Seasonal Distribution of Enteric Opportunistic Cryptosporidium Spp. Oocysts and Giardia Spp. Cysts in a Tropical Water Basin, Cameroon
Ajeagah, GA 1,*; Njine, T1; Bilong Bilong, CF1; Foto, SM1; Wouafo Ndayo Marguerite2;
Nola Moise1; Di Giovanni, GD3; Huw, S4
1 Laboratory of General Biology, P.O. Box 812, University of Yaondé, Cameroon
2 Centre Pasteur du Cameroun, BP 1274, Yaondé, Cameroon
3 Associate Professor, Environmental Microbiology & Waterborne Pathogens, Texas A&M University, Texas, USA
4 Drinking Water Inspectorate, Glasgow, UK
* Correspondence: ajeagahg@yahoo.com
Key Words: seasonality, transmission, Cryptosporidium spp. oocysts, Giardia spp. cysts, surface water
Received: 27 October, 2009; revised 12 January 2010; accepted 7 February; published 19 March 2010; available online 19 March 2010
Summary
The susceptibility of the human host to Cryptosporidium spp. and Giardia spp. (oo) cystic load is due to the immunodepression of the human host, the enteropathogenic transmission, the zoonotic potentialities of the parasites, and the mechanisms of environmental favoritism that influences the contact of these organisms with the host. Seasonal variables were taken into consideration in the determination of the qualitative and quantitative contamination of the Mfoundi River Basin in Yaounde by these parasites. The (oo) cysts were isolated and identified by the Ziehl Neelsen, iodine coloration method and the USEPA Method 1623.The seasonal bio-dynamics of Cryptosporidium oocysts in the streams analyzed indicate that, on average, the highest seasonal values were presented in Abiergue West (110.8 ± 61.33 oocysts/L), followed by Olezoa (88.24 ± 40.32 oocysts/L), Abiergue East (61.73 ± 43.28 oocysts/L), Biyeme (47.28 ± 19.54 oocysts/L), Mingoa (43.13 ± 31.38 oocysts/L) and Ekozoa (26.05 ± 15.15 oocysts/L). The lowest values were assessed in the long rainy season for Abiergue East, Biy eme, Olezoa, Ekozoa and the short dry season for Abiergue West and Mingoa. The highest values were determined in the long dry season for all the streams assessed. The statistical test revealed significant variations of this parameter between the seasons and the sampling sites (P < 0.05). In all the streams sampled, there was a decrease in oocysts density from the long dry season to the short rainy season. The seasonal bio-dynamics of Giardia cysts isolated and identified in the various streams reveal that the highest values were registered in Abiergue West (15.23 ± 7.25 cysts/L), followed by Mingoa (15.04 ± 10.63 cysts/L), Olezoa (13.93 ± 9.82 cysts/L), Abiergue East (12.83 ± 7.0 cysts/L), Biyeme (9.55 ± 4.02 cysts/L), and Ekozoa (8.05 ± 2.04 cysts/L). The variations of the cystic load were significant between seasons (P < 0.05) and not significant between the sampling sites (P > 0.05). From the seasonal point of view, the concentration of the cystic load seemed to decrease with an increase in precipitation, possibly due to the effect of dilution, as the rainfall diluted the concentration of the pathogens in the environment.
Article Outline
Introduction
The aquatic ecosystem harbors many kinds of organisms. Some of these organisms are parasitic protozoa such as Cryptosporidium spp. and Giardia spp., which have recently been recognized as important causes of water and food-borne disease outbreaks associated with fecal contamination (Doron, 2000; Karanis et al., 2006). These pathogens are among parameters to be analyzed in routine drinking water quality assessments. In the United States, the United Kingdom and France, legislation has stipulated that treated water must not contain a concentration of Cryptosporidium and Giardia above 1 (oo) cysts/10 liters. Failure to adhere to this law is considered a criminal offense (USEPA, 1999; AFNOR, 2001; DWI, 2004).
The illnesses caused by these two pathogens are characterized by profuse watery and non-bloody diarrhea, abdominal pain and cramping, nausea and weight loss. These diseases are highly correlated to the immunodepression of the human host, due to the prevalence of the Human Immune Virus (Smith & Rose, 1998; Raccourt et al., 2006) and in children aged 6-36 months, especially among those who are malnourished and depressed (Mor & Tzipori, 2008). These substances can have detrimental effects on human health due to poor hygiene or contaminated food, drinking water, or recreational water (Millard et al., 1994). The use of watersheds is controversial, as it requires a balance between recreational demands and the protection of the water and land resources. The main focus of municipal watershed management has been to keep such areas closed to public use, thereby preventing the contamination of water by pathogenic organisms originating from faecal sources (Gow & Waldner, 2006).
The data on the distribution of pathogenic micro-organisms in different countries will be useful and may contribute to the protection of public health as well as to the effective bio-surveillance strategies of these communicable diseases (Maida et al., 2008). Although little is known about the mechanisms that govern the transport of (oo) cysts in river water, it seems likely that the major determinant is how these resistant forms travel in the water current. A measurement of Turbidity and Suspended solids will give an indication of the relationship between the physical parameters and the transmission of the pathogens, while ammonia values indicate a likely effect of chemical parameters on the (oo) cystic load. Oocysts concentration have been shown to be positively correlated to water flow and turbidity levels of the aquatic ecosystems examined (Medema et al., 1998). Ecological data are prominent in the shaping of intestinal protozoa communities (Bajer, 2008). Emphasis ought to be given to the collection of water quality information that will help assess the sources of fecal contamination rather than focus on the detection of waterborne infections during outbreak investigations. Ensuring quality standards in the hydrosystem is a crucial component in preventing further outbreaks (Tuncay et al., 2008).
