 Change in Physical Properties of Motionally Unperturbed Dilute Aqueous Solutions
Change in Physical Properties of Motionally Unperturbed Dilute Aqueous Solutions
Ivan L. Cameron
Email: cameron@uthscsa.edu
Cellular and Structural Biology Department, The University of Texas Health Series Center at San Antonio, TX 78231
Keywords: water thixotropy, viscosity, conductivity, ions, hydrophilic surfaces, laser light scattering, luminescence, ultraviolet absorption
July 7, 2017; Revised: January 4, 2018; Accepted: January 4, 2018; Published: March 30, 2018; Available Online: March 30, 2018
Abstract
This is a brief review on changes that occur when dilute aqueous solutions are left standing, motionally unperturbed, in a glass storage container from hours to days. This results in an increase in solution viscosity termed thixotropy. Agitation quickly reduces the viscous weak gel-like state back to its original less viscous state. Other changes include increases in electrical conductivity, laser light scattering, luminescence, and UV adsorption. Both the presence of ions and hydrophilic surface have been shown to be factors in the thixotropic phenomenon of water. It is proposed that a supramolecular structure of water develops from minutes to weeks then reaches a plateau. Biological implications are briefly mentioned.
Introduction
This report is divided into two sections dealing with spontaneous changes that occur with increase in duration of storage of motionally unperturbed dilute aqueous solutions. The first section deals with rheology (viscosity) changes and the second section deals with non-rheology changes (electrical conductivity, laser light scattering, luminescence, ultraviolet spectroscopy, circular dichroism and various solution storage conditions). This report does not include discussion of exclusion zones.
Rheology
Vybiral et al. have made the surprising observations that distilled and boiled water when left standing unperturbed for some time (hours to days) developed into a somewhat more viscous weak gel like state (2006, 2007, 2011). He referred to this macroscopic state of distilled water as autothixotropy. It was also observed that if distilled boiled water was first deionized then the unperturbed resting water autothixotropic state did not occur. This lead to the conclusion that low levels of ions present in the distilled water were involved in formation of the thixotropic state. This idea was later confirmed by adding some salt (NaCl) to the deionized water that then demonstrated the autothixotropic effect (2011). Vybiral reported in 2006 that stirring and shaking of water in the autothixotropic state or that boiling it immediately destroyed the autothixotropic state. Based on the evidence that ions are involved in the observed thixotropy it is therefore recommended that the word “autothixotropy” be replaced by the word “thixotropy.”
The main method used by Vybiral (2006, 2007, 2011) for demonstration of water thixotrophy was a plate hung on an elastic filament and immersed into freshly boiled distilled water. Upon twisting of the upper part of the non-immersed filament the immersed plate begins to rotate when a critical angle of rotation was reached. The submerged plate then starts to rotate and reaches a new equilibrium position. This is referred to as a static method of measurement. Given an angular torsion of the filament a force is reached in the water which causes a major change of the angular position of the immersed plate.
A dynamic method of measurement (Vybiral 2006) involved the fall duration of a plastic ball with a given radius and density through a water sample. The duration of ball fall through a given distance was used to determine the samples viscosity. These were two of the three methods used in the experimental studies reported by Vybiral et al. (2006, 2007, 2011)
A reanalysis of data in Vybiral’s 2006 publication on the falling ball duration addresses previously unanswered questions. How long does it take for a distilled and boiled water sample stored in a closed glass container to develop a more viscous, very weak gel like, thixotropic state or to reach a maximum equilibrium state and how much sample perturbance does it take to change this thixotropy state back to its original less viscous state?
The precise timing of thixotropy formation in distilled water or a weak ion containing solution has not previously been determined nor has the amount of time and agitation needed to bring the thixotrophic water state back to its original non-thixotrophic state.
The falling ball method of determining water thixotropy was previously described by (Vybiral 2006). Briefly a liter cylinder with inner diameter of 57 mm and a plastic ball with a non-absorbent surface with a diameter of 28.5 mm (mass of 7.94 g) and density of 1.019 x 103 kg.m-3 was used. This gives 14 mm clearance from beaker wall. The cylinder was filled with the water sample. The experiment was to immerse the ball into the water sample starting with its upper edge 30 mm below water sample surface and then allow the ball to fall over a length of 35 mm. The time it took the ball to fall this distance was measured in seconds with the use of optical equipment with phototransistors and computer controlled scaler. The water sample temperature was maintained at 22° C throughout the experiment. The time for the ball to fall 35 mm was in the 10 second range.
The first step in the experiment was to boil the distilled water sample of about 1 liter for 10 minutes then the sample was allowed to cool down to 22° (about 60 minutes) prior to the first ball fall time measurement.
