 Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 3. Quantum Electrodynamic Analyses of Experimental Data on Solutions of Weak Electrolytes and Non-electrolytes
Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 3. Quantum Electrodynamic Analyses of Experimental Data on Solutions of Weak Electrolytes and Non-electrolytes
Yinnon TA1*, Liu ZQ2
1 K. Kalia, D.N. Kikar Jordan 90666, Israel
2 Department of Physics Qufu Normal University, Qufu, 273165, China
*Correspondence E-mail: lwcdsrc@kalia.org.il
Key Words: Water aggregates; molecular associates; domains; ferroelectric orderings; polar liquid solutions; ultra dilute solutions; serial diluted solutions; biological active liquids.
Received Jan 15th 2015; Revised June 4th; Accepted June 30th; Published Nov 15th; Available online Nov 27th, 2015
Abstract
Molecular associates in very dilute solutions of weak electrolytes or non-electrolytes kept at ambient conditions are studied. Ambient electromagnetic fields affect some of the associates’ types. For concentrations (C) in the range of about 10-7 – 10-20 M, the associates were observed only for solutions with polar solvents, e.g., water, chloroform. At such very low C, sizes of these associates reaching tens of micrometers imply that these mainly are composed of solvent molecules. Some associates’ properties are correlated with the bioactivity of the solutions. As discussed in our two preceding publications in this journal’s issue, electromagnetic fields affecting associates entail electrodynamic forces have to be explicitly described. We employ a quantum electrodynamic model for analyzing experimental data pertaining to the associates’ properties and their impact on the solutions physicochemical characteristics. Our analyses show that associates mediated by electromagnetic fields have the typical characteristics predicted by the model. Our analyses clarifies the prerequisites of polar solvents, serial dilutions and vigorous shaking after each dilution step for stabilizing associates in solutions with C below about 10-7 M. Our analyses provide clues for the domains impact on bio-systems.
Article Outline
Introduction
During the last decade 10-7 – 10-4 m sized molecular associates were observed in polar liquids and their very dilute solutions kept at ambient conditions. For example, in water or chloroform containing non-electrolytic, weak or strong electrolytic solutes, with concentrations (C) in the range of 10-7 – 10-20 M. At such ultra low concentrations (ULC), the huge sizes of these associates imply these are mainly composed of solvent molecules. Some of these solutions are bioactive, e.g., these are antioxidants, plant growth regulators, neuromediators. For reviews see Konovalov and Ryzhkina (2014a) and Elia et al. (2015). Stabilization of associates in ULC solutions have been induced by several perturbative techniques, e.g., serial dilutions with vigorous shaking after each dilution step, iterative filtering liquids, iterative agitating liquids with a Nafion membrane. Independent research groups with a wide variety of techniques evidenced presence of the associates. Therefore it can be ruled out that the relevant data is due to measurement errors. As to impurities released by containers, by filters or by membranes, these affect the perturbed liquids but cannot account for their typical properties — see Elia and Niccoli (2004), Elia et al. (2013, 2015), Yinnon and Liu (2015a,b).
With water, other polar liquids and their solutions playing central roles in many physical, chemical and biological systems, knowledge about the associates’ properties and the physics underlying these is important. Analyses of associates in serial diluted vigorously shaken aqueous strong electrolyte solutions (SDVSASES) we presented in the paper preceding this publication in Water journal. For analyses of iterative filtered water or water agitated with a Nafion membrane (so called Iterative Nafionized Water) see Elia et al. (2015), Yinnon and Elia (2013), Yinnon et al. (2015c in press). The foci of our current manuscript are associates in polar liquids’ solutions which were serial diluted, vigorously shaken after each dilution step and contain weak or non-electrolytic solutes. We denote these Serial Diluted Vigorously Shaken Polar Liquids of Weak Electrolytes or Non-Electrolytes as SDVSPLwe-ne a.
aSDVSPLwe-ne preparation involves serial decimal or centesimal diluting a “stock” solution. Hitherto, mainly aqueous SDVSPLwe-ne have been studied, but effects of other solvents also was investigated. Aqueous SDVSPLwe-ne are prepared with freshly doubly distilled water or water purified by Simplicity®Water Purification Systems – Millipore, with specific electrical conductivity below 2.5 μS/cm. Dust is removed. Stock solution is analyzed for absence of impurities. Concentration of stock solutions are in the 10-1 – 10-3 M range. After each dilution step, SDVSPLwe-ne are vigorously shaken, e.g., with lab dancer shaker, by vertical vortexing or other methods. Plastic or glass vessels are used. Temperature and pressure are kept constant, typically, respectively, at 298 K and about 1 Atmosphere. As controls, the solvent, e.g., water, is serial diluted and shaken after each dilution step, with all experimental parameters identical to those of SDVSPLwe-ne preparation.