Surface water is being exploited for potable water production for the inhabitants of Yaoundé and Mbalmayo. At this latitude, the Mfoundi River Basin is the main tributary of the Nyong River basin, which is the main source for potable water production for the inhabitants of this region.
The objectives of this research were to access the biodynamics of Cryptosporidium and Giardia; to determine the morphometry of Cryptosporidium and Giardia, respectively; to determine the ecological factors of the aquatic ecosystem, such as ammonia, turbidity, and suspended solids; and to analyze the correlation between the seasonal loads of the (oo) cysts and the ecological factors of the medium.
Materials and Methods
I. Surface Water Sampling Sites
This research was carried out in the surface water of the Yaoundé Municipality. This hydrosystem is situated at latitude 3o50`North and Longitude 11º32` East, with an altitude of 700-800m above sea level. The climate is equatorial and the soil is mainly ferralitic. The streams analysed are Abiergue E, Abiergue W, Biyeme, Ekozoa, Olezoa and Mingoa 1 before the Municipal lake, and Mingoa 2 after the Municipal lake as presented in Fig. 1. The samples are transported to the laboratory of General Biology of the University of Yaoundé I for physico-chemical and parasitological examinations as described below. Sampling was carried out on a monthly basis for a period of one year.
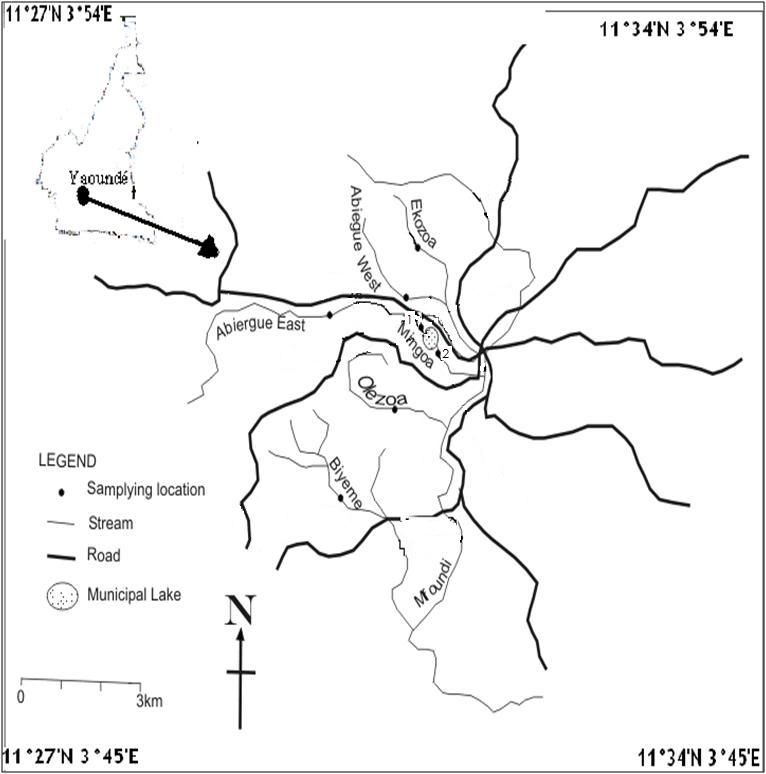
Figure 1: Map of the Mfoundi River Basin indicating sampling points
II. Measurement of Physico-Chemical Parameters
One chemical and two physical parameters, which could influence the biodynamics and transportation of the resistant forms of the pathogens in the aquatic ecosystems assessed, were analyzed alongside the (oo) cysts densities. These ecological factors are Turbidity, Suspended solids and Ammonia.
II. 1. Ammonium Ion
The assessment of this parameter was done with the aid of the Nessler method in which 1mL of Rochelle salt is added to 1mL of Nessler reagent (K2HgI) and 25mL of sample. The values are read in a spectrophotometer and recorded in mg/L of the ammonium ion.
II. 2. Suspended Solids
Measurement of suspended solids was carried out with the aid of a spectrophotometer Hach model. The values are directly read in mg/L.
II. 3. Turbidity
Measurement was carried out with the aid of a spectrophotometer Hach model. The values are expressed in Formazine Turbidity Units (FTU).
III. Measurement of the (oo) Cystic Load
The identification and counting of the (oo) cysts took place by the coloration method of Ziehl Neelsen for the Cryptosporidium oocysts, the lugol-iodine coloration for Giardia cysts. The USEPA Method 1623 was applied as a control method to verify the presence of the pathogens in the surface water analyzed.
III. 1. Application of the USEPA Method 1623
Ten liters of water are sampled and filtered through an Envirochek filter (Pall cooperation). An elution buffer comprised of Laurel-12, Tris EDTA, Antifoam A, and distilled water is applied in the recovery of the oocysts. The eluent is centrifuged at 1500g for 15 minutes. The paste is introduced into L-10 tube containing Buffer A, Buffer B and dynabeads anti-Cryptosporidium and anti-Giardia (Invitrogen). This solution is vortexed and then mix in a dynal sample mixer for 1 hour at 15 turns per minute. It is placed in MPC-1 and MPC-S for immunomagnetic separation in the presence of HCl. They are vortexed for 5 seconds, allowed to stand for 5 minutes and then vortexed for 5 seconds. Well slides are prepared on dynal spot on slides with NaOH. The slides are dried in a desiccant overnight and they are stained with FITC, Giardia spp. and Cryptosporidium spp. monoclonal antibodies are applied (Invitrogen, Oslo, Norway), PBS (phosphate buffer saline), mounting medium. The slides are incubated for 1 hour at 37.5ºC before microscopic epifluorescence observation at 40X and 100X .