Two different experiments were done on ball fall duration. The first experiment involved an increase in time of water sample rest at 22° C for up to 17 days. It was expected that the ball fall duration would slow down with time of water samples rest due to increase in water sample thixotropy. Measurements were also done on long term stored water sample before and after intense mechanical stirring.
The second experiment was to measure the ball fall duration in distilled water stored for 7 days. Multiple measures of the 7 day rested water sample at 5 minute intervals between multiple ball fall measures was done to measure the effect of water perturbed by increased mixing due to increased number of falling ball drops.
The statistical analyses used were one way analysis of variance and linear and one phase exponential regression analyses. The falling ball data used in this report were published by Vybiral in 2006. These data were subjected to further statistical analyses to provide additional new information. Table 1 summarizes data used to determine time dependent changes in development of water thixotropy. Freshly boiled and stirred distilled water give the stirred and distilled ball fall duration values in Table 1. The ball fall duration measured at increasing times of the unperturbed water sample rest were subjected to additional statistical analysis. The stirred and the initial ball fall duration were found to be significantly faster than all longer rest durations as judged by the lack of overlap of 95% confidence interval (CI) values. The ball fall duration of the 7-day rest value was significantly faster than the 17-day ball fall time values. Thus the ball fall time continued to significantly decrease during the standing water rest period over a period of 17 days.
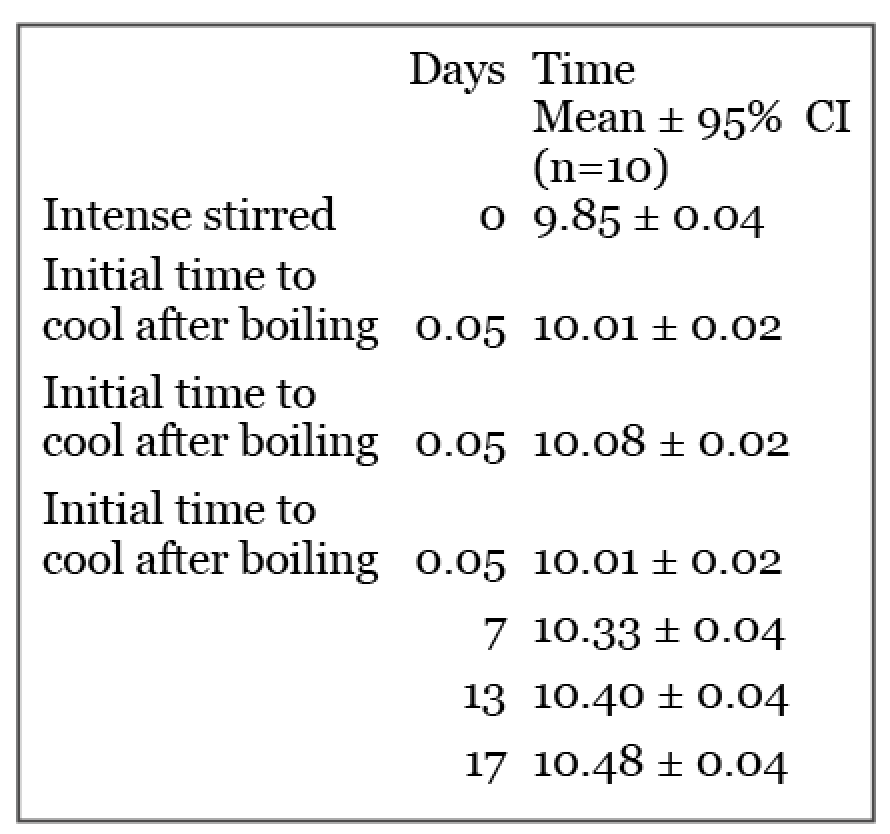
Table 1. Ball Fall Duration – 351 mm (seconds)
The duration of change with time was subjected to regression analyses by both a linear analysis fit and then a non-linear exponential fit. Both methods showed good R2 fits but the non-linear exponential fit was better R2 0.9148 than the linear R2 0.8699. Plus the exponential fit gave an end point plateau value of 10.50 sec. This value is not significantly different than the 17 days ball fall duration value. These findings suggest that the water thixotropy is near a maximum plateau value by 17 days of aqueous solution rest.
Initial values allow for cool down to 22° C following boiling. No overlap between 95% confidence interval values indicates significant differences. Thus intense stirred and initial values differ significantly and were both significantly faster than all longer rest periods and the 7 days of rest sample is significantly faster than the 17 days of rest sample.