Molecular association in ULC SDVSPLwe-ne initially was revealed with calorimetric, electric conductivity (χ) and pH measurements (Elia and Niccoli, 1999, 2000, 2004). Vigorous shaking after each dilution step is a crucial perturbation required for inducing such association. Serially diluting solutions while omitting the vigorous shaking do not lead to molecular association for C below a solute type dependent critical concentration (Ccrit), i.e., these solutions have the customary infinite diluted solutions characteristics. Typically ~10-10 M < Ccrit <~10-6 M. Not all solutes facilitate molecular association in ULC SDVSPLwe-ne (Konovalov, 2013). The required solute attributes are not yet clarified.
The sizes of associates in ULC SDVSPLwe-ne, their electrokinetic potential (ζ-potential) and their impact on the liquid’s dielectric permittivity were first uncovered with dynamic light scattering (DLS), electrophoresis and dielcometric titrations (Konovalov et al., 2008; Ryzhkina et al., 2009a). Stabilization of the associates occurs during about 1-18 hours after preparation of SDVSPLwe-ne. For solute type dependent C ranges, correlation was observed between ULC SDVSPLwe-ne’s bioactivity, χ, their associates’ effective hydrodynamic diameter and ζ-potential; all these properties non-linear depend on C and are reproducible (Ryzhkina et al., 2009a,b, 2010a,b, 2011a-e, 2012a-d, 2013; Konovalov et al., 2014a-c).
The origins of the physical, chemical, catalytic and bio-active properties of associates in ULC SDVSPLwe-ne are not yet clarified. As to forces underlying the molecular association in ULC SDVSPLwe-ne, electro-dynamic ones play a crucial role. As first demonstrated in 2011 by Konovalov’s group, on storing samples under hypo electro-magnetic conditions, i.e., in a Permalloy container with residual field of 10 nano Tesla, no associates are observable for 10-20M<C<Cthr; a weak electro-magnetic field (EMF) influences the associates for Cthr <C<~10-4 M (Ryzhkina et al., 2011d, 2012a, c, 2013; Konovalov, 2013, 2014a-c). The threshold concentration Cthr is solute type dependent, typically 10-10 M<Cthr<10-6 M. These findings signify explaining ULC SDVSPLwe-ne phenomena necessitates electrodynamic theory. The quantum electrodynamic (QED) model for SDVSPLwe-ne proposed by Yinnon and Yinnon (2011) has provided consistent explanations for various phenomena, e.g., SDVSPLwe-ne’s heat of mixing, χ and their dependence on time and volume (Yinnon and Elia, 2013).
The goals of this paper are: (a) employing the QED model of SDVSPLwe-ne for explaining recently observed (and to the best of our knowledge yet unexplained) characteristics of the various associate types present in these liquids; (b) elucidating the associates’ impacts on these liquids’ properties, e.g., their dielectric permittivity, spectra and bioactivity. This paper is the third of a series on associates in serial diluted solutions printed in this journal’s issue. In the first paper of the series, we presented a summary of the main QED theory aspects of polar liquids in general, and water in particular, relevant to serial diluted solutions. Hitherto this theory mainly is employed for explaining special phenomena and therefore readers may be unfamiliar with these aspects. In the second paper: we summarized the model proposed by Yinnon and Yinnon (2011) for serial diluted vigorously shaken aqueous strong electrolyte solutions (SDVSASES), and we showed that the model’s predictions pertaining to the properties of the associates present in these liquids conform to the measured ones. In the current paper, in its Theory section we concisely summarize the SDVSPLwe-ne model proposed by Yinnon and Yinnon (2011). The model is similar to that of SDVSASES, but varies from it in several aspects. For preventing duplication, we will refer to the two preceding papers for detailing and explaining some QED properties of associates in polar liquids. In the Discussion Section we focus on recently reported measured SDVSPLwe-ne properties and show these conform to those predicted by the model. At the end of this section we conjecture possible mechanisms for serial diluted vigorously shaken solutions affecting biosystems. We stress we do not present any new experimental results — our discussions pertain to previous reported experimental data. A list with abbreviations is presented at the end of this paper.