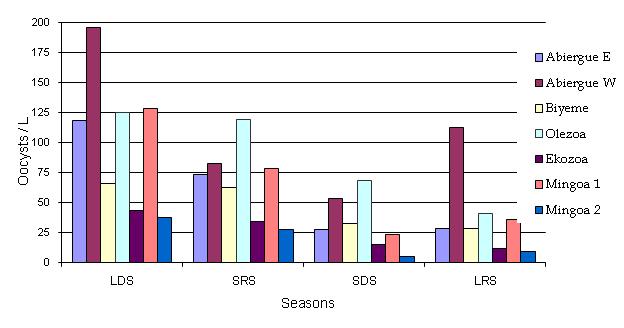
Figure 2: Seasonal distribution of Cryptosporidium spp oocysts in the Mfoundi River Basin
III. 2. The Ziehl Neelsen Coloration Method
Ten liters of samples were allowed to settle for 24 hours. The sediments are centrifuged for 1000g for 10 minutes. Zinc sulphate is added, centrifuged for 2500g for 2 minutes. The oocysts become lighter and float at the surface, they are withdrawn, diluted in distilled water and centrifuged for 1500g for 10 minutes. Slides are prepared with the paste. They are fixed with Methanol, stained with Phenic Fucshine, rinsed with sulphuric acid, counter stained with malachite green, rinsed with tap water, dried in air. The oocysts are observed in the microscope at 40X and 100X.
III. 3. The Iodine Coloration Method
Ten liters of sample are allowed to settle for 3 hours, the upper part is aspirated and fixed with Formaldehyde. The solution is centrifuged for 600g for 5 minutes and 3 drops of Lugol iodine colorant and Zinc sulphate are added. They are centrifuged for 650 g for 3 minutes and the cysts are observed in the microscope at 40X and 100X.
III. 4. Statistical Analysis of Results
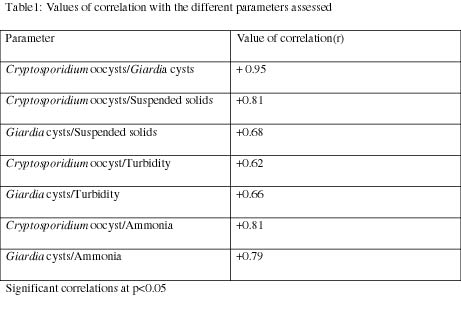
The repartition of the various points with respect to each other was evaluated in-order to verify the possibility of a relationship between the various entries. This was followed by an analysis of the correlation coefficient (R) using the logiciel SPSS (Table 1). The significance between the various physical, chemical and biological parameters in time and in space were evaluated by the two variable Anova statistical tests to calculate the Fischer value F and compare it to the Fischer tabulated value at p > 0.05. In the cases for which the spatio-temporal variation was significant, the Student t test was applied to assess the degree of variation of the various components, taken two by two.
Results and Discussion
The spatio-temporal distribution of the ecological variables and the oocystic load is presented in the form of LDS (Long Dry Season) from mid-November to mid-March, SRS (Short Rainy Season) from mid-March to mid-June, SDS (Short Dry Season) from mid-June to mid-August, LRS (Long Rainy Season) from mid-August to mid-November. The results are presented in the form of the mean ± standard deviation. The average results for Mingoa 1 and Mingoa 2 are considered in our analysis. The seasonal variation of ammonia in the streams, as illustrated in Fig. 6, shows that independently of the months considered, the highest value was recorded in Abiergue West (3.15 ± 1.36 mg/L) followed by Biyeme (2.31 ± 0.23 mg/L), Olezoa (1.81 ± 0.47 mg/L), Abiergue East (1.75 ± 0.68 mg/L), Mingoa (1.73 ± 0.57 mg/L) and Ekozoa (1.47 ± 0.37 mg/L), respectively. In general, in the LDS and the SRS, the highest value was obtained in Biyeme, Abiergue West, Olezoa, Ekozoa, Mingoa 1, Abiergue East and Mingoa 2, while for the SDS and LRS, the highest value was recorded in Abiergue West. The statistical test revealed a significant variation (P < 0.05) of this parameter between the seasons, and a non-significant variation (P > 0.05) between the sampling sites.
On a seasonal basis, the lowest values of Turbidity as presented in Fig 5 were recorded in the LDS in Ekozoa and Abiergue West, SDS in Abiergue West, LRS in Abiergue West, and Olezoa and Mingoa and SRS in Biyeme. The highest value was recorded in the LDS for Abiergue East , Biyeme, and Olezoa, the SRS in Abiergue West, and the SDS in Ekozoa. In the LDS, the highest value ranges from Abiergue E, Biyeme, Mingoa, Olezoa and Ekozoa. In the SRS the highest value was for Abiergue West, Abiergue East, followed by the other streams evaluated in the river basin. The statistical test revealed a significant variation of turbidity between the seasons and the sampling sites (P < 0.05). There was a remarkable rise in turbidity in Abiergue East during the LDS, possibly due to the activities of man, such as the dumping of waste in the watercourse.
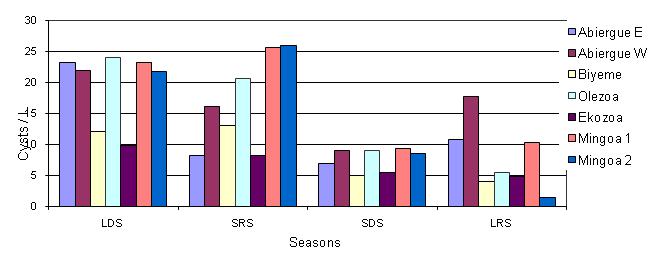
Figure 3: Seasonal distribution of Giardia spp. cysts in the Mfoundi River Basin
The seasonal distribution of suspended solids in the streams sampled is represented in Fig 4. Independently of the seasons considered, the highest values were determined in Olezoa (235.68 ± 83.02), followed by Biyeme (230.63 ± 161.20 mg/L), Abiergue East 204.7 ± 82.48 mg/L), Abiergue West (176.83 ± 93.07 ), Mingoa (181.4 ± 79.87) and Ekozoa (127.13 ± 99.79 ), respectively.