The second set of data analysis concerns the loss or disruption of the water thixotropy with multiple falling ball drops measured at 5 minute intervals. The data on ball fall duration over a distance of 351 mm after increase in number of repeated ball drops is shown in Figure 1.
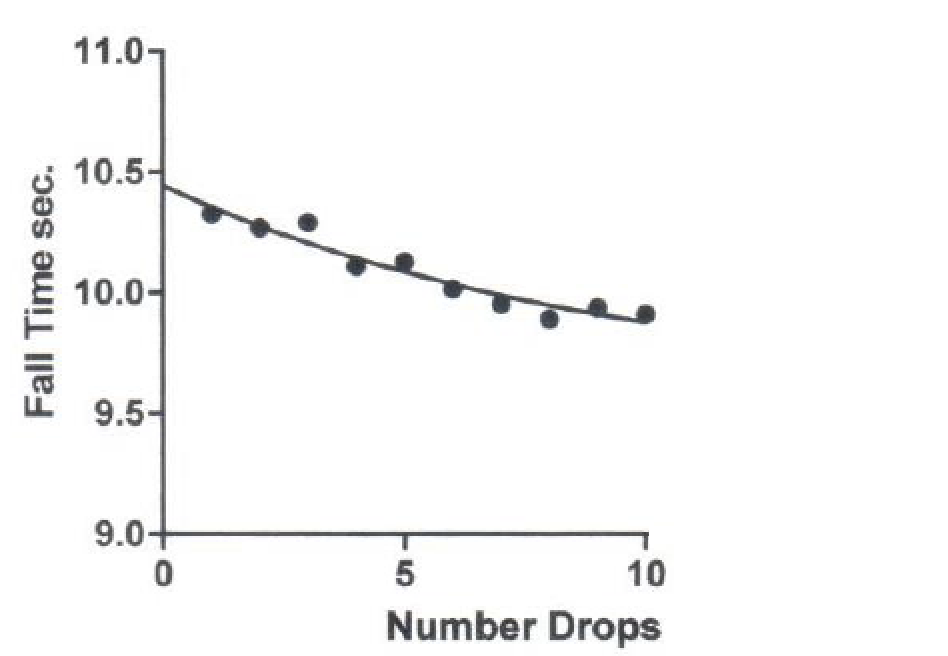
Figure 1. Evidence that distilled water that had developed thixotropy with rest loses thixotropy when exposed to repeated ball movement through the sample.
Rested distilled water thixotropy was disrupted by adding balls with the same specifications as before. Balls were added at 5-minute intervals and duration of fall over a length of 351mm was measured. Both linear and non-linear (one phase exponential decay) regression analysis were performed. The non-linear exponential fit was better than the linear fit (R2 0.9277 vs R2 0.9075) and was selected for further analysis.
Repeated falling ball drops every 5 minutes speeds the falling ball duration indicating disruption of thixothropy. The non-linear data indicates the change in fall duration with repeated ball falls is projected to reach a plateau of about 9.580 seconds and has a zero intercept value of 10.44 of unstirred long standing distilled water which is near the plateau value of 17 days of unperturbed water rest 10.48 sec. and the calculated plateau of maximum water thixotropy 1.50 sec. thus a value in the 10.44 to 10.50 sec. range appears to represent a maximum extent of thixotropy of distilled water sample and an increase in rest beyond the 17 days of unperturbed water rest is not likely to significantly further increase the thixotropic state.
Boiled degassed deionized water stored motionally unperturbed in a sealed glass container, to limit exposure to air, failed to show evidence of thixotropy upon rest. If the observed increased ball fall duration is due to leaking of unidentified ions from the glass container wall or to contact with the hydrophilic glass container wall upon storage in deionized water it might have contributed to the increase in ball fall duration with storage duration. It did not (Vybiral 2011). If leaking of ions, contamination from the air or exposure to the hydrophilic glass surface of the container wall are not the cause of increase in the falling ball time, as reported in Vybiral’s 2011 torsion experiments, the increase in ball fall duration with storage might be attributed to interactions in the distilled water. The author’s supposition on the possible contribution of ions in the distilled water was supported by the fact that NaCl salt added at 0.4 g / 400 ml to deionized water did demonstrate the thixotropic effect (Vybiral 2011).
These observations lead to the conclusion that there was a long term water structure development (water thixotropy) in the presence of some residual ions in the re-boiled closed container of distilled water used in the experiments reported by Vybiral 2011.
Non-Rheology
This section summarizes non-rheological measures of resting (unperturbed) dilute aqueous solutions.