Theory
Properties of ULC SDVSPLwe-ne containing molecular associates considerably differ from those predicted by the customary theories of equilibrated polar liquids and their ULC solutions. According to the customary theories: below a solute type dependent concentration all solutes solvate; solvated solutes distribute homogenously, move independently and randomly; polar solvent molecules (except solvation shells’ solvent molecules) move randomly; for aqueous solutions, its water molecules (H2O) form flickering hydrogen-bond networks; electromagentic radiation, serial dilutions or vigorous shaking do not affect ULC solutions (Horne, 1972). These customary models explicitly include electrostatic forces and assume electrodynamic ones can be treated perturbatively. In contrast, QED theory explicitly includes electrodynamic forces.
QED indicates that EMF interactions with electrolytic solutes, polar solute molecules or with solvent molecules with sufficiently large electric dipole moments may lead to formation of various QED domains types. Formation of the domains occurs only in specific C ranges (including ULC). These ranges depend on solute type. The domains were generally denoted as “CD” — a shortening for “coherence domains”. CD may agglomerate into supra-domains (supra-CD). Supra-CD are not ensembles of molecules but agglomerates of domains, like domains in liquid crystals. Details of the QED theory of polar liquids and their various hitherto identified CD, their schematic pictures, their properties, the physics underlying CD formation’s dependence on concentration, citations of experimental data verifying aforementioned and the reason underlying the name “coherence domain” are presented in the first paper of this series (Yinnon and Liu, 2015a).
Coherence domains
The CD types relevant to the SDVSPLwe-ne model include CDplasma, IPDplasma, CDrot and CDelec. Below we concisely summarize their properties. Some of these vary from those for solutions of strong electrolytes, summarized in Yinnon and Liu (2015b).
• CDplasma — these domains only form when the solvent molecules have a sufficiently strong electric dipole moment. CDplasma are composed of few solvated solutes and numerous solvent molecules (Del Giudice et al., 2000). The plasma oscillations of these solvated solutes are coherent. Interactions between the solutes and tetra Herz to mega Herz EMF underlie the coherence. CDplasma are not micelles b.
bA micelle is an aggregate of surfactant molecules. In aqueous solutions, its molecules’ hydrophilic “head” regions are in contact with surrounding solvent, sequestering their hydrophobic single “tail” regions in the micelle’s centre. The critical concentration of the surfactant molecules above which micelles form and all additional surfactants added to the system go to micelles is denoted critical micelle concentration.
CDplasma are very stable domains. Energy gained by a solute particle on its incorporation in CDplasma amounts to a few eV. Weak electrolytes only organize in CDplasma for ![]() <C<
<C<![]() . [This is not the case for solutions of strong electrolytes, in which CDplasma form at all concentrations above
. [This is not the case for solutions of strong electrolytes, in which CDplasma form at all concentrations above ![]() ]. Solute characteristics determine the transition concentrations
]. Solute characteristics determine the transition concentrations ![]() and
and ![]() . Typically:
. Typically: ![]() <1M (Del Giudice et al., 2000); is of the order of 10-4 – 10-6 M (Yinnon and Yinnon, 2012).
<1M (Del Giudice et al., 2000); is of the order of 10-4 – 10-6 M (Yinnon and Yinnon, 2012). ![]() is the concentration at which the distance between identical nearest neighbor solutes equals the Debye length. As to non-electrolytes, only those with sufficiently large permanent or by the solvent induced electric dipole moments can organize in CDplasma. Just as for weak electrolytes this only occurs for
is the concentration at which the distance between identical nearest neighbor solutes equals the Debye length. As to non-electrolytes, only those with sufficiently large permanent or by the solvent induced electric dipole moments can organize in CDplasma. Just as for weak electrolytes this only occurs for ![]() <C<
<C<![]() . The diameter of CDplasma is of the order of 10-6 m. It is an inverse function of concentration, i.e., when the concentration decreases, the size of CDplasma increases, the number of its solvated solutes diminishes and the number of its solute molecules enhances.