On the seasonal basis, the lowest values were recorded in the LDS for Olezoa, in the SDS for Abiergue East and Ekozoa, in the SRS for Biyeme, and in the LRS for Abiergue West and Mingoa. The highest value was analyzed in the short dry season for Olezoa and the LDS for all the other streams analyzed. There is a slight decrease in the concentration in suspended solids from the LDS to the LRS (Fig. 4). The seasonal biodynamics presents four different profiles: that of Abiegue East and Biyeme, which present a minimum in the SDS and the highest in the LDS; that of Abiergue West, Ekozoa and Mingoa 2, which have the lowest in the SDS and the highest in the LDS; that of Olezoa, which has the lowest densities in the LRS and the highest in the SDS; and the profile presented by Mingoa 1, which has the lowest value in the LDS and the highest in the LRS. The statistical test revealed a significant variation of this parameter between the seasons and the sampling sites (P < 0.05).
Figure 4: Seasonal distribution of suspended solids
The seasonal bio-dynamics of Cryptosporidium oocysts in the streams analyzed is represented in Fig. 2. The dynamics indicate that, on average, the highest seasonal values were presented in Abiergue West (110.8 ± 61.33 oocysts/L), followed by Olezoa (88.24 ± 40.32 oocysts/L), Abiergue East (61.73 ± 43.28 oocysts/L), Biyeme (47.28 ± 19.54 oocysts/L), Mingoa (43.13 ± 31.38 oocysts/L) and Ekozoa (26.05 ± 15.15 oocysts/L). The lowest values were assessed in the LRS for Abiergue East, Biyeme, Olezoa, Ekozoa and the SDS for Abiergue West and Mingoa. The highest values were determined in the LDS for all the streams assessed. In general, in the LDS and the LRS, the oocysts’ densities decreased in Abiergue W, Mingoa 1, Olezoa, Abiergue East, CHU, and Ekozoa; in the SRS and the SDS the densities decreased from Olezoa, Abiergue West, Mingoa 1, Olezoa, CHU, and Ekozoa and Mingoa 2, respectively. The oocystic load diminished from the LDS to the LRS. The concentrations in Abiergue West were higher than in other streams as represented below: The statistical test revealed significant variations of this parameter between the seasons and sites (P < 0.05). In all the streams sampled there was a decrease in oocysts density from the LDS to SRS.
The seasonal bio-dynamics of Giardia cysts, isolated and identified in the various streams sampled, is presented in Fig 3. The values disclose that on average, the highest values were registered in Abiergue West (15.23 ± 7.25 cysts/L) followed by Mingoa (15.04 ± 10.63 cysts/L), Olezoa (13.93 ± 9.82 cysts/L), Abiergue East (12.83 ± 7.0 cysts/L ), Biyeme (9.55 ± 4.02 cysts/L ), and Ekozoa (8.05 ± 2.04 cysts/L). On a monthly basis, the lowest values were identified in the SRS in Abiergue East, Abiergue West, Olezoa, and in the LRS in Biyeme, Ekozoa and Mingoa. The highest values were generally registered in the LDS. In the LDS, the cysts density was highest in Olezoa, followed by Mingoa, Abiergue West, Biyeme and Ekozoa. In the SRS and SDS, the streams with the highest density were Mingoa, Abiergue East, Olezoa, Abiergue West, Biyeme, and Ekozoa, correspondingly. In the LDS, Abiergue West presented the highest value, followed by Abiergue West, Abiergue East, Mingoa, Olezoa and Ekozoa, respectively. The variations of this parameter were significant between seasons (P < 0.05) and insignificant between the sampling sites (P > 0.05). From a seasonal point of view, the concentration of the cystic load seemed to decrease with an increase in rainfall, due to the effect of dilution.
Chemical parameters play a primordial role in the distribution of Cryptosporidium and Giardia oocysts. A high concentration in ions such as ammonium have been well-known to inactivate these parasites in aquatic medium, whereas pH and the redox potential of the medium play a relatively weak role in their repartition in aquatic ecosystems (Sattar et al., 1999). High concentrations of the ammonium ion have been identified to infiltrate into the walls of the (oo) cysts and the membrane of the sporozoite, thereby reducing the viability of the pathogens (Jenkins et al., 1998). In this study, the concentrations in ammonia increased from the long dry season to the long rainy season (Fig. 6), whereas (oo) cysts dynamics evolved in the reverse direction (Fig. 2 & 3).
