New Physicochemical Properties of Water. Experimental Study of Physicochemical Changes in Pure Water by Iterative Flowing Procedure Induced by Peristaltic Pump Apparatus
Click here to view a message from Dr. Gerald Pollack, Editor-in-Chief
Vittorio Elia1, Elena Napoli1, Roberto Germano2, Rosario Oliva1,3, Daniele Naviglio1, Angela Longo4, Mariano Palomba4, Raffaele Vecchione5
1Department of Chemical Science, University “Federico II”, Complesso Universitario di Monte Sant’Angelo, Via Cintia, I-80126 Napoli, Italy.
2PROMETE S.r.l., CNR Spin off, P. le V. Tecchio, 45, 80125 Napoli, Italy.
3Physical Chemistry I – Biophysical Chemistry, Faculty of Chemistry and Chemical Biology, TU Dortmund University, Otto-Hahn-Strassen 4a, 44227, Dortmund, Germany.
4Institute for Polymers, Composites and Biomaterials-National Research Council (IPCB-CNR), SS Napoli/Portici, Piazzale Enrico Fermi, 1, 80055 Portici, Italy.
5Center for Advanced Biomaterials for Health Care (CABHC), Istituto Italiano di Tecnologia, Largo Barsanti e Matteucci 53, 80125 Napoli, Italy
Submitted: November 1, 2022
Revised: May 23, 2024
Accepted: August 2, 2024
Published: December 30, 2024
Abstract
Water is the most studied substance in the world, but its properties still amaze us. This work reports the experimental results of electrical conductivity measurements, χ (µScm-1) and pH of bidistilled water subjected to the forced movement induced by a peristaltic pump. The results of this physical perturbation, unforeseeable until today, are extremely significant: Increases in electrical conductivity χ of 100-150 (µScm-1) and variations of pH values from 5.6 to 8.2 were observed. The variations in these two physicochemical parameters were measured after a few hours of water flow obtained through a peristaltic pump that removed the liquid and then returned it to the same container. With this configuration, we obtained an iterative procedure (IPW-MW: Iteratively Perturbed Water-Moving Water). The rate of the phenomenology depends substantially on the volume of treated water. The lower the volume of water, the shorter the time needed to measure a stationary state. In fact, after a first rapid growth of the χ value, the system reaches a plateau. The value of the conductivity of the plateau is, unexpectedly, a function of the volume of bidistilled water treated. The same phenomenon was observed for pH as well.
These results indicate the discovery of a new phenomenology: “the volume effect.” Many other techniques and measurements were carried out to support this discovery, including:
1) Optical microscopy, identifying the presence of polymers of micrometric dimensions.
2) Lyophilization of perturbed bidistilled water, producing a soft white solid.
3) Thermogravimetric Analysis (TGA) of this solid, showing a very high thermal stability of an important fraction of it (50%), up to almost 1000°C.
4) Fluorescence spectra, clearly showing the presence of new chemical bonds that are not present in pure water.
5) IR (infrared) spectrum, confirming the presence of new compounds that appear as a consequence of the peristaltic procedure we used.
6) ICP-MS (Inductively Coupled Plasma Mass Spectrometry) measurements, not finding impurities to explain the extraordinary new properties of the perturbed water.
Introduction
The study of water is an exciting field of research, as it helps us understand the properties of the substance in which life was born on our planet. In this work, we revisit two procedures or perturbing methods that elicit unknown behaviors, citing experiments already known in the literature, and we introduce a novel third procedure yielding still completely unknown results. The procedures are:
1) The use of insoluble hydrophilic polymers of either organic or inorganic nature.
2) The iterative procedure of water filtration, using sintered glass filters.
3) The use of a simple peristaltic pump.
These procedures are very similar in that we subjected the modified water continuously to the same iterative procedure (IP).
During the last ten years our research team has been interested in the aspects of the changes in the physicochemical properties of pure water (bidistilled) submitted to some physical, low-energy perturbations (Elia et al., 2013b; Capolupo et al., 2014). The key to the relative success of these trials was to put iterative procedures into practice. We noticed that when you start to submit the water to physical perturbations you do not get significant changes in its physicochemical parameters. Thus, you are likely to abandon the procedure! In some specific cases, however, the iteration produces small variations in the measured properties, and the perturbed water can accumulate these effects until their value reaches a plateau, which depends on the water volume. The iterations significantly increase and enhance the variations of the physicochemical parameters under study. Fortunately, the small changes induced in electrical conductivity (χ) are among the easiest and fastest to measure, a fact that has worked to our benefit.
Chronologically, we obtained the first interesting results using filtration procedures, through sintered glass or cellulose (Millipore) filters, i.e., perturbing method 2. The filtered pure water recovered from a filtration underwent a new filtration, and so on and so forth, iteratively. This iterative technique produced significant experimental results. After a relatively small number of iterations on samples of low volume (1-10 ml), the electrical conductivity χ became high enough to be easily detected (10-200 µS cm-1). Of course, the measured parameters were not limited to electrical conductivity, but they included a variety of chemical and physical parameters, such as the pH, the heat of mixing with acids and bases, and the density (Elia et al., 2013b; Capolupo et al., 2014).
Perturbing Method 1:
Use of Insoluble Hydrophilic Polymers and Inorganic Materials (IPW-N, IPW-CE, etc.)
A significant change in the possibility of interpretation of the above experimental results occurred with the discovery of the Exclusion Zone (EZ) by Pollack (Zheng et al., 2006; Huszàr et al., 2014). The EZ is a modified water that forms at the liquid-solid interphase, e.g. between a hydrophilic polymer (Nafion®) and the liquid. EZ has properties that are more like those of a liquid crystal than a liquid. This discovery has allowed us to hypothesize that the iterative techniques lead to the accumulation of clumps of EZ (Elia et al., 2013a), producing measurable changes in the chemical and physical parameters of perturbed water.
To obtain the perturbed water, we have used a large number of either natural or synthetic – water insoluble – hydrophilic polymers: Nafion®, Cellulose, Cellophane, Crabyon, Hemp, Wool, Silk and Bamboo, as well as two inorganic materials: the Yellow Neapolitan Tuff (Elia et al., 2022b) and a sintered, very porous inorganic material (Signanini et al., 2019). These studies employed an iterative procedure of successive hydrations and dehydrations of polymers (Elia et al., 2018; Elia et al., 2019). By varying the chemical nature of the polymers used, we found many similarities and some peculiarities in the variations of the measured physicochemical parameters.
Of course, current paradigms cannot explain these variations, because conventional changes of state fail to consider the low energy transformation of water molecules. One example, above all, highlights the need to find a working hypothesis useful for the scientific understanding of the new phenomenology. The simple measure of electrical conductivity, after numerous iterations, shows huge increases, up to four orders of magnitude. The polymers used in the experiments are all insoluble in water, while the multiple washing processes carried out on these materials guarantee that the release of soluble compounds, like ions, is negligible. These iterations cannot determine an increase in the concentration of any electrolytes, if not at the level of impurities. The phenomenology is not dependent on an accidental increase in the concentration of electrolytes, since the number of variations is incompatible with trivial contamination. This statement finds decisive support in the observation that different polymers give quantitatively different results. At the same time, the results obtained with the use of the same polymer sample for several years are reproducible. It is a non-trivial physicochemical phenomenology.
In the context of this example of the extraordinary increase in electrical conductivity, one of our working hypotheses sees the formation of polymers of water molecules that favor the mobility of proton and hydroxyl ions. Fluorescence, optical, atomic force, and electronic microscopy techniques highlighted experimentally the presence of polymers in perturbed pure water (Elia et al., 2014a). The polymers would lead to an increase in proton and hydroxyl mobility due to the increase in the length of the jump proposed two centuries ago by Grotthuss (Grotthuss, 1806). To our knowledge, this is the first time that the idea of an increase in mobility of the two ions derived from water determines an increase in the electrical conductivity of the liquid water itself. It must be underlined that the chemical composition of the liquid has remained unchanged due to the insolubility of the perturbing polymers used. Thanks to measurements of pH, mixing heat, density, and various microscopy techniques, this working hypothesis is widely validated.
From the processed liquids, it is possible to obtain a solid by a simple freeze-drying procedure. The quantity of solid obtained is strictly reproducible: up to about 6 grams per liter of freeze-dried IPW (Iteratively Perturbed Water)!
Hence, we named the solid obtained, Xerosydryle (Elia et al., 2022a), from ancient Greek: (yle) material, consisting of (xeros) dry water (ydro):
• Xerosydryle exhibits exceptional resistance to high temperatures. Thermogravimetric Analysis (TGA) highlighted this property. The curves of the TGA clearly and indisputably distinguish the different chemical nature of the perturbing polymer from that of the Xerosydryle.
• Further contributions to the hypothesis of the presence of a new substance derive from the measurements of Differential Thermal Analysis (DTA), fluorescence and IR spectra of the various Xerosydryles obtained experimentally.
• Unlike the perturbing polymers, insoluble in water, the Xerosydryles are soluble in water.
• Xerosydryles are a new class of materials.
We came across an unknown and fascinating topic, as this introduction clearly shows. The most studied liquid in the world is still capable of surprising us.
Perturbing Method 2:
Iterative Procedure of Water Filtration (IPW-F)
This method involves the iterative procedure of filtering the water using sintered glass filters, with various diameters of the holes in the filters.
We took the filtered liquid and iterated the filtration procedure many times over. We observed that at each step, the electrical conductivity systematically increased. The incremental increase in this parameter depends on the diameters of the holes and on the volume of the liquid. The smaller the diameter of the holes and the lower the volume of the liquid, the higher the increase in the parameters measured (Elia et al., 2013b; Elia et al., 2013a; Elia and Napoli, 2011).
Perturbing Method 3:
Iteratively Perturbed Water-Moving Water (IPW-MW) Using Peristaltic Pumps
The new working hypothesis from our latest experimental results opened wide fields of research. The techniques we used are extremely simple and at the disposal of any researcher working in physicochemical labs. The experiments described do not require complex and/or expensive instruments to show the new phenomenology. After a series of attempts, we concluded that water is very sensitive to physical perturbations.
Also, the experimentation we propose for the first time in this work is extremely simple. It requires the use of a conductivity meter and a pH meter, i.e., the scientific equipment to follow the variations in the electrical conductivity and the proton concentration (pH).



