringent particles surrounded intervening non-domain phase with fewer particles. The present study demonstrates that the unperturbed gel (domain and non-domain) excludes dye. The thin albumen sol fraction which flows through the filter sieve, includes dye and therefore differs from both the domain and no-domain phases of the thick albumen gel (Figure 2). The thick gel has some dye which could be dissolved in a thin fraction held between the fibers but with the same concentration as in the thin fraction. This possibility seems unlikely as the thick gel fraction was 27% domain and 73% non-domain of the total volume as determined by morphometric measurements. If the non-domain area of thick albumen gel did not exclude dye the total thick albumen fraction would be expected to be much more blue than was observed. Summarizing the physical characteristics of thick albumen, prior studies revealed that the thick albumen gel has elastic properties (Brooks and Hale 1959) and demonstrates thixotropy (Cameron 2010). Cameron also demonstrated that modest shear force applied to thick gel was enough to cause loss of the dye exclusion property.
Impact of globular protein docking (polymerization) on the proton spin-lattice (T1) relaxation rate
Some background on this subject helps explain the T1 relaxation rate differences observed between thick and thin albumen and between the gel and sol state of thick albumen. Polymerization of globular proteins has an effect on T1 relaxation times (Fullerton et al. 1987, Fullerton and Cameron 1998). The effect can either cause an increase or decrease in T1. The basis for the effect is that proteins that dock with one another during polymerization displace a portion of the hydration sheath. This causes an increase in the bulk-water relative to the bound-water fraction and structured-water fractions, and will cause a decrease in the relaxation rate. The group of docked molecules is now however equivalent in mass to a much larger globular protein.This polymer rotates and translates much more slowly; it thus contributes to a more rapid T1 relaxation. These two influences are simultaneous and opposite to one an other in influence on T1. In vitro studies on actin and tubulin, two globulin proteins frequently participating in polymerization-depolymerization reactions in vivo, showed that changes in macromolecular motion are most important, the relaxation rate increases with the polymerization reaction. A study of lysozyme indicated that the displacement hydration water can also be of critical importance. Both changes are important in vivo and in vitro (Bray 1993, Lynch 1983, Fullerton et al. 1987, Fullerton and Cameron 1998).
This conclusion is confirmed by a study of the influence of NaCl on the spin-lattice relaxation of a 2 g lysozyme/5 ml of 0.1 M NaCl solution during a salting-out relaxation. As shown in Figure 4 the initial addition of salt shortened the relaxation time of the solution due to increased effective molecular weight caused by molecular docking. Over a period of several days, the relaxation time recovered and then exceeded the original relaxation time. This was due to continued molecular docking, which formed protein crystals that further slowed the motion of the lysozyme to the point where it was no longer effective in acting as a relaxation sink for the solution. This was simultaneous with an increase in the bulk-water fraction, due to additional displaced hydration water. These studies confirm that changes in the macromolecules from monomer, to polymer, to crystal can have profound effects on the T1 relaxation time.
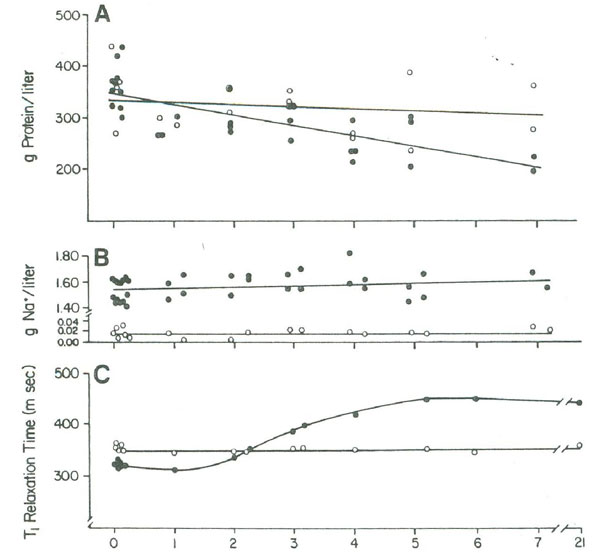
Figure 4:(A) The lysozyme concentration and (B) the sodium ion concentration in the supernatant in days after mixing together 2 g of lysozyme in 5 ml of water (open circles) or in 5 ml of 0.1 M NaCl (closed circles), (C) T1 measurements were made with all reactants (including crystals) in the vessel. Figure 4 is reproduced from Fullerton and Cameron (1988) with permission of Wiley VCH Publishers, Inc.