Due to their comparatively small size, low specific densities, and particular surface properties, these enteroproto-parasites are generally considered to move through watersheds from their source to drinking water reservoirs with little attenuation in their dynamics in the stream channel. Nevertheless, the transportation of oocysts in surface water may be mediated by interactions with suspended sediments and subsurface filtration and removal in streambed sediments. Organic matter inhibits microbes transport due to hydrophobic interactions between these organisms and grain surfaces that are coated with this organic matter (Kinoshita et al., 1993). Adsorption of pathogens onto mobile colloids also eases their transport potentials (De Jonge et al., 2004). Attachment and detachment of oocysts on suspended particles are believed to control this displacement of (oo) cysts in surface water as in the case of the Mfoundi River Basin. A variety of chemical (electrostatic, sedimentation and interceptions) and biological factors can play a role in oocysts-suspended particles and oocysts–oocysts interactions (Dai & Boll, 2003). According to Brush et al. (1999), the oocysts are not associated with large particles but exist in faecal run-off as distinct oocysts and hence have a low settling velocity in the ecosystem analyzed. The implication of this low settling velocity on the protozoan risk reduction within water supply reservoirs corresponds to a three dimensional model and challenges the mechanism of sedimentation as a water purification strategy (Brooks et al., 2006). (Oo) cysts entrained in overland flow interact with particulate matter as a result of electrostatic, Van de Waals, Lewis acid base or hydration forces (Grasso et al., 2002). The oocysts are stable due to the hydrogen bonding and sterric stabilization that exist between them and the particulate matter in the hydrosystem (Plummer et al., 1995; Brush et al., 1988). Searcy et al., (2006), described the leaching of oocysts through sands and sediments as a convective spreading transport method in conjunction with sorption–desorption process. Our analysis of the water samples attests to the ubiquity of the (oo) cysts in the surface water, at relatively high proportions, which can be the basis of an outbreak. A significant seasonal pattern was observed in river water used for drinking water in Paris and its environs. Positive samples for Cryptosporidium were found to be more frequent in autumn than spring, summer or winter; positive samples for Giardia were found to be less frequent in summer (Mon et al., 2009). Meanwhile, the seasonality of Giardia and Cryptosporidium among diarrheic patients in the Philippines had a distinct peak in September with (oo) cystic load more prevalent in the rainy season (2.6 percent) than in the dry season (0.9 percent), according to Navividad et al., (2008). In the Quets River Watershed, the high prevalence of Giardia from upstream to downstream was related to intense human activity and the high prevalence of this pathogen in animals around the municipality (Ongerth & Pecoraro, 1995; Chauret et al., 1998). The distribution of (oo) cysts in the Mfoundi mainstream increases in a similar model as the above-mentioned watershed. This may be due to the anthropogenic activities carried out by waterside inhabitants, such as opening their toilets directly into the streams, throwing refuse into the waterways, as well as a lack of restoration projects to repair the polluted ecosystem. This leads to a large number of contaminated subjects who are directly related to the low hygienic and sanitary situation of the assessed environment (Standish–lee and Loboschefsly, 2006; Garrett et al., 2008). In Italy, the low presence of (oo) cysts in some wastewater could be directly linked to the low prevalence (1.9%) of cryptosporidiosis and giardiasis in the community (Pezzoti et al., 1999).
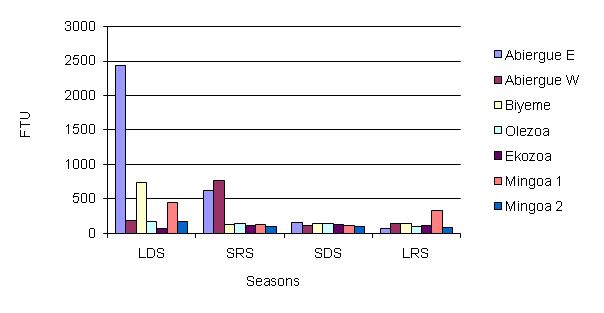
Figure 5: Seasonal distribution of turbidity in the water basin
The high values in the concentration of suspended particles in the aquatic ecosystem consequently influences an increase in turbidity, as presented by the significant correlation (+ 0.66 to + 0.83) of these parameters as expressed in Table 1, with an increase in humidity from the long dry season to the long rainy season in the samples analyzed. Hsu and Yeh, (2003), had in a similar study determined a significant correlation between water turbidity and (oo) cyst dynamics in three pilot scale plant processes located in Southern Taiwan. The same research also obtained similar results between the resistant forms of the pathogens and suspended particles ranging from 3–10 μm. This might elucidate the possibility of specific receptor sites on the oocysts and cysts for inorganic and organic particles in water.
The high correlation recorded between the cysts and oocysts dynamics in the surface water (0.95), as presented in Table 1, could suggest the predirection of these parasites for immunodepressed systems as there is a six percent prevalence in Yaoundé. This is also due to the zoonotic and anthroponotic potentials, as humans and animals contaminate the aquatic ecosystem, the presence resistant forms of parasites in the environment, and the mode of contamination, which is principally fecal-oral as they can be found in food and water. These values are in line with the findings of Shun-Hwu et al. (2007), who calculated an r value of 0.55 between these parameters and the (oo) cysts in the Nakdong river in Korea; Falabi et al., (2002), registered similar findings in the waste water effluent in Arizona, USA. A significant correlation was determined between the oocysts and the cysts in the LRS; the highest incidence of enteric protozoan parasites was observed in the rainy season in Varanasi, India (Tuli et al., 2008). This is an indication that the geo-climatic characteristics of the environment influence their distribution in the ecosystem. The abundance and prevalence increase downstream of the Mfoundi River Basin, probably due to an increase in anthropogenic contamination of the watercourse and hydrodynamic characteristics of the streams. The high-density population in Yaoundé could correlate with the population dynamics of the resistant forms of the pathogens in the hydrosystem. There is also the possibility of peril faecal contamination of the water system when rainwater transports Giardia cysts and Cryptosporidium oocysts from faecal deposition in the environment and then into the surface water (Arnone and Walling, 2006; Harter et al., 2008). This could be one of the factors influencing the numerical increase in (oo) cysts along the Yaoundé drainage basin during the rainy season (Figs. 2-3). The statistical analysis (of the student t) test for the time variation revealed a prominence of these (oo) cysts during the LDS and SRS from February to April, as correlated by the discharge of the pathogens into the River Basin by the riverside–inhabitants, who evacuate fecal matter into the hydrosystem from their toilets during the advent of this season. Wuhip et al. (1994) had associated the high prevalence of Cryptosporidium spp. and Giardia spp. in the environment to the rainy season (p < 0.005). Muchiri et al. detected more oocysts at the end of the rainy season in a watershed in Kenya and this coincided with the timing of human infection in the given municipality. According to Kistemann et al. (2002), this increase could be due to the movement of the microbial load from non point sources to the water systems in the runoffs associated with storms.