In these experiments, the iteration of the procedure plays the determining role. In the initial experiments, we performed the iterations for moving water manually. Later, we introduced automated iterations for moving water. The measurability of the phenomenon depends on the accumulation of the effects of the perturbation in the liquid subjected to experimentation. Most likely the phenomenology is no different from that of the iterative filtrations (Elia et al., 2013b; Elia et al., 2013a; Elia and Napoli, 2011). Electrical conductivity is, once again, the principal measure of the variation because of its simplicity. In the very first experiments, we manually put about 5 ml of bidistilled water in a 1-liter Pyrex glass bottle. Then we transferred the liquid into a second identical bottle. The procedure simply consisted of alternately transferring water from one bottle to the other. After hundreds of steps, this strange procedure led to measurable variations in electrical conductivity. We passed from the initial value, very close to the unit, to values of tens of µS cm-1. As in the iterative filtration (IPW-F) experiments, we determined that the perturbation (IPW-Moving Water, IPW-MW) increased the conductivity value both when increasing the number of iterations and when decreasing the volume of water to be perturbed. For these reasons, we surmised that the mere flowing of water into glass containers, using a common peristaltic pump, was the needed iteration. We enriched the liquid through a “perturbation” that consisted of taking the liquid out with the pump and reinserting it into the same container (Image A). In this experiment, the observed phenomenology is more significant for the smaller volumes. Measurable results are obtained within a few hours (2-3). By varying the type of the pump (peristaltic or membrane), we did not observe qualitatively different effects, but peristaltic pumps were more reliable and introduced some peculiarities, depending on the frequency of the peristaltic movement. A characteristic of this new perturbation lies in the fact that the variations in electrical conductivity, using bidistilled water, reached a plateau value of about 130 µS cm-1 as compared to the initial value of 1-2 µS cm-1. The time needed for reaching the plateau is, however, strongly dependent upon the volume to be perturbed (Fig. 1 and Table 1).


In Figure 1, we report some experiments using peristaltic pumps in which we measured the electrical conductivity as a function of time. Something new appears! This is due to the extraordinary dependence on the volume of water perturbed. The phenomenon is clearly repeatable but not reproducible because of the very low energy involved in the process. Considering the high number of parameters involved (flux, peristaltic frequencies, volume of the samples, concentration of CO2, etc.), it is very difficult to obtain real reproducibility. Still, we put the new phenomenology into evidence without any doubts.
Figure 1 shows the experimental values of the electrical conductivity χ as a function of time for different volumes of bidistilled water (15-100 ml), in long experiments (about 15 hours). It shows the very different curves obtained, and how the phenomenology is nevertheless repeatable. Notice how the lower the volume, the lower the time to reach a plateau. In addition, the plateau values are not the same, but depend on the volume of the processed sample, and not simply on the pumping time. This is a new, unknown phenomenology not compatible with orthodox behavior: what we call the “volume effect.” All these experimental results were obtained using different peristaltic pumps, even if at approximatively the same flux, and involved many parameters (the flux, the diameter of the tubes, the volume of treated water, the CO2 concentration in the atmosphere, etc.). Therefore, it is not possible to perfectly control this very low energy process. For this reason, the experimental results appear repeatable but, until now, not perfectly reproducible. Under some favorable conditions, we can assume that we are also giving the same mechanical perturbation, or at least, the same energy, to the very different water volumes. Even in these conditions, it is probable that a simple mathematical relation between χ and time exists. It is interesting to note that looking at either Table 1 or Figure 1 it is possible to see a relationship between electrical conductivity and time. After seven hours, as seen in Figure 2, a very good linear correlation appears. In any case, the behavior of these systems cannot be explained using known orthodox theories.

Figure 3 shows micrographs by optical microscopy (50x magnification) of drops taken from the same 50 ml sample, at three different times during the peristaltic procedure, thus showing different conductivity values: 35, 57 e 148 µS/cm-1. Note the synchronous increase in conductivity and in the number and size of the objects visible with the optical microscope. This very new experimental result confirms the hypothesis that the concentration of water polymers and their size increases with the period of peristaltic pump use. We believe that this result opens new research opportunities regarding the properties of water, the substance in which life was born on our planet. It is worth noting that the micrographs show two phenomena: the increase in the number of polymers per unit area and the increase in their size. This type of experiment strongly supports the validity of the results.

Given the extraordinary nature of the phenomenon, we decided to make the results public as soon as possible. At a later stage, we will introduce any further improvements. Using containers or bottles of different materials, such as glass, polyethylene or polypropylene, for example, the phenomenology qualitatively reproduces the same results. If we stop the flux of water, the system maintains the values of the parameters. When we restart the pump, the phenomenology continues along the same trend. These results demonstrate that the perturbation is not a reversible phenomenon but is more likely a state function based on a very low energy process; for this reason, the phenomenology is repeatable but not reproducible. These results are also a very good indication of the stability of the perturbed liquid! The lyophilization of this perturbed liquid (IPW-MW) leads to a solid that appears as flakes with a soft appearance and clearly white color (Image C).