. The diameter of CDplasma is of the order of 10-6 m. It is an inverse function of concentration, i.e., when the concentration decreases, the size of CDplasma increases, the number of its solvated solutes diminishes and the number of its solute molecules enhances.
• IPDplasma — these domains only form when the solvent molecules have a sufficiently strong electric dipole moment. As to solutions of non-electrolytic compounds, IPDplasma only form when the solvated solutes have sufficiently strong electric dipole moments. IPDplasma formation requires C is below the transition concentration ![]() (Yinnon and Yinnon, 2012). IPDplasma are composed of few solvated solutes and numerous solvent molecules. IPDplasma are not micelles. The plasma oscillations of the solutes in IPDplasma are in phase i.e., an IPDplasma is a special CD — an In-Phase Domain. Also the plasma oscillations of its solvent molecules are in phase. Interactions between its molecules and tetra Herz to mega Herz EMF underlie all these in phase plasma oscillations. IPDplasma are crystalline structured. The dipole moments of their solvent molecules are spherical symmetric aligned around their crystalline ordered solvated solutes. IPDplasma are very stable domains, slightly more stable than CDplasma. On diluting below
(Yinnon and Yinnon, 2012). IPDplasma are composed of few solvated solutes and numerous solvent molecules. IPDplasma are not micelles. The plasma oscillations of the solutes in IPDplasma are in phase i.e., an IPDplasma is a special CD — an In-Phase Domain. Also the plasma oscillations of its solvent molecules are in phase. Interactions between its molecules and tetra Herz to mega Herz EMF underlie all these in phase plasma oscillations. IPDplasma are crystalline structured. The dipole moments of their solvent molecules are spherical symmetric aligned around their crystalline ordered solvated solutes. IPDplasma are very stable domains, slightly more stable than CDplasma. On diluting below ![]() , CDplasma transform into IPDplasma, i.e., the coherent plasma oscillations of the domains’ solvated solutes become in phase. The diameter of IPDplasma equals that of CDplasma at
, CDplasma transform into IPDplasma, i.e., the coherent plasma oscillations of the domains’ solvated solutes become in phase. The diameter of IPDplasma equals that of CDplasma at ![]() , i.e., about 10-6 m. In contrast to the case for CDplasma, the diameter of IPDplasma does not significantly change with concentration. On diluting solutions below , the number of IPDplasma diminishes.
, i.e., about 10-6 m. In contrast to the case for CDplasma, the diameter of IPDplasma does not significantly change with concentration. On diluting solutions below , the number of IPDplasma diminishes.
• CDrot — these domains only form when the solvent molecules have a sufficiently strong electric dipole moment. These domains are composed of ferroelectric ordered solvent molecules (Del Giudice et al., 1988; Del Giudice and Vitiello, 2006). The molecules constituting CDrot coherently oscillate between two rotational states. CDrot formation results from the dipole moments of their molecules interacting with Far Infra Red (FIR) EMF. CDrot have an electric dipole moment due to the ferroelectric ordering of their solvent molecules. In bulk water and most other polar liquids at ambient conditions CDrot do not auto-organize. However, immersing objects with sizable asymmetric charge distributions (e.g., macromolecules, hydrophylic membranes) may induce their formation, resulting in a permanent time dependent polarization. Solutes are pulled into CDrot. Few solute particles can locate in CDrot and do not wreck their host. Many solute molecules destroy CDrot. Solute and solvent types determine critical C below which CDrot persist (![]() ). CDrot’s diameter is of the order of 10-4 – 10-5 m.
). CDrot’s diameter is of the order of 10-4 – 10-5 m.
• CDelec — these domains are composed of electronically excited solvent molecules. Only for water the characteristics of these domains were derived (Arani et al., 1995; Bono et al., 2012 ). The CDelec present in water were denoted by ![]() . These domains are composed of solvent molecules only.
. These domains are composed of solvent molecules only.![]() cannot contain solutes. Solvated solutes or aggregates of solutes (e.g., CDplasma or IPDplasma) locate adjacent to
cannot contain solutes. Solvated solutes or aggregates of solutes (e.g., CDplasma or IPDplasma) locate adjacent to ![]() . The H2O constituting coherently oscillate between their electronic ground |0⟩ state and an excited |b⟩ state.