Figure 6: Seasonal distribution of ammonia in the water basin
Conclusion
Ammonia values increase from the dry season to the rainy season. There is a high concentration of suspended solids in the samples assessed. This trend is the same with the values of turbidity recorded in the surface water points. The values of suspended solids and turbidity are higher in the dry season, with respect to the rainy season, due to the effect of dilution of rainwater on the ecosystem. These pathogens are liberated in the tributaries of the Mfoundi River Basin, where they are transported to other regions of the hydrosystem.
An assessment of (oo) cysts density in the Mfoundi Mainstream reveals the presence of Cryptosporidium spp. oocysts and Giardia spp. cysts in all the sampling sites and at all the sampling periods. There is nonetheless a remarkable increase in the density of the resistant forms of the parasites from upstream to downstream. This indicates an accumulation of the effects of anthropogenic activity and a likely zoonotic contamination of the ecosystem.
The statistical test revealed a significant prevalence of the (oo) cysts in the aquatic ecosystem during the period from the long dry season and the short rainy season, probably due to the accumulation of peril fecal matter in the streams, and the contamination of the streams by riverside inhabitants who open their toilets during this period to evacuate their fecal matter.
There was a significant correlation observed between ammonia, turbidity, suspended solids, and the (oo) cysts population dynamics during the long rainy season, demonstrating that the organic and inorganic substances in water might influence the distribution and transmission of these pathogens in the aquatic ecosystem.
The presence of Giardia cysts and Cryptosporidium oocysts in the Yaoundé Watershed is a potential health risk to the population. These results show that an elaborate program for the treatment, distribution, and protection of the Mfoundi River System is imperative, so as to evade an outbreak of giardiasis and cryptosporidiosis in Yaoundé and its vicinity, which are urban centers that are plagued with a high prevalence of the Acquired Immune Deficiency Syndrome. A definitive diagnosis of these etiological agents is imperative in order to avoid cases of empirical treatment.
References
AFNOR (2001). Qualité de l’eau. Recherche et dénombrement d’oocystes de Cryptosporidium et des kystes de Giardia. Norme T90: 455.
Arnone, RD; Walling, JP (2006). Evaluating Cryptosporidium and Giardia concentrations in combined sewer overflow. J Water Health 4(2): 157-65.
Bajer, A (2008). Between year variation and spatial dynamics of Cryptosporidium spp and Giardia spp infections in naturally infected rodent populations. Parasitolog 135(14): 1629-49.
Brooks, JD; Davies, CM; Hipsey, MR; Antenucci, P (2006). Association of Cryptosporidium with bovine faecal particles and implications for risk reduction by settling within water supply reservoirs. J Water Health 4(1): 87-98.
Brush, CF; Walter, MF; Anguish, LJ; Ghiorse, WC (1988). Influence of pre-treatment and experimental conditions on electrophoretic mobility and hydrophobicity of C.parvum oocysts. App Environ Micro 64(11): 4439-4445.
Brush, FC; Ghiorse, WC; Lynne, JA; Parlange, JV; Ghiorse, HG (1999). Transport of Cryptosporidium parvum oocysts through saturated columns. J Environ Qual 28: 809- 815 .
Chauret, C; Springthorpe, S; Sattar, S (1999). Fate of Cryptosporidium oocysts, Giardia cysts, and microbial indicators during waste water treatment and anaerobic sludge digestion. Canadian J Micro 45: 257- 262.
Dai, X; Boll, J (2003). Evaluation of attachment the of Cryptosporidium parvum and Giardia lamblia to soil particles. J Environ Qual 32: 296-304.
De Jonge, LW; Kjaergaad, C; Moldrup, P (2004). Colloids and colloid facilitated transport of contaminants in soils. An introduction. Vodose Zone J 3: 321-25.
Doron, M (2000). Cryptosporidium dans l’environment aquatique.consequence pour les eaux de distribution. (Service National d’information et de documentation sur l’eau. (SNIDE).
DWI (2004). Standard operating protocol for the monitoring of Cryptosporidium oocysts in treated water supplies to satisfy the water supply regulations.
Falabi, JA; Gerba, CP; Karpiscak, MM (2002): Giardia and Cryptosporidium spp removal from waste-water by duckweed (Lemna gibba L.) covered pond. Lett App Micro 34(5): 384- 387.
Garrett, V; Ogutu, P; Mabonga, P; Ombeki, S; Mwaki, A; Aluoch, G; Phelan, M; Quick, R (2008). Diarrhoea prevention in a high-risk rural Kenyan population through point of use chlorination, safe water storage and rainwater harvesting. Epid Infect 21: 1-9.
Gow, S; Waldner, C (2006). An examination of the prevalence of and risk factors for shedding of Cryptosporidium spp and Giardia spp in cows and calves from Western Canadian cow-calfs herds. Vet Parasit 137(1-2): 50-61.
Grasso, D; Subramanian, K; Butkus, MA ; Bergendahl, J (2002). A review of non–DLVO interactions in environmental colloidal systems. R Environ Science Bio/Tech I :17-38.
Harter, T; Atwill, E; Hou, L; Karle, B; Tate, K (2008). Developing risk models of Cryptosporidium transport in soils from vegetated , tilted soil box experiments. J Environ Qual 37(1):245-58.
Hsu, M; Yeh, H (2003). Removal of Giardia and Cryptosporidium from drinking water. Water Research 37 (5): 1111-1117.