The new iterative procedure used in this perturbing method (moving water) is simpler than the previous ones. In addition, a single experiment obtains measurable results in a few hours and these results are very similar. The value of χ increases as does the value of pH. The nature of the container (Pyrex glass) and the short time needed for each experiment excludes all possible explanations based on the release of impurities or the growth of bacteria or seaweed. The latest results obtained with peristaltic pumps are consistent with the new paradigm that water can change its physicochemical parameters because of physical perturbations of low energy. We think that the very high increase of the pH parameter and the corresponding increase of χ are indicative of a peculiar new property of water.
Considering the difficulty in explaining the rich phenomenology with too simple or naïve chemical hypotheses, we propose a new paradigm: “The iterative perturbation method produces polymers of water molecules.” Their nature and dimensions depend on the nature of the perturbation, as well as on the chemical composition of the perturbing material. For example, Nafion® as perturbing polymer produces increments of χ and a decrease of pH (Elia et al., 2013a; Elia et al., 2014b; Elia et al., 2013b). All the other iterative perturbations, including iterative filtration, contact with hydrophilic polymers (Elia et al., 2014a; Elia et al., 2020; Elia et al., 2015) or moving water, the new procedure described in this paper, that use a peristaltic pump, produce an increase of χ and pH. The numerical variations of the cited parameters are very different between the two methods and depend on the iterative perturbation used. Previous procedures (Elia et al., 2020; Elia et al., 2015) depend on the EZ clumps identified by Pollack. Instead, in the peristaltic pump procedure, a new phenomenology of mechanical nature likely appears.
The working hypothesis we propose (described below) for the increase in electrical conductivity and pH depends on the presence of polymers of water molecules that allow liquid water to partly behave like a solid (Elia et al., 2014a; Elia et al., 2013a; Elia et al., 2017; Elia et al., 2018). The increase of χ depends on the increase in mobility of the H+ (Grotthuss jump mechanism) and/or of the OH– ions, making longer jumps possible in the presence of water polymers. This phenomenon could explain the mechanism of water transportation into very tall plants, where water could be transferred from the soil after dissociation into proton and hydroxyl ions (Signanini et al., 2019). This is the first time that the increase of electrical conductivity of water is attributable to the increase of mobility of the two ions in which water dissociates. No increase in ionic strength is needed. In the case of Nafion® as perturbing hydrophilic polymer, pH reaches the very low value of 3.0. In the other cases studied, the experimental measurements lead to very alkaline liquids (pH 8-9) using the methods of iterative filtration, iterative procedure of hydration and dehydration of hydrophilic polymers, and moving water. It must be emphasized that to obtain these very large changes of pH, at least until now, the only way would be to add an acid or a base to the sample. The moving water procedure excludes this possibility because of its simplicity and the short time of the procedure to obtain the cited results. We face a very intriguing new phenomenology concerning the physicochemical properties of water. The coherence of the new results in this work with literature allows us to harmonize all these similar results under the same new paradigm.
The working hypothesis of formation of water polymers that have chemical affinity to the H+ can be tested in the case of the very simple procedure that uses the peristaltic pump. We simply used the perturbing method with peristaltic pumps on different samples, obtained adding different quantities of HCl to the bidistilled water before perturbation, to start the experiment with the wanted pH value.
SEM-EDX Measurements
Figure 4 (a,b) reports the SEM image at two magnification values of a sample obtained by perturbing 200 ml bidistilled water via peristaltic pump. Measurements were obtained using FESEM Ultra-plus (Zeiss) Scanning microscope using 10 kV accelerating voltage. The lyophilized sample was redispersed in bidistilled water to be spotted onto the substrate and coated with sputtered gold. An aluminum stub was used to avoid sources of carbon deriving from the holder. SEM images show a granular morphology of the material with a feature size below 200 nm.
Figure 4c also reports the Energy Dispersive X-ray (EDX) pattern displaying several peaks. Excluding the Al peak of the stub, the Au peak of the coating of the sample and some impurities, we focused on oxygen, carbon as well as calcium considering that one possible source of carbon can be calcium carbonate. The following table reports the list of their Wt%:

Very interestingly it emerged that most of the material is made of oxygen, corroborating the hypothesis of a material born from liquid water. However, there is also a consistent amount of carbon that cannot only be justified by the calcium carbonate considering the excess of carbon. Most probably some of this carbon comes from the atmosphere. (“Process and apparatus for the capture and storage of the carbon of CO2 in the structure of the Xerosydryle,” Italian Patent pending: 102022000020472). Further experiments in a controlled environment are ongoing.
It is obvious that, together with the already performed ICP MS measurements on the perturbed water in liquid phase (see Table 9) this EDX analytic result on the solid material (Xerosydryle) – obtained by iterative flowing procedure induced by peristaltic pump apparatus – removes any imaginative “contamination hypotheses.”
The Role of H+ Concentration in the New Phenomena
In Figures 5, 6, 7, 8 and 9 we measured both χ and pH of the same sample as time passed while the peristaltic pump was moving the water. Similarly to χ, pH also varied. The sample had HCl added before being pumped to reach the wanted initial pH value (e.g. pH = 6 in Table 2 and Figure 5).
Normally, to obtain the increase in χ, an electrolyte must be added to water and to obtain the increase in pH, a basic substance must be added to water. This new phenomenology stops after a certain time interval, when it reaches a plateau. This means that, as for low soluble salts, a maximum concentration of water polymers at the plateau value was reached.
The phenomenon is repeatable even if you reuse the same container in the next experiment. Hence, the hypothesis of impurity release from the container is not credible. The synchronous behavior of the two parameters is very interesting.






Possible Working Hypothesis
The movement produced by the peristaltic pump induces the formation of polymers of water molecules, as seen in the optical microscopy image (Image B). The same polymers of water molecules (or in general polymers of water molecules) could also explain the solid obtained by lyophilization of the perturbed water (Image C).
The presence of polymers determines, via Grotthuss jump mechanism, an increase of mobility of both ions deriving from water dissociation, i.e., proton and hydroxyl ions, as already described previously. For this reason, an increment of χ is observed in pure water. At the same time (Elia et al., 2019) the binding constant of the two ions, H+ and OH–, to the polymers determines the formation of complexes having charge separation. If the prevailing complex depends on H+, the liquid assumes a pH greater than 7 (8, 9 and 11). The opposite is true in the case of complex formation with OH–. Figure 4 clearly shows the synchronous phenomena! The increase of pH stopped a little before the increase in χ. When the concentration of the free H+ decreases (pH increases), the complex formation stops because of the very low concentration of the protons. The simultaneous increase of conductivity is probably due to the contribution of OH– via jump mechanism, until the concentration of polymers reaches its maximum. It must be emphasized that the measured increase in pH is due to the reduction of the concentration of free proton ions (bonded ions) and not to the increase of the OH– ones. For the conductivity, the increase does not stop at the same time because of the presence of free OH– ions that also can exploit the jump mechanism. A saturation of the concentration of polymers of water molecules should determine the two plateaus in χ and pH. The working hypothesis agrees with the formation of the complex between H+ and the polymers of water molecules. When the concentration of free H+ reaches very low values, due to the very high value of measured pH, the formation of the complex proton water polymers cannot occur because of their very low concentration. Therefore, the increase of pH stops. The coherence with the working hypothesis seems acceptable.
To check the working hypothesis and understand the role of the presence of H+, we measured the effect of the use of the peristaltic pump on a solution of HCl in bidistilled water. The presence of a large quantity of free H+ should modify the phenomenology. The system reaches a lower acid level compared to the one compatible with the presence of the added HCl because of the new possibility of complex formation between the polymers of water molecules and the added free protons. It seems that the stability of the polymer depends on the presence of a higher quantity of free protons. When the concentration of protons is approximately lower than 10-8 moles per liter (pH = 8), the phenomenology stopped (Figures 5-9). For all the systems, we used 50 ml of acidic solutions via the presence of HCl and the same peristaltic pump to reduce the influence of possible variability of the perturbing system.
It is very interesting to note that at the beginning of the procedure in pure water, there are very rapid and noticeable increases of the two parameters χ and pH. Both increases are consistent with the working hypothesis. The rapid increase of pH depends on the binding process of free H+ ions on the formed polymers of water molecules. The rapid increase of pH then rapidly reduces because of the very low values of free H+. For the increase of conductivity, we must consider that both the ions produced in water dissociation contribute to the jump mechanism and the large concentration of the free OH– ions contributes to increase conductivity.
In this case of a quite neutral control at pH=6 (pure bidistilled water) we obtained an increase of both parameters and for a long time, the system reached two plateaus (see Figure 5). The working hypothesis explains the increase of pH with the formation of polymers of water molecules that can bind the free protons, and it explains the increase of conductivity with the increase of mobility via the jump mechanism. When the concentration of free protons is about 10-8 moles/liter the binding phenomena disappears. The entire phenomenology depends on the presence of free protons.





The value of χ increases as the value of the pH increases. To check the validity of this working hypothesis we conducted the iterative procedure in the presence of added HCl to verify if the pH increases much more. We conducted many experiments in the presence of HCl. See Figures 5-9.
After observing the experimental results, it is astonishing to note that at a very low pH of 2.6 (Figure 6) the production of polymers of water molecules can reduce, via binding the free protons, the conductivity from 1380 to 500 µS cm-1 and cause an increase of pH from 2.6 to about 7.0. It means that the concentration of water polymers may be very high. This may be a variation of five orders of magnitude for the proton concentration.
In the case of an initial pH of 3.2, the production of water polymers can reduce the initial conductivity of 410 to about 150 µS cm-1 and produce an increase in pH from 3.2 to about 8.0.
In the case of the pH of 3.4, the initial conductivity reduced from 140 to 80 µS cm-1 and caused an increase in pH of about five orders of magnitude. This case was very similar to the previous case.
Increasing the value of the initial pH, in other words, moving toward the neutrality of pure distilled water, the variations of conductivity and pH reduce, as is the case in Figure 9. The conductivity varies from 80 to about 60 while the pH varies from 3.6 to about 5.0.
In the case of non-acid water, the phenomenology is quite different! Both conductivity and pH start to increase immediately (see Figure 5). χ varies from 2 to about 140 µS cm-1 while pH increases from about 6-7 to 8.2. These results show that the system “water” has an equilibrium constant for the formation of water polymers and so the maximum quantity for the formation of Xerosydryle is regulated by thermodynamic parameters (∅G, ∅H, ∅S); nature prevents the complete formation of water polymers in a determined water system.
There is agreement with the working hypothesis.
In Figure 10, we report the trend of the variation of the values of pH for all the measurements. As we can see, the increase of pH stopped when reaching the value of 8-9. The increase of the measured pH happens rapidly. At the same time, if we look at Figure 10 (red curve, pH=6.0) that depicts the behavior of pure water, we can see that after reaching a relative maximum, the pH values start to decrease. We could hypothesize that the moderate decrease of the pH value is caused by the accumulation of CO2 during the operation of the pump.