. The H2O constituting coherently oscillate between their electronic ground |0⟩ state and an excited |b⟩ state. ![]() formation is mediated by ultraviolet (UV) EMF. One electron of an H2O residing in its |b⟩ state is almost free (binding energy of about 0.4 eV). Hence, a
formation is mediated by ultraviolet (UV) EMF. One electron of an H2O residing in its |b⟩ state is almost free (binding energy of about 0.4 eV). Hence, a ![]() is a pool of ~106 quasi-free electrons located at their boundary, and correspondingly an ensemble of quasi free protons (the partners of the quasi-free electrons). At ambient conditions, in bulk water: the fraction of H2O included in
is a pool of ~106 quasi-free electrons located at their boundary, and correspondingly an ensemble of quasi free protons (the partners of the quasi-free electrons). At ambient conditions, in bulk water: the fraction of H2O included in ![]() is about 20 percent; H2O continually adsorb on
is about 20 percent; H2O continually adsorb on ![]() while simultaneously H2O desorb, causing a ~10-14 s timescale flickering landscape. Thus
while simultaneously H2O desorb, causing a ~10-14 s timescale flickering landscape. Thus ![]() observation requires fast resolution probes. CDrot, CDplasma, IPDplasma or other aggregates of solutes may stabilize
observation requires fast resolution probes. CDrot, CDplasma, IPDplasma or other aggregates of solutes may stabilize ![]() , i.e., reduce their flickering and ease their observation.
, i.e., reduce their flickering and ease their observation. ![]() and supra-
and supra-![]() may get encapsulated in CDrot and supra-CDrot . Such assemblies we denote [supra-CDrot <supra-
may get encapsulated in CDrot and supra-CDrot . Such assemblies we denote [supra-CDrot <supra-![]() >]. The state of H2O belonging to both CDrot and
>]. The state of H2O belonging to both CDrot and ![]() is a superposition of the state typifying the H2O constituting CDrot and the state typifying the H2O constituting
is a superposition of the state typifying the H2O constituting CDrot and the state typifying the H2O constituting ![]() . The diameter of
. The diameter of ![]() is about 10-7 m.
is about 10-7 m.
To give the reader an intuitive feeling for the relative sizes of the CDrot, CDplasma and ![]() , we note their ratios are similar, respectively, to those of the sun, earth and moon.
, we note their ratios are similar, respectively, to those of the sun, earth and moon.
Superfluidic domains
CDrot, IPDplasma and ![]() are superfluidic domains, i.e., their molecules do not collide (see Yinnon and Liu, 2015a). CDplasma are not superfluidic. The superfluidity of CD has implications for the liquid’s properties, e.g., its electric conductivity.
are superfluidic domains, i.e., their molecules do not collide (see Yinnon and Liu, 2015a). CDplasma are not superfluidic. The superfluidity of CD has implications for the liquid’s properties, e.g., its electric conductivity.
Schematic pictures of aqueous solutions according to QED
Figure 1 presents schematic pictures of serial diluted aqueous solutions of weak- or non-electrolytic compounds for 10-20 M<C<1 M. The (a) and (b) series pertain to solutions which, respectively, were not vigorously shaken and those which were vigorously shaken after each dilution step. Figure 1 is similar to the schematic picture of aqueous solutions of strong electrolytes presented in Figure 1 in the preceding paper (Yinnon and Liu, 2015b). The main differences are that in solutions of weak- or non-electrolytic compounds: none or only few solutes solvate for C above ![]() ; solvated solutes only organize in CDplasma for
; solvated solutes only organize in CDplasma for ![]() <C<
<C<![]() . Hence Figure 1ia communicates that for concentration above
. Hence Figure 1ia communicates that for concentration above ![]() the solvated solutes move randomly, i.e., do not organize in CDplasma, and aggregates of non-solvated solutes are present. Figure 1 iia shows that for
the solvated solutes move randomly, i.e., do not organize in CDplasma, and aggregates of non-solvated solutes are present. Figure 1 iia shows that for ![]() <C<
<C<![]() solvated solutes organize in CDplasma, and
solvated solutes organize in CDplasma, and ![]() and supra-
and supra-![]() are stabilized by CDplasma. Figure 1iia and Figure 1iiia exhibit the transition of CDplasma into IPDplasma. On comparing Figure 1iiia, Figure 1iva and Figure 1v, one discerns that the diameter of IPDplasma does not significantly change with concentration. Instead on diluting, the number of IPDplasma diminishes. On diluting also the number of non-solvated solutes diminishes. Figures 1 vi-viiia illustrate that in very dilute solutions, IPDplasma do not form and all solvated solutes locate randomly.