Jenkins, MB; Bowman, DD; Ghiorse, WC (1998). Inactivation of Cryptosporidium parvum oocysts by Ammonia. Appl Environ Micro 64: 784-788.
Karanis, P; Karenti, C; Smith, H (2006). Waterborne transmission of protozoan parasites .a review of world wide outbreaks and lessons we learnt. J Water and Health 5: 1-38.
Kinoshita, T; Bales, RC; Maguire, KM; Greba, CP (1993). Effect of pH on bacteriophage transport through sandy soils. J Contam Hyd 14: 55-70.
Kistemann, T; Claben, T; Koch, C; Dangendorf, R; Fischeder, R; Gebel, J; Vacata, V; Exner, M (2002). Microbial load of drinking water reservoirs tributaries dyring extreme rainfall and run-off. App Environ Micro 68 (5): 2188- 2197.
Maida, CM; Benedetto, MA; Firenze, A ; Calamusa, G ; Di Piazza, F ; Milici, ME; Romano, N (2008). Surveillance of the sanitary conditions of a public swimming pool in the city of Palermo (Italy).] Igiene e sanita pubblica 64(5):581-93.
Medema, GJ; Schets, FM; Teunis, P (1998). Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. App and Environ Micro 64: 4460-4466.
Millard, PS; Genscheiner, KF; Addis, DG; Sosin, DM; Beckett, GA; Houck–Jankoski, A; Hudson, A (1994). An outbreak of Cryptosporidiosis from fresh–pressed apple cider. JAMA 272: 1592-1596.
Mons, C; Dumetre, A; Gosselins, C; Moulin, L (2009). Monitoring of Cryptosporidium and Giardia River contamination in Paris area. Water Research 43(1): 211-17.
Mor, SM; Tzipori, S (2008) Cryptosporidiosis in children in sub-Saharan Africa; A lingering challenge. Clin Infect Dis 47(7): 915-21.
Muchiri, JM; Ascolille, L; Mugambi, M; Ward, HD; Naumova, EN; Egorov, AI; Cohens, S; Else, JG; Griffiths, JK (2009). Seasonality of Cryptosporidium oocysts detection in surface waters of Meru ,Kenya as determined by isolation methods followed by PCR. J Water Health 7(1): 67-75.
Natividad, FF; Buerano, CC; Lago, CB; Mapua, CA; De Guzman, BB; Serpe, EB; Samentar, CP; Endot, T (2008). Prevalence rates of Giardia and Cryptosporidium among diarrheic patients in Philippines. Southeast Asian J Trop Med Public Health 39(6): 991-99.
Ongerth, JE; Pecoraro, JP (1995). Electrophoretic mobility of Cryptosporidium oocysts and Giardia cysts. J Environ Engng-AQF 112(3): 228-231.
Pezzoti, P; Serraino, D; Rezza, G; Dai Maso, L; Vaccher, E; Lepri, AC; Franceschi, S (1999). The spectrum of AIDS – defining diseases: temperal trends in Italy prior to the use of highly active antiretroviral therapies, 1982-1996. Intern J Epid 28: 975–981.
Plumer, JD; Edzwald, JK; Kelley, MB (1995). Removing Cryptosporidium by dissolved air flotation. J AWWA 87: 85-95.
Raccourt, CP; Pannier, SC; Eyma, E; Verdier, RI; Totet, A; Pape, JW (2006). Enteric parasites and AIDS in Haiti. Utility of detection and treatment of parasites in family members. Med Trop 66(5): 461-4.
Satter, SA; Chauret, C; Springthorpe, VS; Battigelli, D; Abbaszadegan, M; LeChevalier, M (1999). Giardia cyst and Cryptosporidium oocyst survival in watersheds and factors affecting inactivation. J AWWA 907(61): 1-2.
Searcy, K; Packman, A; Atwill, E; Harter, T (2006). Deposition of Cryptosporidium oocysts in streambeds. App Environ Micro 72 (3): 1810-1816.
Shun-Hwa, L; Chul-Hee, Lee; Yun–Hee Kim; Ju –Hee Do; Seung-Hyun Kim; Seung–Hyun Kim (2007). Occurrence of Cryptosporidium oocysts and Giardia cysts in the Nakdong River and their removal during water treatment. J Water and Health 5(1): 163-169.
Smith, HV; Rose, JB (1998). Waterborn Cryptosporidiosis. Current status. Parasite Today 14: 14-22.
Standish-Lee, P; Loboschefsky, E (2006). Protecting public health from the impact of body-contact recreation. Wat Sc Tech 53 (10): 201-7.
Tuli, L; Gulati, AK; Sundar, S; Mohapatra, TM (2008). Correlation between CD4 counts of HIV patients and enteric protozoan in different seasons-an experience of a tertiary care hospital in Varansi, India. BMC Gastroent 20: 8-36.
Tuncay, S; Songuil, D; Tonay, I; Leyla, O; Murat, OA; Ciler, A; Umit, A (2008). An outbreak of Gastroenteritis associated with intestinal parasites. Turkiye Parazitoloji Dergisi 3: 249-252.
USEPA (1999). Method 1623: Cryptosporidium and Giardia in water by filtration /IMS/FA.Publication EPA, 82-12-99-006. US Environmental Protection Agency, Office of Water, Washington D.C.
Wuhip, T; Silva, TM; Newman, RD; Garcia, LS (1994). Cryptosporidial and Microsporidial infections in human immunodeficiency virus-infected patients in Northeastern Brazil. J Infect D 170(2): 494-497.
Discussion with Reviewers
Mayuna Srisuphanunt1: You used the Envirocheck capsule for water sampling, and you also used Ziehl Neelson and Iodine coloration. Can you explain how these three methods are different or similar?