In Figure 11 and Table 8, ∆pH is the difference between the initial values of the pH (pump off) and the maximum values of Figure 10. In Figure 11, it appears that starting at the initial pH (no HCl added) of pure water, that is pH=6.6 at the right of the figure in blue, the effect of pumping produces a clear increase in the difference between the initial pH and the maximum pH (see Table 8). Each experimental data point represents a new measurement corresponding to a different water solution, to which a varying amount of HCl has been added and that is subjected to pumping with the peristaltic pump.


Figure 9 minus pHi, the initial one).
At pH values higher than 3.5, the linear trend has a negative slope, while the opposite happens for the lower values: The two trends determine a break point. This is similar to equivalent points in many kinds of acid-base titrations.
If we add HCl (lower pH, higher concentration of free protons), the ΔpH should increase, and in fact it does. Consequently, there is a direct relation between the concentration of protons and the increase of ∆pH.
Therefore, the polymers of water molecules substantially produce complexes with excess of protons. Naturally, we expect that the maximum concentration of polymers is a finite one. In fact, the system reached saturation, and the increase stopped. All available polymers have already formed complexes with the protons from the acid. Then, the excess of protons reflects the finite concentration of the polymers that leaves many free protons, as in a normal acid-base titration. Consequently, pH decreases. The break point in Figure 11 gives us information on the maximum concentration of polymers (solubility). From Figure 11, it is possible to find the pH of the break point at about pH=3.5. It means that at this H+ concentration of the added HCl, the system cannot bind more free protons. Depending on the stoichiometry of the binding process, we may have information on the concentration of the polymers. In more acidic solutions, at a pH lower than 3.5, the concentration of protons is higher than the concentration of polymers. Therefore, the reduction of free protons via binding to the polymers stops and the value of ΔpH reduces. It is interesting to note that at the lowest pH (pH=2) the ∆pH is zero. In the liquid (aqueous solution of HCl), there is a very high number of free protons, and the polymers can bind only to a limited number of them.
The coherence of our working hypothesis with the observed experimental behavior induced us to develop some new proofs, capable of removing any possible uncertainty about the presence of polymers of water molecules.
One of these supporting procedures is the lyophilization of the water that has been perturbed via the use of the peristaltic pump, which results in the formation of Xerosydryle. It is very difficult to determine the chemical nature of this solid. Thus, we suppose the presence of something very similar to a polymer of water molecules or in general polymers of molecules of water. As of this date, we cannot make a coherent hypothesis on these chemical bonds. Therefore, we cannot perform a chemical analysis.
Then we introduced the ICP-MS procedure to study the ion composition of pure water and perturbed water. This way we can determine if there is a known quantity of solutes that justifies the physicochemical parameters of the perturbed liquid (χ = 130 µS/cm-1 and pH higher than 8). The ICP-MS is a procedure that breaks the chemical bonds of the molecules of the liquid, forming atomic ions that can be identified by the detector. Comparing the results (see Table 9) of this procedure – applied both to the perturbed water and to the simple bidistilled water – confirms, without any doubt, that no elemental chemical substances capable of modifying χ and pH are present in the liquid (IPW-MW). Thus, the chemical composition is pure water. The chemical composition of Xerosydryle is also pure water.
Despite this experimental result, obtained with the latest generation technology (ICP-MS), that confirms that the composition of the samples is pure water, the χ and pH parameters have undergone very significant numerical variations. Such an increase of electrical conductivity cannot derive from an alteration of the elemental composition of pure water. What we found experimentally agrees with the working hypothesis of the formation of polymeric molecules deriving from water.
The mineral composition of samples of IPW was assessed by ICP-MS. Table 9 reports the elemental species. This elemental composition cannot be responsible for the high conductivity shown by samples of water that underwent the iterative procedure. This is a further confirmation of the presence of water polymers (very stable polymeric water molecules) that allow protons to move more rapidly, and, in this way, produce the increment in conductivity that was observed.

To confirm the hypothesis of very stable water molecules polymers, or simply the presence of polymeric water, we tried to recover the “water polymers” with the lyophilization methodology. We obtained quite incredible results. The lyophilization procedure determines the ability to see the formation of a white solid, named by us as Xerosydryle (Elia et al., 2022a). It is soluble in water and exhibits extraordinary stability to the increase of temperature.
As can be seen from Figure 12, a thermogravimetric diagram, two kinds of solids are present: The first one is stable until 600°C and the second one until 1000°C. All these extraordinary properties confirm the possibility of obtaining a water polymeric substance using low mechanical energy. The chemical composition of this new substance is pure water.

Figure 13 shows the IR spectrum determined on the same solid (Xerosydryle, see Image C). It is possible to see the clear reduction of the transmittance on the left of the figure compared to liquid water. We suppose that the clear reduction depends on the reduction of free OH– passing from liquid to solid via lyophilization. All experiments point to the formation of water polymers.

To support our hypothesis, see Figure 14, showing the fluorescence emission spectrum of the perturbed and pure untreated water, upon excitation at 280 nm. The two spectra are clearly different.