are stabilized by CDplasma. Figure 1iia and Figure 1iiia exhibit the transition of CDplasma into IPDplasma. On comparing Figure 1iiia, Figure 1iva and Figure 1v, one discerns that the diameter of IPDplasma does not significantly change with concentration. Instead on diluting, the number of IPDplasma diminishes. On diluting also the number of non-solvated solutes diminishes. Figures 1 vi-viiia illustrate that in very dilute solutions, IPDplasma do not form and all solvated solutes locate randomly.
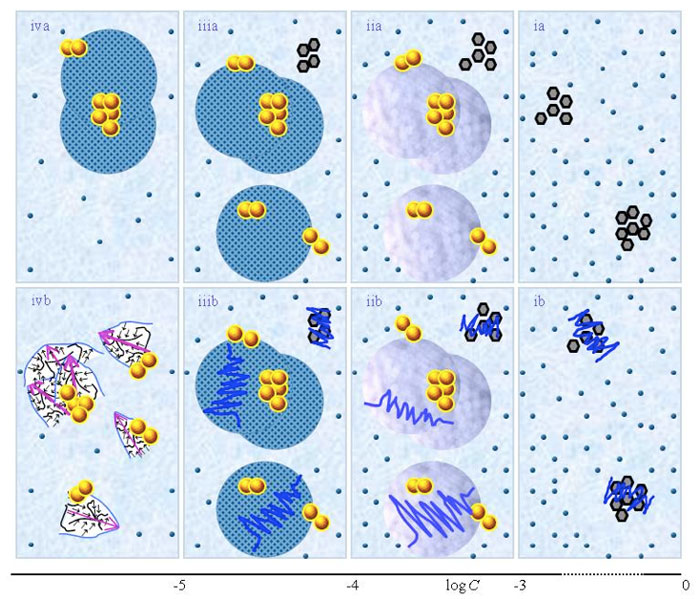
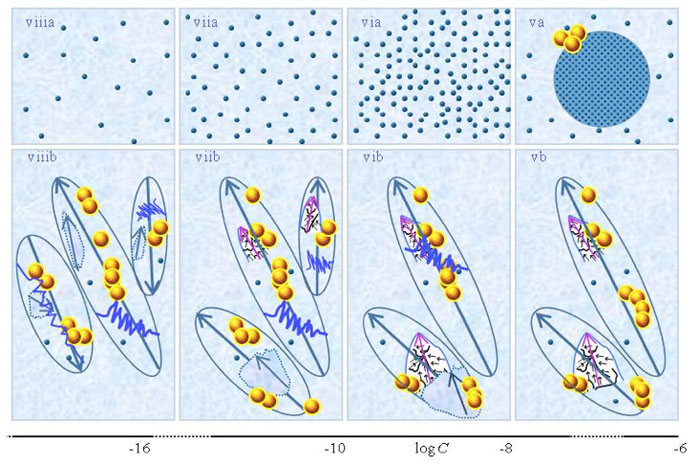
Figure 1: This figure presents a schematic picture of serial diluted solutions of weak electrolytes or non-electrolytic compounds. The top row (a) and bottom row (b) series pertain to solutions which, respectively, were not vigorously shaken and those which were vigorously shaken after each dilutions step. Figure ia illustrates that for C>![]() all solvated solutes move randomly, i.e., do not organize in CDplasma. The tiny blue balls represent randomly moving ~10-9 – 10-8 m solvated solutes. The irregular shaped bunches of black hexagons represent aggregates of non-solvated solutes. Figure iia illustrates that on dilution below
all solvated solutes move randomly, i.e., do not organize in CDplasma. The tiny blue balls represent randomly moving ~10-9 – 10-8 m solvated solutes. The irregular shaped bunches of black hexagons represent aggregates of non-solvated solutes. Figure iia illustrates that on dilution below ![]() , solvated solutes organize in CDplasma (symbolized with purple-blue colored balls). The yellow-brown balls and their agglomerates represent, respectively, ~10-7m
, solvated solutes organize in CDplasma (symbolized with purple-blue colored balls). The yellow-brown balls and their agglomerates represent, respectively, ~10-7m ![]() and supra-
and supra-![]() stabilized by CDplasma. Figures iia and iiia illustrate the transformation of CDplasma, into IPDplasma at C~
stabilized by CDplasma. Figures iia and iiia illustrate the transformation of CDplasma, into IPDplasma at C~![]() . Figures ia-iiia illustrate that on dilution the non-solvated solutes diminish, i.e., solvate. Figures iiia-va illustrate that on dilution the diameter of IPDplasma does not change, but the number of IPDplasma diminishes. Figures via-viiia illustrate that below a certain concentration there are insufficient solutes to form IPDplasma. The concentration below which no IPDplasma form has yet not been theoretically derived. Figures via-viiia illustrate that whenever there are too few solutes to form IPDplasma, the solution has the characteristics predicted by the customary models, i.e., all solvated solutes move randomly and their number diminishes on dilution. In the Figure 1b series, the blue zigzag curves symbolize that shaking excites or cracks domains and aggregates. Figure iib illustrates that excitations or cracking does not significantly alter the internal