The above description of T1 relaxation time changes due to docking of globular proteins during aggregation is consistent with observed measure of proton T1 relaxation time difference between thick and thin egg white albumen fractions and between the gel and sol state of thick albumen. The T1 relaxation time of fresh unperturbed thick albumen has significantly longer T1 relaxation time than thin albumen which has a significantly higher water content 9.6 g water/g DM (dry material) vs. 7.3 g/g DM for thick albumen (Cameron 2010). This difference in T1 relaxation between thick and thin albumen is not therefore attributed to the water content but is attributed to extreme slowing of proteins in the domain structure of the thick albumen gel. Apparently the rotational and translocation motion of the proteins and particles in the gel is slowed to the extent that the proteins and particles form a relatively immobile domain structure which is no longer effective in acting as a relaxation sink for protons in solution. The fragmentation of the large domains of the gel state of thick albumen by agitation (shaking) might be expected to have a shorter T1 relaxation time due to increased mobility of proteins released by dissolution (depolymerization) of the large, relatively immobile domains. Data in Table 2 indicates that gel domain fragmentation does have a shorter mean T1 relaxation time as predicted.
Summary and Conclusion
We conclude that hen egg white structure is a useful model to study factors impacting heterogeneous distributions of particles, proteins, metabolites, salts and other small molecules in cellular structures of biology due the following observations:
1. The thick albumen gel, as visualized by the polarized light microscopy, is composed of elongated weakly birefringent domains enriched in strongly birefringent particles separated by areas with fewer particles causing heterogeneous distributions of these particles.
2. Thick gel agitation fragments the large domain structure of the gel and is associated with a more homogenous distribution and increased mobility of these birefringent particles.
3. Flow of thick albumen gel through the sieve following mechanical agitation (transition to sol) shows depolymerization of protein filaments to create smaller domains due to domain fragmentation that correlates with redistribution of proteins molecules and particles.
4. Shorter proton NMR T1 relaxation times following mechanical agitation of the thick albumen gel imply de-polymerization of protein structures induced by mechanical forces implying very weak binding mechanisms.
5. Exclusion of blue dye by thick albumen gel and loss of dye exclusion following mechanical agitation confirms that cosolute concentrations are correlated with protein aggregation caused by polymerization.
6. In a prior report the gel to sol transformation of thick albumen was demonstrated to be reversible following a long (4 day) period of rest (Cameron 2010) which demonstrates self association similar to the process of tendon formation by spontaneous association of collagen molecules.
7. The gel to sol and back to gel phase transformation of hen thick albumen appears to be similar to the contraction induced non-Newtonian cytoplasmic flow characteristics observed in amoeba and Physarum (Taylor and Condeelis 1979, Kamiya 1981, Bray 1993).
8. The vital dye excluding property of the thick albumen hydrogel resembles that of the cytoplasmic “antidomain” structures of a giant algal cell (Shepherd 2006).
9. We conclude that the formation of hydrogels by the spontaneous interaction of cellular protein causes heterogeneous distributions of particles, proteins and cosolutes and is likely a alternative mechanism to active membrane “pump” transport causing heterogeneous distributions of ions, cosolutes and other biologically active molecules in biology; these spontaneous, cytoplasmic pump mechanisms can be studied using the hen egg white model demonstrated in this study.
Acknowledgements
Dr. Yongsook Lee’s help with NMR measurements is greatfully acknowledged as is the secretarial help of Cathy A. Bunnell.
References
Bray, D. Cell Movement. Garland, NY. 1993.
Brooks, J. and Hale, H.P. The mechanical properties of the thick white of the hen’s egg. Biochem. Biophys. Acta. 1959. 32: 237-250.
Brooks, J. and Hale, H.P. The mechanical properties of the thick white of the hen’s egg II: the relationship between rigidity and composition. Biochem. Biophys. Acta. 1961. 46: 289-301.
Cameron, I.L. Dye exclusion and other physical properties of hen egg white. Water. 2010. Volume 2. pp. 83-96.
Fels, J., Orlov, S.N., Grygorczyk, R. The hydrogel nature of mammalian cytoplasm contributes to osmosensing and extracellular pH sensing. Biophys. J. 2009. 96: 4276-4285.
Fullerton, G.D. and Cameron, I.L. Relaxation of biological tissues. Biomedical Magnetic Resonance Imaging. 1988. pp. 115-155. Eds. F.W. Webili, D. Shaw, J.B. Kneeland. Vch Publishers, Weinheim.
Fullerton, G.D., Finnie, M.F., Hunter, K.E. et al. The influence of macromolecular polymerization on proton NMR T1 relaxtion of water solutions. J. Magn. Reson. Imag. 1987. 77: 426-445.
Hiidenhovi, J. Chapter 9 ovomucin. Bioactive Egg Compounds. 2007. pp. 61-68.
Hiidenhovi, J., Makinen, I., Huopalahti, R., and Ryhanen, E.L. Comparison of different egg albumen fractions as sources of ovomucin. J. Agric. Food Chem. 2002. 50: 2840-2845.