280 nm. The spectra were recorded by using a 1 cm path length of the quartz cuvette at the temperature of 25°C.
The emission spectrum of the sample has a broad emission band centered at about 334 nm. In contrast, the control sample did not show any band in this spectral region. The sharp peak at around 310 nm is due to the Raman peak of water. Indeed, this peak is present in both spectra. In the sample, the Raman peak is more intense with respect to the control water. This can be due to the presence of micrometer-sized polymers that enhance its intensity due to light scattering.
The presence of a fluorescence emission spectrum of the sample is indicative of the presence of molecular species possessing a π electron system. Indeed, at the excitation wavelength used, normally only n → π* and π → π* transitions can be promoted. In addition, fluorescent compounds are mainly aromatic, meaning that an extended π electron system is needed. In addition, some linear, conjugate molecules can exhibit fluorescence spectra (for a deep discussion, please refer to chapter 4 of Valeur and Berberan-Santos, 2012). Thus, according to the fluorescence data and the other data reported above (FT-IR, Thermogravimetric analyses, ICP-MS and Optical microscopy), it is possible to conclude that the molecular species responsible for the observed spectrum should contain an extended π electron system. Since our ICP-MS analysis did not highlight the presence of any external substances with respect to pure water, the species responsible for the fluorescence spectrum has the same composition as that of pure water. In a molecule of water, H2O, the oxygen atom (with a sp3 hybridization, so there are four sp3 orbitals) is bonded to two hydrogen atoms through sp3 (O)-1s (H) bond (σ-σ bond). Instead, two electron lone pairs occupy the remaining two sp3. Thus, in principle, the oxygen atom in each water molecule has the capability to form, with other oxygen atom of another water molecule, σ-σ (single bond) and π-π (double bond) interactions. However, the nature and the kind of molecular bonds of such molecular species remain unknown.
A discussion about several clues pointing to the spontaneous quantum origin of these structures induced in liquid water by low energy physical perturbations was advanced in a paper of one of the authors (R.G.) a few years ago (Germano, 2015), strongly suggesting the possibility that these structures are the matrix of life.
Conclusions
It is evident that some unknown properties of water identified through low energy processes have been discovered, through rather simple experimental procedures. This fact opens very interesting research scenarios because the phenomenon cannot be explained by conventional laws. The phenomenology of this new topic is very much like that involving perturbed water (IPW) via iteration of the procedure of hydration and dehydration of insoluble hydrophilic polymers. On the other hand, the similitude to the iterative procedure of iterative filtration permits us to remain within the same paradigm that concerns new physical and chemical characteristics of water. These results confirm the working hypotheses of the presence of polymeric water molecules, the role of free protons in their binding process, the impossibility to polymerize all molecules of water contained in a water system, and finally the presence of a solid after the lyophilization procedure. These findings give a response to two important mechanism of water transport: transferring water molecules from soil to the top of very tall plants, and the exchange of water molecules between cellular compartments; water polymers accelerate the processes via the Grotthuss jump mechanism. Results shown in this paper highlight that the chemical composition of the perturbed liquid (IPW-MW) cannot explain the very high values of χ and pH. Therefore, the chemical composition of the solid is the same as that of the liquid: polymers of water molecules. The performed analyses exclude contaminations; in particular, the simplicity of the procedure and the very short time needed to obtain the formation of the polymers excludes any contamination of biological origin.
Acknowledgments
We thank Valentina Mollo for her support in the acquisition of SEM images and EDX data.
References
Capolupo A, Del Giudice E, Elia V, Germano R, Napoli E, Niccoli M, Tedeschi A, Vitiello G (2014) Self-similarity properties of Nafionized and filtered water and deformed coherent states. Int J Mod Phys B 28 (03): 1450007. https://doi.org/10.1142/S0217979214500076
Elia V, Napoli E (2011). Nanostructures of water molecules in iteratively filtered water. Key Eng Mater 495: 37-40. https://doi.org/10.4028/www.scientific.net/KEM.495.37
Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M (2013a). Experimental evidence of stable polymers of water at room temperature and normal pressure after iterative contact with a Nafion® polymer membrane. Water 5: 16-26.
Elia V, Marchettini N, Napoli E, Niccoli M (2013b). Calorimetric, conductometric and density measurements of iteratively filtered water using 450, 200, 100 and 25 nm Millipore filters, J Therm Anal Calorim 114: 927-936. https://doi.org/10.1007/s10973-013-3046-y
Elia V, Napoli E, Niccoli M (2013c). Physical-chemical study of water in contact with a hydrophilic polymer: Nafion. J Therm Anal Calorim 112: 937-944. https://doi.org/10.1007/s10973-012-2576-z
Elia V, Napoli E, Niccoli M (2013d). Calorimetric and conductometric titrations of nanostructures of water molecules in iteratively filtered water. J Therm Anal Calorim 111: 815-821. https://doi.org/10.1007/s10973-011-2164-7
Elia V, Ausanio G, De Ninno A, Germano R, Napoli E, Niccoli M (2014a). Experimental evidences of stable water nanostructures at standard pressure and temperature obtained by iterative filtration. Water 5: 121-130.
Elia V, Lista L, Napoli E, Niccoli M (2014b). A Thermodynamic characterization of aqueous nanostructures of water molecules formed by prolonged contact with the hydrophilic polymer Nafion. J Therm Anal Calorim 115: 1841-1849. https://doi.org/10.1007/s10973-013-3371-1
Elia V, Germano R, Napoli E (2015). Permanent dissipative structures in water: the matrix of life? Experimental evidences and their quantum origin. Curr Top Med Chem 15: 559-571. https://doi.org/10.2174/1568026615666150225102531
Elia V, Yinnon TA, Oliva R, Napoli E, Germano R, Bobba F, Amoresano A (2017). Chiral micron-sized H2O polymers in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water. Water 8: 1-29.
Elia V, Oliva R, Napoli E, Germano R, Pinto G, Lista L, Niccoli M, Toso D, Vitiello G, Trifuoggi M, Giarra A, Yinnon TA (2018). Experimental study of physicochemical changes in water by iterative contact with hydrophilic polymers: A comparison between Cellulose and Nafion. J Mol Liq 268: 598-609. https://doi.org/10.1016/j.molliq.2018.07.045
Elia V, Napoli E, Germano R, Oliva R, Roviello V, Niccoli M, Amoresano A, Naviglio D, Ceravolo M, Trifuoggi M, Yinnon TA (2019). New chemical-physical properties of water after iterative procedure using hydrophilic polymers: the case of paper filter. J Mol Liq 296: 111808.https://doi.org/10.1016/j.molliq.2019.111808
Elia V, Napoli E, Germano R, Roviello V, Oliva R, Niccoli M, Amoresano A, Toscanesi M, Trifuoggi M, Fabozzi A, Yinnon TA (2020). Water perturbed by Cellophane: Comparison of its physicochemical properties with those of water perturbed with cotton wool or Nafion. J Therm Anal Cal 146: 2073-2088. https://doi.org/10.1007/s10973-020-10185-0
Elia V, Napoli E, Germano R, Naviglio D, Ciaravolo M, Dal Poggetto G, Caputo D, Oliva R, Yinnon TA (2022a). New physicochemical properties of liquid water resulting from recurrent contact with hydrophilic polymers. Characteristics of the resulting supramolecular polymers: the Xerosydryle. WATER 12: 72-85.
Elia V, Napoli E, Germano R, Naviglio D, Ciaravolo M, Dal Poggetto G, Caputo D, Oliva R, Yinnon TA (2022b). A study on the changes in physical properties of distilled water put in contact with porous hydrophilic materials: experimental evidences on Neapolitan Yellow Tuff. WATER 12: 119-129.
Germano R (2015). Water’s Permanent Dissipative Structures Quantum Origin and Life. Electromagn Biol Med 34 (2): 133-137. https://doi.org/10.3109/15368378.2015.1036074
Grotthuss T (1806). Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Ann Chim 58: 54-73.
Huszàr IN, Màrtonfalvi Z, Laki AJ, Ivàn K, Kellermayer M (2014). Exclusion-zone dynamics explored with microfluidics and optical tweezers. Entropy 16 (8): 4322-4337 https://doi.org/10.3390/e16084322
Signanini P, Vessia G, Elia V, Napoli E, Germano R (2019). A study on the changes in physical properties of demineralized water put in contact with porous hydrophilic materials: experimental evidences on metabrick material. J Porous Media 22 (12): 1609-1625.https://doi.org/10.1615/JPorMedia.2019026816
Valeur B, Berberan-Santos MN (2012). Molecular Fluorescence: Principles and Applications, 2nd Edition. Wiley-VCH. Weinheim, Germany. https://doi.org/10.1002/9783527650002
Yinnon TA, Elia V, Napoli E, Germano R, Liu Z-Q (2016). Water ordering induced by interfaces: an experimental and theoretical study. Water 7: 96-128
Zheng JM, Chin WC, Khijniak E, Khijniak E Jr., Pollack GH (2006). Surfaces and interfacial water: evidence that hydrophilic surfaces have long-range impact. Adv Colloid Interf Sci 127: 19-27. https://doi.org/10.1016/j.cis.2006.07.002
Zheng JM, Pollack GH (2006). Solute exclusion and potential distribution near hydrophilic surfaces. In Pollack GH, Cameron IL, Wheatley DN (Eds.). Water and the Cell. Springer. Dordrecht, The Netherlands: 165-174 https://doi.org/10.1007/1-4020-4927-7_8
Discussion with Reviewers
Reviewers’ Comments
Critical assessment of the properties of iteratively perturbed water – all of which can be explained by different types of contamination
Torsten Walther & Anne S. Ulrich
Karlsruhe Institute of Technology (KIT), IOC and IBG-2, Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany.
Correspondence: anne.ulrich@kit.edu
The present manuscript by Elia et al. is a continuation of a series of studies on iteratively perturbed water, in which the authors claim to have discovered a novel form of “polymeric” water. In their hands, repeated perturbation of pure water (here: by peristaltic pumping) leads to massive changes in its physical properties (here: conductivity, pH), which is attributed to polymer formation. Furthermore, lyophilization of such solutions yields substantial amounts of solid remnants, which are asserted to consist of “solid water” (termed “Xerosydryle” or “IPW”). These interpretations, however, do not live up to critical examination, as there exist classic explanations for essentially all of the described phenomena. According to careful experiments carried out more recently in our laboratory, the “novel” properties can be explained in terms of simple contaminations, stemming from different sources depending on the method of sample preparation.
Methods of sample preparation
As outlined in the introduction, Elia et al. have used three different methods of repetitive perturbation in the course of their cumulative work:
1. Exposure to hydrophilic materials (Nafion, cellulose, cellophane, Crabyon, wool, silk, etc.)
2. Filtration (through cellulose or sintered glass)
3. Use of a peristaltic pump (this work).
In each case, the dominant contaminant seems to have escaped their detection, either because the authors did not explicitly search for it, or because the analytical methods were not appropriately applied or interpreted. In the following, the relevant types of contamination and analytical pitfalls shall be summarized, following a chronological order of reported observations. Experimental evidence and a more detailed discussion of the critical aspects can be found in a recent publication from our group (Greil, 2023), and is backed up by further unpublished data from our lab that will be submitted soon. A close collaboration with the Elia team constituted a key aspect of our studies, which were accompanied by regular discussions and an exchange of all available data. Therefore, we sincerely regret that our colleagues in Naples no longer wish to be part of these jointly envisaged publications.
Potential sources of contamination
When a water sample has been extensively treated using one of the above methods (1 – 3), it is essential to exclude the most obvious sources of potential contamination, as there are:
A. Microbial growth, when working under non-sterile conditions
B. Molecules derived from the starting material to which the water was repeatedly exposed
C. Microscopic particles from the air, or rubbed off from surfaces.
Each type of possible contamination needs to be assessed specifically, using designated analytical techniques.
(A) Contamination due to microbial growth
A few years ago, our group had been intrigued by the steady series of reports from Naples on iteratively perturbed water (“IPW”). We were keen to collaborate and characterize these remarkable solutions (supposedly containing accumulated EZ water) and the lyophilized solid material (supposedly consisting of polymeric H2O) by means of solid-state NMR (1H, 2H, 17O). Using Nafion membranes as a starting material for method (1), we initially tried to prepare IPW samples in our lab in Karlsruhe, under the guidance of the Naples group and according to their published protocols. However, working under sterile conditions in a laminar flow-bench, we did not see any significant changes in conductivity, nor did we succeed in obtaining any lyophilized material, even after many weeks of daily treatments. Only the pH of the solutions would decrease successively, as expected from the highly acidic sulfonic acid groups in Nafion that will release protons into solution upon wetting (Greil, 2023). One remarkable exception occurred when the experimenter had a cold, whereupon significant changes in conductivity were observed. This sample, however, contained lots of bacteria, as seen under the microscope.
To be able to proceed with the planned NMR experiments, the Naples group was so kind to provide us with several different IPW samples (liquids and lyophilized solids) for comprehensive analysis, including microbiological assays that they had never performed so far. To exclude any bacterial contamination, we checked these samples using methylene-blue staining for light microscopy and DAPI staining for fluorescence microscopy. As described in Greil et al. (2023), numerous round and rod-shaped bacteria were detected, as well as microalgae such as diatoms, which are known to be highly abundant in the form of aerosols, especially in coastal areas as in Naples. Electron microscopy and ATR-FTIR spectroscopy confirmed the presence of these microbiological contaminants (including the silica shells of diatoms), besides particulate matter that most likely represented synthetic fibers and dust.