Verdel et al. (2012) reported that the electrical conductivity of long term storage of unperturbed extremely dilute aqueous solutions significantly increased. Conductivity measured at 1000 Hz also significantly increased when the surface to volume ratio of the storage container was increased. They speculated that both ions and contact with the hydrophilic glass container surface could be conditions causing the increased conductivity.
Recently Verdel and Bukovec (2016) also reported on the increase in conductivity upon storage of aqueous solutions of various cation chlorides. Conductivity increased faster in solutions with Cs and Li (weak salting out, chaotropes) than with solutions of strongly salting out hydrophobes (kosmotropes) like K and Mg. Silica did leak from the glass container wall with storage time and gave a slight increase in conductivity. They noted that this silica increase in rate of conductivity was not enough to change their conclusions on the conductivity increase rates by the various cation chloride solution with increase in storage time nor did dissolution of CO2 from air. It was found that the chaotropes Cs and Li gave a faster rate of increase in conductivity than the kosmotropes K and Mg. It was concluded that the increase in conductivity with time of solution storage was due to greater water structuring around the chaotropes that better facilitates proton or hydroxyl hopping and therefore an increase in conductivity. They concluded that a weak thixotrope state of water is “triggered” by hydrophilic surfaces and by ordering of hydration shell layers around nucleating solutes over extended time with little or no mechanical aggregation (rest).
Are there optical changes in dilute aqueous solutions with motionally unperturbed storage? Lobyshev et al. (1999) reported storage time dependent increase in water luminescence in the visible and near ultraviolet ranges. It was concluded that water and dilute aqueous solutions are self-organizing polymorphous systems.
Laser light scattering (LLS) and other methods for study of aqueous dilute solutions caused formations of 100 nm sized domains (Verdel and Bukovec 2014), Sedlak 2006a, 2006b, 2006e, Sedlak and Rak 2013, Yinnon and Yinnon 2012, Yinnon and Elia 2013).
Elia et al. 2017 recently reported that double distilled Milli Q water can form chiral aggregates of water molecules when exposed to a Nafion membrane. The method used involved exposure of the water to a Nafion membrane then stirring the water and turning over the membrane. This process is repeated 10 times and the membrane removed and dried. The procedure is then repeated 10-20 times with the dried membrane. This procedure is referred to as the iterative Nafionized membrane (INM) procedure. Analysis of the INM treated water was subjected to circular dichroism spectral analysis and to other methods of analysis and revealed homochirality not attributed to impurities released by the membrane. Multiple 200 nm size aggregates were observed by fluorescent microscopy and SEM. Other research groups have reported H2O aggregates but not their chiral supramolecular structure. If chiral supramolecular water structure is common in the in vitro and in vivo systems is yet to be reported.
Summary and Implications
The rheology section of this report is devoted to the experimental results of Vybiral 2006, 2011 on time dependent viscosity changes in stored (motionally unperturbed) water. Viscosity increased in stored distilled water but not in deionized water. Addition of salt (NaCl) to the deionized water increased its viscosity. Stirring/shaking or boiling of the viscous samples quickly reduced their viscosity. Reanalysis of data from the resting storage samples showed an exponential decrease in the rate of viscosity increase to a plateau value at 17 days. Vybiral proposed that thixotropy is due to macroscopic clusters of water around ions that evidently fill the whole container.
The main studies reviewed in the non-rheology section deal with electrical conductivity of aqueous solutions by Verdel et al. 2012, 2016. They reported significantly higher conductivity in unperturbed aged solutions except those stored frozen. They also found increase in conductivity as the surface to solution volume ratio increased. Thus they propose the excess conductivity was due to the hydrophilic glass surface and the ions present. Their 2016 report focused on the role of aqueous solutions of various cation chlorides. They report that Cs and Li increase conductivity at a faster rate than K or Mg. Silica species also built up in the glass vessel during storage which complicates but did not invalidate interpretation of the cation chloride results. They propose that structuring of hydration shells around Cs and Li maybe enhanced more than around K and Mg with storage and this better accelerates proton or hydroxyl hopping, i.e. electrical conductivity.
Optical changes in motionally unperturbed aqueous solutions have been reported. The changes include luminescence and laser light scattering (LLS). The LLS studies reveal formation of heterogeneous domains of 30 to 500 nm that increase in number from minutes to weeks.
The time for development of rheological and non-rheological changes in unperturbed aqueous solutions appear to extend over a relatively long duration from days to weeks which then apparently reaches a plateau. Shaking or boiling the thixotropic state rapidly returns the specimen to its original non-thixotropic more sol like state. These time dependent changes have important implications as discussed below. Clearly many important unanswered questions arise about the physical properties of motionally unperturbed water solutions as it changes from a free flowing sol state to a weak gel like state.