Gideon Ajeagah: The Envirochek filter, or the USEPA method 1623, was applied as a confirmatory diagnosis of pathogens in water, while routine analysis for Cryptosporidium oocysts were carried out by Ziehl Neelsen and Giardia cysts by Iodine coloration. All these methods are acknowledged in the assessment of the parasitic load in water and wastewater.
Srisuphanunt: Since you used Envirochek for confirmatory tests, can you say how you confirmed the water samples? Did you confirm every water sample or only the positive sample by routine methods? How do you know when you will use Envirochek to collect the water sample in each location site? In my experience, it’s neccessary to use Envirocheck with the power pump for water sampling in each of the field locations. Envirocheck is costly, especially for developing countries such as Cameroon or Thailand, etc., and I can’t imagine it’s possible to use Envirocheck for every water sampling site, is it? I wonder how you select the location site for water sampling?
Ajeagah: It is evident that the USEPA Method 1623 is an elaborate, time-consuming, expensive process. Since our research was based on seasonal dynamics, we carried out confirmatory tests on samples per season/stream and sample/watershed, and analyzed these samples before proceeding with the coloration methodology for the routine biodynamics of the (oo) cystic densities.
Srisuphanunt: As you said: “There is nonetheless a remarkable increase in the density of the resistant forms of the parasites from upstream to downstream. This indicates an accumulation of the effects of anthropogenic activity and a likely zoonotic contamination of the ecosystem.” What do you mean by the “resistant forms of the parasite”? What is the meaning of “upstream and downstream”?
Ajeagah: The resistant forms of the parasites are the oocysts for Cryptosporidium spp. and the cysts for Giardia spp. In lotic aquatic ecosystems, the streams are classified from Crenon (source), rhithron (the midzone), and Potamon (the lower streamcourse). In our research we assimilated the crenon and the rithron as the upstream and the Potamon as the downstream.
Srisuphanunt: Did you investigate these parasites by DIC and vital forms (e.g., DAPI)?
Ajeagah: With the coloration methods, we were keen on the internal structure of the (oo) cysts; the morphology and the morphometry were taken into account. The FITC conjugated monoclonal antibodies were applied to give more sensitivity to the identifications. However, our research is more or less pioneering research in this field in Cameroon, and we were more interested in the isolation and identification of the pathogens in environmental samples, so the part using DIC and DAPI for viability studies has been reserved for further studies.
Srisuphanunt: As you said: “There was a significant correlation observed between ammonia, turbidity, suspended solids, and the (oo) cysts population dynamics during the long rainy season demonstrating that the organic and inorganic substances in water might influence the distribution and transmission of these pathogens in the aquatic ecosystem.” What are the “organic and inorganic substances” you used in the study? And why? Is it possible to use other organic and inorganic substances different from yours?
Ajeagah: The physico-chemical variables, such as suspended solids, turbidity and ammonia, are indirect methods used to detect the presence of organic and inorganic substances in water. They do not give a precise indication of the type of substances present in the medium. Other methodologies to characterise in-organic and organic substances in water, such as Biochemical Oxygen Demand, Chemical Oxygen Demand, and Oxydability tests, are applicable.
Srisuphanunt: What are the mechanisms of environmental favoritism that enhance the transmission of entero-pathogenic Cryptosporidium oocysts and Giardia cysts in the aquatic environments?
Ajeagah: The (oo) cysts can remain viable and infectious in water, soil or animal excreta for many months. They have a great amplitude of variation to environmental factors, such as heat, pH, freezing and drying. They are resistant to disinfectants, such as Chlorine and ozone, that are usually applied in developing countries.
Srisuphanunt: Why do you think that it is appropriate to include these pathogens in the Neglected Diseases Initiative?
Ajeagah: All diseases included in the world health organization’s Neglected Diseases Initiative have a common link with poverty. As the current view is to take a comprehensive approach to all these diseases, both Giardia and Cryptosporidium were included in 2004. These pathogens are significant causes of diarrhea and nutritional disorders in communities in developing countries. This is due to the very low economic and sanitary status of the population.
Srisuphanunt: What is the impact of meteorological variation on the transmission of infectious diseases?
Ajeagah: The Infra red and Ultrviolet radiation have a limited impact in the disinfection of these pathogens in the aquatic ecosystem. This may be due to the structure of the oocysts, which is double walled, or the architecture of the cysts, which can resist solar irradiation. That may explain why we assessed a majority of (oo) cysts during the long dry season, when there are higher temperatures in tropical environments. This could be due to the stability of the hydro-systems and the persistence of parasitic forms in the medium. In the long rainy season, we attributed the low (oo) cystic load to the effect of dilution by rainwater.
Srisuphanunt: How can the dissemination of waterborne and foodborne diseases be prevented? In the case of Yaoundé in Cameroon, which presents numerous (oo) cyst densities in streams and rivers, is there a direct sanitary risk of Cryptosporidiosis and Giardiasis incurred by the community?
Ajeagah: Parasites are easily transmitted through contaminated water, dirty hands, undercooked meat, unwashed fruit and vegetables, pets, toilet seats and even from the briefest of bodily contact. To prevent parasites, always wash fruit and vegetables, cook meat and fish well, drink filtered water, never let pets lick your face or eat from your crockery, wash your hands after handling pets or working outdoors, and clean toilet seats regularly. We believe that the sanitary conditions are not the best, so there is a continuous input of oocysts and cysts into the streams, which then contaminates the population in one way or the other, making the incidence of cryptosporidiosis and Giardiasis, as well as other infectious diseases, higher.
1 Associate Professor, Department of Parasitology, Faculty of Public Health, Mahidol University, Thailand