It is not surprising that bacteria, aquatic microalgae and dust have accumulated in the course of sample preparation in Naples, which involved the repeated wetting and air-drying of Nafion sheets in large open containers on the lab bench. Given that biofilm formation on Nafion membranes is a well-known problem in microbial fuel cells, it is not surprising that biofouling will affect the physicochemical parameters recorded by the Elia team to monitor IPW sample preparation. Bacterial multiplication cycles can thus explain the irregular and unreproducible fluctuations in conductivity, pH, density and light scattering that have been documented over days and weeks in their earlier work using method (1). Also, the corresponding data from circular dichroism and thermogravimetry analyses can be readily explained by the above biological and organic (see below) contaminants found in the different IPW samples.
(B) Contamination from the starting material
The preparation of IPW according to method (1) typically involves repeated rubbing of the hydrophilic starting material (Nafion, cellulose, cellophane, Crabyon, hemp, wool, silk, bamboo), supposedly in order to enhance the collection of EZ water from these surfaces (Yinnon, 2016). It is conceivable that such physical treatment leads to a chafing off of the starting material into solution – either as fragments of the hydrophilic macromolecules, and/or as a suspension of microscopic particles. In both cases, solid-state NMR spectroscopy should be able to detect the presence of the organic starting materials in the lyophilized samples, by analyzing the 13C-NMR spectra under magic angle sample spinning.
We had gratefully received several different types of lyophilized IPW samples from Naples for collaborative NMR analysis, prepared with Nafion, silk, wool, and cellulose. Except for the Nafion-derived specimen (that was only available in small quantity, close to the threshold of detection), these samples produced distinct solid-state 13C-NMR spectra that differed from one another. The respective patterns correspond largely to the fingerprint spectra of the organic starting materials, which implies that debris from silk/wool/cellulose constitute the major portion of these IPW samples (manuscript to be submitted). These rubbed-off starting materials in fact represent the main contamination, as any microbiological organic material would have otherwise shown up quantitatively as a different kind of dominant fingerprint. Interestingly, the Nafion-derived IPW gave only a weak 19F-NMR signal, despite the high sensitivity of the fluorine nuclei contained in the polymer. Hence, the main contamination in this case can be attributed to aerosols and microbial growth, which – notably – also takes longer to accumulate and yields lesser amounts of IPW (personal communication from Naples).
(C) Microscopic particles as contamination
Inorganic particulate matter, finally, would seem to explain the findings reported by Elia et al. in the present manuscript, which is their first presentation based on method (3), i.e., sample preparation by peristaltic pumping. Particle shedding from peristaltic pump tubing is a well-recognized problem in the manufacturing of pharmaceutical grade products, as reported by other groups. For example, Saller et al. (2015) showed that the silicone tubing releases particles of around 200 nm, next to a small fraction in the lower micrometer range, which tend to aggregate further upon standing. The continual shedding of particles under physical stress was proven by SEM and 3D laser scanning microscopy, demonstrating morphological alterations of the inner tubing walls after pumping. This unavoidable release of silicone particles from the peristaltic pump thus appears to be a sufficient explanation of the observations described here by the Elia team. It is not clear whether silicon (Si) was probed for in their elemental analysis, though the particulate matter might not even be ionizable in standard liquid-state ICP-MS.
Furthermore, the reported changes in pH and conductivity are plausible in view of the siloxane building blocks and possibly traces of the polymerization initiators. Finally, the thermogravimetric analysis of the lyophilized material obtained in the present manuscript is fully consistent with the well-known exceptional thermal stability of silicones. The following TGA profiles from the literature provide good evidence that the various lyophilized samples produced by Elia et al. actually consist of exactly those contaminations proposed above, instead of any polymeric “solid water.” The panel on the left, panel a, compares an earlier Nafion-derived IPW sample from Naples (Yinnon, 2016) with that of a typical protein (bovine serum albumin) (Gomez-Rico, 2005). The panel on the right, panel b, compares the new type of IPW obtained by peristaltic pumping with the TGA analysis of silicone elastomers from the literature (Arkles, 2015). In both cases, the distinct thermogravimetric profiles of the IPW samples correspond rather well to those of the dominant contaminations proposed by us.
Hence, given all the arguments presented above, there is no need to invoke a special novel phase of water that can be isolated at room temperature. It would thus seem wise to let go of the “IPW” hypothesis, and instead focus on some of the other fascinating properties of water in future research.
References:
Arkles B, Sulaiman S (2015). Silicone Elastomers with Exceptional Elongation. Conference Paper presented at the 188th Technical Meeting of Rubber Division, ACS / International Elastomer Conference, Paper # 124
Gómez-Rico MF, Font R, Fullana A, Martín-Gullón I (2005). Thermogravimetric study of different sewage sludges and their relationship with the nitrogen content. J. Analyt. Appl. Pyrolysis 74: 421.
Greil F, Punampalam R, Walther TH, Heißler S, Ulrich AS (2023).”Iteratively nafionated water” in its solid phase at room temperature is in fact a mixture of lyophilized biological and non-biological contaminants. J Mol Liq 385: 122351.
Saller V, Matilainen J, Grauschopf U, Bechtold-Peters K, Mahler HC, Friess W (2015). Particle shedding from peristaltic pump tubing in biopharmaceutical drug product manufacturing. J. Pharm. Sci. 104: 1440.
Yinnon TA, Elia V, Napoli E, Germano R, Liu ZQ (2016). Water ordering induced by interfaces: an experimental and theoretical study. Water 7: 96.
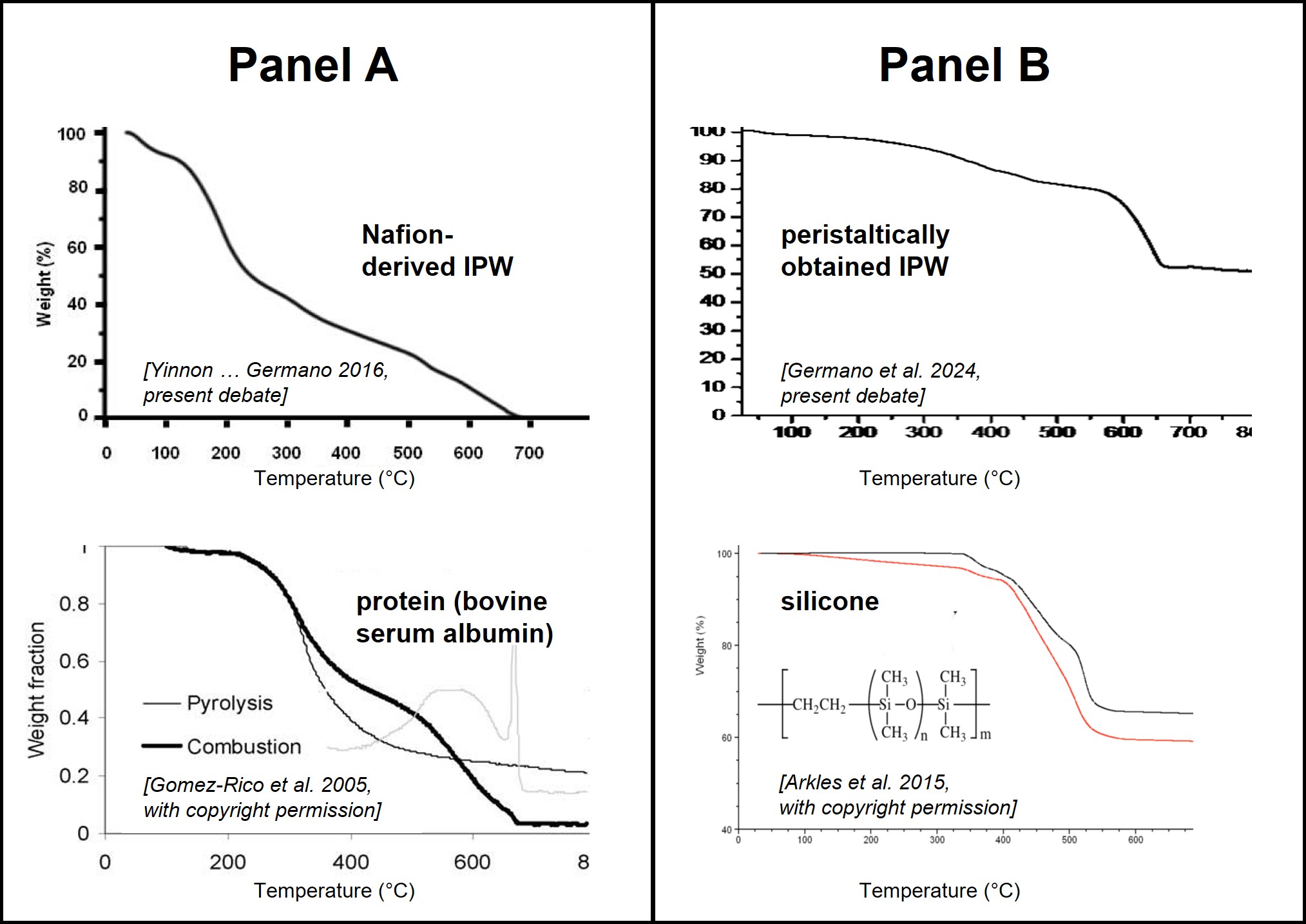
Panel B (lower TGA-curve): Reprinted from Conference Paper presented at the 188th Technical Meeting of Rubber Division, ACS / International Elastomer Conference, Paper # 124, Barry Arkles, Jonathan Goff, Santy Sulaiman, “Silicone Elastomers with Exceptional Elongation”, 2015, https://www.researchgate.net/publication/287998678_Silicone_Elastomers_with_Exceptional_Elongation, with permission from the author.
Response to Reviewers
“Iteratively Perturbed Water” Nature Cannot Be Misidentified as Contaminants
Vittorio Elia1, Elena Napoli1, Roberto Germano2*, Rosario Oliva1,3, Daniele Naviglio1, Angela Longo4, Mariano Palomba4, Raffaele Vecchione5
1Department of Chemical Science, University “Federico II”, Complesso Universitario di Monte Sant’Angelo, Via Cintia, I-80126 Napoli, Italy.
2PROMETE S.r.l., CNR Spin off, P. le V. Tecchio, 45, 80125 Napoli, Italy.
3Physical Chemistry I – Biophysical Chemistry, Faculty of Chemistry and Chemical Biology, TU Dortmund
University, Otto-Hahn-Strassen 4a, 44227, Dortmund, Germany. 4Institute for Polymers, Composites and
Biomaterials-National Research Council (IPCB-CNR), SS Napoli/Portici, Piazzale Enrico Fermi, 1, 80055 Portici, Italy.
5Center for Advanced Biomaterials for Health Care (CABHC), Istituto Italiano di Tecnologia, Largo Barsanti e Matteucci 53, 80125 Napoli, Italy
*Corresponding author: Tel.: +39 349 784 82 06
E-mail address: germano@promete.it
This is a response to the comments made by reviewers, Torsten Walther and Anne S. Ulrich, regarding our paper entitled, “New Physicochemical Properties of Water. Experimental Study of Physicochemical Changes in Pure Water by Iterative Flowing Procedure Induced by Peristaltic Pump Apparatus,” submitted for publication to WATER Journal. In their article, “Critical assessment of the properties of iteratively perturbed water– all of which can be explained by different types of contamination,” they are citing their paper (Greir et al., 2023) in which they reject not only the reviewed paper, but even all our experimental findings published in a dozen international publications.
In this circumstance, it is useful to start by defining the context of our experimental findings, otherwise one can’t see the forest for the trees.
We reported evidence of micron-sized chiral supramolecular H2O aggregates in water at ambient conditions for the first time in our paper, entitled, “Chiral micron-sized H2O aggregates in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water,” published in WATER Journal (Elia et al., 2017).
These aggregates were generated by physically perturbing pure water through iterative immersion of a hydrophilic membrane (NAFION®) in bidistilled water, stirring, membrane removal and drying. The circular dichroism spectra of such perturbed water exhibit similarities to β-sheet ordered biomolecules. Moreover, lyophilization of the perturbed water resulted in the formation of a solid residue. To exclude the possibility of impurities (released by the membrane, organic or bio-contaminants) as the cause of these phenomena, we employed advanced analytical techniques such as Matrix-Assisted Laser Desorption/Ionization Time of Flight, Gas Chromatography both coupled with Mass Spectroscopy and Ion Chromatography. This ”iteratively nafionated water” was found to contain only 10-6 M Fluorine and Sulfate ions, released by the membrane, as documented on page 29, table S1 in Elia et al. (2017) and negligible amounts of contaminants. Furthermore, we showed also that UV absorbance and fluorescence spectra of the ”iteratively nafionated water” could not be attributed to contaminants or molecules released by the membranes (Elia et al., 2017).
Our observations revealed that aggregates formed in water adjacent to a hydrophilic material can adopt and maintain a stable chiral configuration (Elia et al., 2017). A few months after the publication of our paper, this finding was reinforced by the discovery of DNA’s Chiral Spine of Hydration (McDermott et al., 2017), which represents a significant advancement in the understanding of water properties, with profound implications for biological science. The results reported in McDermot et al., (2017), demonstrate that water in close proximity to DNA, similar to our observations with other hydrophilic compounds, exhibits plasticity and the ability to adapt to a robust supramolecular structure conforming to DNA’s chirality.
What we are describing in our paper, “New Physicochemical Properties of Water. Experimental Study of Physicochemical Changes in Pure Water by Iterative Flowing Procedure Induced by Peristaltic Pump Apparatus,” are very different experimental conditions than those described in the paper by Greil et al., (2023) and that we have spoken to them about in an attempt to improve their experimental procedures. We have, in fact, published a dozen papers, encompassing around 300 pages, describing many different experimental results (see e.g., the references in Elia et al., 2022), reporting something very different from their description about our experiments and from their replication attempt. It is important to mention that our recent research is the natural experimental progress of the work described in about 50 papers previously published internationally by us (by V Elia and E Napoli) showing evidence of the roots of these new physicochemical phenomena obtained perturbing pure liquid water.
There is a fundamental consideration that we believe essential: In some cases, for some typology of hydrophilic materials in contact with pure water, we get up to 6 g/L of Xerosydryle (water supramolecular aggregates – solid residue – [see Elia et al., 2022]) from double distilled water (i.e., 1.5 g from 250 ml) in a few hours. So, let’s imagine a 1 m sided cubic aquarium filled with double distilled water: How would you get 6 kg of bacteria and diatoms in a few hours? The described phenomenon is characterized by this gigantic magnitude, not to be confused with a hypothetical reproduction of protozoa or bacteria (in distilled water).
Besides this, the basic component of the Xerosydryle is nanometric in nature (2 nm) (see Fig.1), or in any case, below 200 nm (see Fig.3) and not at all composed of the cadavers of single-celled beings that are at least of micrometer size.
Furthermore, none of the different typologies of analyses reported in all our experimental papers (essential results for all referees) showed evidence (in quantities exceeding “traces”) of nitrogen, phosphorus or silicon. What are diatoms and bacteria made of? If these elements are not highlighted by very sensitive analytical methods, it means that, although some microorganisms may be present, they contribute in an irrelevant way to the total mass of Xerosydryle and therefore to the general experimental phenomenology.
Even if our measurements show that the thermal properties of the Xerosydryle resemble those of biological macromolecules (so called “denaturation” behavior of biological macromolecules), they are much more heat resistant. Moreover, their chirality is not affected (“denaturation” behavior) by the addition of sodium hydroxide (NaOH) or hydrogen chloride (HCl) in amounts sufficient to induce pH change up to 13 or down to 3, respectively, differently from the biological macromolecules that are much more fragile.
Amazingly, a large fraction of these aggregates in the dried state are stable up to 970°C, while the carbon bonds are totally broken at 500°C.
In addition, these features are undoubtedly demonstrating that, apart some possible not relevant contaminations (always possible in traces), the supramolecular structures (Xerosydryle) that are self-organizing from the “iteratively perturbed water” cannot coincide at all with biological contaminants.
Besides, we can’t be silent about the particularly embarrassing “juxtaposition” of thermogravimetric curves by Torsten Walther and Anne S. Ulrich, trying to compare two typologies of Xerosydryle with a typical protein (bovine serum albumin) in one case and with silicone elastomers (from the literature) in another case, as possible contaminants forming the whole mass of Xerosydryle. This is unreasonable as far as sensitive analytical results reported in our published papers show clearly no contamination.