Kamiya, N. Physical and chemical basis of cytoplasmic streaming. Annu. Rev. Plant Physiol. 1981. 32: 205-236.
Laghi, L., Cremonine, M.A., Plaucci, G., Sykora, S., Wright, K., and Hills, B. A proton NMR relaxation study of hen egg quality. Magn Reson. Imag. 2005. 23: 501-510.
Lynch, L.J.. Water relaxation in heterogeneous and biological systems. Magn Reson. Imag. 1983 pp. 248-304. Wiley: New York.
Rabouille, C., Aon, M.A., Muller, G., Cartaud., J, Thomas, D. The supramolecular organization of ovomucin. Biochem. J. 1990. 266:697-206.
Rabouille, C., Aon, MA, Thomas, D. Interactions involved on ovomucin gel-forming properties: a rheological-biochemical approach. Arch Biochem Biophys. 1989. 270: 495-503.
Robinson, DS, Monsey, JB. Studies in the composition of egg-white ovomucin. Biochem J. 1971. 121: 537-547.
Robinson, DS and JB Monsey. Changes in the composition of ovomucin during liquefaction of thick egg white: the effect of ionic strength and magnesium salts. J. Sci. Food Agric. 1972. 23: 893-904.
Shepherd, VA. Coherent domains in streaming cytoplasm of a giant algal cell. Water and The Cell. 2006. pp. 71-92. Eds. G.H. Pollack, I.L. Cameron and D.H. Wheatley. Springer, Dordrecht.
Taylor, DL and Condeelis, JS. Cytoplasmic and contractility in amoeloid cells. Int. Rev. Cytol. 1979. 56: 57-144.
Discussion with Reviewers
Mae Wan Ho1: Would you comment on hydrogel acting as an alternative to pump transport?
Ivan Cameron and Garry Fullerton: Clearly the hen thick albumen hydrogel excludes vital dye like methylene blue. Such vital dye exclusion, in cells, is usually attributed to a membrane. The thick albumen hydrogel excludes the same vital dye without a membrane pump. The hydrogel is proposed to exclude the vital dye due to the presence of non-dye-solvent water. Agitation of the albumen hydrogel converts the gel to sol state which causes loss of its dye excluding property but with time for rest the sol state can return to a dye excluding gel state. Thus the thick albumen hydrogel is an alternate to a cell membrane pump transport system.
Ho: Why do you suppose that the thick gel albumen consist of globular aggregates rather than extended protein aggregated, as suggested by Gilbert Ling?
Cameron and Fullerton: We have not made this supposition but will comment that Gilbert Ling’s association induction process requires binding of a cardinal adsorbent, like ATP, to induce a transformation of a folded (introverted) globular protein state to the unfolded (extroverted) state. This transformation requires a cardinal adsorbent , like ATP, that allows multilayer of polarized water with solute excluding properties to form over the exposed hydrophilic surface. Loss of ATP, causes to transformation back to the folded non-solute excluding globular protein state. As far as is known, the solute (dye) excluding property of the thick albumen gel does not depend on the presence of a “cardinal adsorbent” like ATP. All that may be needed is the presence a hydrophilic “water loving” gel surface to forms a solute excluding zone over the hydrophilic surface of a globular protein monomer or aggregate.
Ho: Is it possible that you may have more than one NMR relaxation time in the gel phase?
Cameron and Fullerton: Yes. For example water protons in a protein crystal can become so immobile that their water proton NMR relaxation rate is too fast to be detected. This happens with water in the form of ice. However addition of cold liquid water to an ice cube gives only the water proton NMR relaxation rate/time of the cold liquid water. Both relaxation times occur but only one is measureable. The hen egg white gel is also proposed to have more than one NMR relaxation rate fraction but one fraction may relax too fast to be measured.
Ho: Are your results on dye exclusion related in a way to Pollack et al.’s exclusion zone next to hydrophilic gels?
Cameron and Fullerton: Yes as just alluded to in my response to the previous question. Pollack’s gel exclusion zone extends over the hydrophilic gel surface and grows with time, can be destroyed by physical perturbations, and can then re-grow with time. These are the same properties observed in the thick hen egg white gel.
Ho: Do you think transition between heavy and light water could be involved in liquid and gel phases of albumen?
Cameron and Fullerton: We assume by heavy and light water you refer to water density. We have not made measures of water density in the hen albumen fractions however we assume that water hydrogen bonded to a hydrophic surface is more densely packed than bulk water. Thus changes in protein folding and aggregation is expected to change the proportion of dense to less dense water between the gel and the sol phase of the thick albumen.
1 Visiting Professor of Biophysics, Catania University, Sicily