Might a weak thixotropic gel state also exist in biological organisms? If so what might it’s physiological implications be?
Evidence is accumulating that cell cytoplasm is a water-immiscible substance when squeezed from a cell into a dilute solution. Cytoplasm has also been shown to exclude dyes. What these observations indicate is that water in the cell is structured/ordered differently than ordinary bulk water.
Given that water in cytoplasm exists in an immiscible gel state one might question if a major agitation would result in change from a gel state to a sol state. This is what has been suggested to occur with a traumatic hammer blow to the head of a rabbit (Gallyas and Pal 2008). In this ultrastructural study of the rabbit’s cortical brain in a region of the hammer blow to the head, the observations showed that some of the neurons underwent a major shrinkage of 50 to 60% throughout the entire cell body including its dendrites and axon process. The authors concluded that the affected neurons “dark neuron” had undergone a general gel to sol change resulting in a major loss of cell water. It seems possible that the loss in volume may be due to a decrease in the osmotically unresponsive properties of the gel state to the more osmotically responsive properties of the sol state. This type of traumatic concussion response may be what occurs in boxers, soccer and football players.
Based on finding on thixotropy in a report by Ooigawa et al. 2006 indicates that dark shrunken neurons form instantly after a traumatic brain injury (TMBI) but return to a larger non-dark state in 30-180 min. The non-biological reports cited in the current review indicate that development of a thixotrope (weak gel) state can take days to weeks. It is proposed that the shorter recovery time of dark neurons to normal appearing neurons vs in vitro formation of a thixotropic (weak gel) state may be due to the much greater surface to water volume ration in vivo than in the in vitro studies.
References
Vybiral, B. 2006, The comprehensive experimental research on the autothixotropy of water. In Water and the Cell: Pollack, G.H., Cameron, I., Wheatley, D. Eds.; Springer; New York, NY, USA.
Vybiral, B. and Voracek, P. Long term structural effects in water autothixotropy of water and its hysteresis Homeopathy, 2007, 183-188
Vybiral B. Autothixotropy of water and its possible importance for the cytoskeletal structures 2011 J Physics 329:1-11
Verdel, N.; Jerman, I.; Krasovec, R.; Bukovec, P.; Zupancic, M. Possible time-dependent effect of ions and hydrophilic surfaces on the electrical conductivity of aqueous solutions. Int. J Mol. Sci. 2012, 13, 4048-4068
Verdel, N., Bukovec P. Correlation between the increasing conductivity of aqueous solutions of cation chlorides with time and the “salting-out” properties of the cations 2016 Entropy 18: issue 3
Lobyshev, V.I., Shilklinskaya, R.E.; Ryzhikov, B.D. Experimental evidence for intrinsic luminescence of water. J. Mol. Liq. 1999, 82, 73-81
Verdel N., Bukovec P. 2014 Possible further evidence for the thixotropic phenomenon of water entropy 2014, 16, 2146-2160
Sedlak, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: I. Light scattering characterization. J. Phys chem B 2006a, 110, 4329-4338
Sedlak, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: II. Kinetics of the formation and long-time stability. J. Phys. Chem B 2006b, 110-4339-4345
Sedlak, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: III. Correlation with molecular properties and interactions. J. Phys. Chem B 2006c, 110, 1396-13984
Sedlak, M.; Rak, D. Large-scale inhomogeneties in solutions of low molar mass compounds and mixtures of liquids: Supramolecular structures or nanobubbles? J. Phys. Chem. B 2013, 117, 2495-2504
Yinnon, T.Al; Elia, V. Dynamics in perturbed very dilute aqueous solutions: Theory and experimental evidence. Int. J. Mod. Phys. B 2013, 27, 1350005-1350040
Yinnon, T.A.; Yinnon, C.A. Domains of solvated ions in aqueous solutions, their characteristics and impact on electric conductivity: Theory and experimental evidence. Mod. Phys. Lett. B 2012, 26, 1150006-1150020
Gallyas,F., Pal J. 2008 Whole-cell transition of neurons and its possible role in apoptotic cell death Pp 63-73 Phase Transition in Cell Biology eds Pollack G.H., Chen W.-C. Springer Dordrecht
Ooigawa, H., Nawashiro, H., Fukui, S.Otani, N., Osumi,A., Toyooka, T., Shima, K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate, Acta Neuropathol (2006) 112:471-481
Elia, V., Yinnon, T.A., Oliva, R., Napoli, E., Germana, R., Bobba, E., Amoresano, A. Chiral micron-sized H2O aggregates in water:Circular dichroism of supramolecular H2O architectures created by perturbing pure water, WATER 2017, 8, 1-29