Measurement of perturbed water at 100 nm scale. The false color bar expresses the height in nanometers. (Elia et al., 2017).

Finally, there is a general epistemological consideration that is very important from our point of view. Since the same type of experimentation – iterative perturbation of pure water – produces (Elia et al., 2022):
• Completely reproducible results on different types of water physicochemical characteristics,
• Completely analogous behaviors for different insoluble materials used to perturb the water (e.g., electric conductivity increases with the number of procedure iterations),
But, at the same time,
• Very specific results for each type of used material (e.g., in one case pH becoming strongly alkaline – like, among others, in the case of the peristaltic pump treatment – and, in another case becoming strongly acid, and in another case remaining neutral).
This indicates the irrelevance of any possible disturbing factors on the general phenomenon highlighted.
In fact, to obtain the increase in χ, normally an electrolyte must be added to water. And, normally, to obtain the increase in pH, a basic substance must be added to water. But, in our case, this new phenomenology stops after a certain time interval, when it reaches a plateau. This means that, as for low soluble salts, a maximum concentration of water structures at the plateau value was reached. The phenomenon is repeatable even if you reuse the same container in the next experiment. Hence, the hypothesis of impurity release from the container is not credible. The synchronous behavior of the two parameters is very interesting. The possible explanation is the following: The presence of aggregations determines, via the Grotthuss jump mechanism, an increase of mobility of both ions deriving from water dissociation, i.e., proton and hydroxyl ions. For this reason, an increment of χ is observed in pure water. At the same time the binding constant of the two ions, H+ and OH–, to the aggregates determines the formation of complexes having charge separation. If the prevailing complex depends on H+, the liquid assumes a pH greater than 7 (8, 9 and 11). The opposite is true in the case of complex formation with OH–.
Moreover, as explicitly requested by one of the reviewers of our submitted paper, “New Physicochemical Properties of Water. Experimental Study of Physicochemical Changes in Pure Water By Iterative Flowing Procedure Induced by Peristaltic Pump Apparatus,” we performed very recently SEM investigation of a sample obtained by perturbing 200 ml of bidistilled water via peristaltic pump – see Fig. 3 (a,b). Measurements were performed by a FESEM Ultra-plus (Zeiss) Scanning Microscope, using 10 kV accelerating voltage.


The lyophilized sample was redispersed in bidistilled water to be spotted onto the substrate and coated with sputtered gold. It was used with an aluminum stub to avoid sources of carbon deriving from the holder. SEM images show a granular morphology of the material, with a feature size below 200 nm.

As can be seen, most of the material is made of oxygen, corroborating the hypothesis of a material born from liquid water. However, there is also a consistent amount of carbon that cannot be justified only by the calcium carbonate considering the excess of carbon. Most probably some of this carbon comes from the atmosphere (“Process and apparatus for the capture and storage of the carbon of CO2 in the structure of the Xerosydryle,” Italian Patent pending: 102022000020472). Further experiments in a controlled environment are ongoing.
Several clues indicate that this material is not a residue from bacteria or other micro-organisms. First, they are not visible by SEM and, moreover, in the case of direct SEM analysis of the lyophilized sample, it appears with long and intricate filaments typical of polymeric structures. In Figure 4, we present examples of SEM on a lyophilized sample. It is deposited on the stub by a carbon adhesive layer that compromises the quantification of the carbon by EDX; this is why, for the EDX, we chose to redisperse the sample in bidistilled water to dispense and dehydrate a droplet of sample directly on an Al stub.
So, it is obvious that, together with the already performed ICP MS measurements on the perturbed water in liquid phase (see Table 9 of our submitted paper: “New Physicochemical Properties of Water. Experimental Study of Physicochemical Changes in Pure Water by Iterative Flowing Procedure Induced by Peristaltic Pump Apparatus”), these last SEM with EDX analytic results on the solid material (Xerosydryle) – obtained by iterative flowing procedure induced by peristaltic pump apparatus – removes the imaginative “contamination hypotheses” clumsily put forward to discredit our extensive experimental results.
Finally, we would like to mention that our general experimental findings were independently replicated by Pollack et al., (2023). In this study, the Exclusion Zone of water (EZ water), formed against three chemically distinct surfaces, Nafion, ghee, and Whatman-5 filter paper, was extracted, characterized by UV–Visible absorbance spectroscopy, and solidified either by lyophilizing or evaporation in an oven. The resulting highly stable solid was analyzed by mass spectroscopy, verifying the absence of any ionizable contaminants that could reproduce the characteristic “signature EZ” spectra in the three liquid preparations, or in the solids formed from desiccated EZ water that had been reconstituted in deionized water. This study reports, therefore, independently from us, that a solid form of EZ water exists indeed at room temperature.
In conclusion, we would like to underline that for every experiment, there is always a threshold of competence below which that experiment turns out to be irreproducible.
References
Elia V, Yinnon TA, Oliva R, Napoli E, Germano R, Bobba F, Amoresano A (2017). Chiral micron-sized H2O aggregates in water: Circular dichroism of supramolecular H2O architectures created by perturbing pure water, WATER 8: 1-29.
Elia V, Napoli E, Germano R, Naviglio D, Ciarovolo M, Dal Poggetto G, Caputo D, Oliva R, Yinnon TA (2022). New physicochemical properties of liquid water resulting from recurrent contact with hydrophilic polymers. Characteristics of the resulting supramolecular aggregates: the Xerosydryle. WATER 12: 72-85.
Greil F, Punampalam R, Walther T, Heiβler, Ulrich AS (2023). ” Iteratively nafionated water” in its solid phase at room temperature is in fact a mixture of lyophilized biological and non-biological contaminants, J.Mol.Liq. 385: 122351.
McDermott ML, Vanselous H, Corcelli SA, and Petersen PB (2017). DNA’s Chiral Spine of Hydration, ACS Cent. Sci., 3(7): 708-714.
Sharma A, Traynor-Kaplan A, Pollack GH (2023). Solid water at room temperature? Arabian Journal of Chemistry 16(3): 104537.