structure of CDplasma, which just as in Figure 1iia are represented with purple-blue colored balls. Figures iib and iiib illustrate the transition from CDplasma to IPDplasma, with the latter pictured as blue-crystalline balls just as in Figure iiia. Figures iiib and ivb illustrate that shaking excites or breaks up IPDplasma. The excited or broken IPDplasma pieces, which in the text we denoted electric dipole aggregates (EDAIPDplasma), are pictured as irregular shaped aggregates in (ivb). Their aligned black arrows orderings symbolize EDAIPDplasma’s distorted ferroelectric H2O orderings. The purple arrow in the EDAIPDplasma symbolizes these domains’ dipole moments. Figures ivb and vb illustrate that on diluting below a solute type dependent critical concentration (
. Figures ia-iiia illustrate that on dilution the non-solvated solutes diminish, i.e., solvate. Figures iiia-va illustrate that on dilution the diameter of IPDplasma does not change, but the number of IPDplasma diminishes. Figures via-viiia illustrate that below a certain concentration there are insufficient solutes to form IPDplasma. The concentration below which no IPDplasma form has yet not been theoretically derived. Figures via-viiia illustrate that whenever there are too few solutes to form IPDplasma, the solution has the characteristics predicted by the customary models, i.e., all solvated solutes move randomly and their number diminishes on dilution. In the Figure 1b series, the blue zigzag curves symbolize that shaking excites or cracks domains and aggregates. Figure iib illustrates that excitations or cracking does not significantly alter the internal structure of CDplasma, which just as in Figure 1iia are represented with purple-blue colored balls. Figures iib and iiib illustrate the transition from CDplasma to IPDplasma, with the latter pictured as blue-crystalline balls just as in Figure iiia. Figures iiib and ivb illustrate that shaking excites or breaks up IPDplasma. The excited or broken IPDplasma pieces, which in the text we denoted electric dipole aggregates (EDAIPDplasma), are pictured as irregular shaped aggregates in (ivb). Their aligned black arrows orderings symbolize EDAIPDplasma’s distorted ferroelectric H2O orderings. The purple arrow in the EDAIPDplasma symbolizes these domains’ dipole moments. Figures ivb and vb illustrate that on diluting below a solute type dependent critical concentration (![]() ) CDrot get stabilized by EDAIPDplasma, i.e., the irregular shaped EDAIPDplasma are located within the elongated ovals representing CDrot. The dark blue arrows symbolize the dipole moment of CDrot. Figure vib shows that vigorous shaking excites or breaks up CDrot, thus creating EDACDrot. The chunk outlined with an irregular shaped broken curve and located at the bottom of one of the left CDrot represents the EDACDrot. Figures vib-viib show that at certain concentrations both EDAIPDplasma and EDACDrot are present within CDrot, though the sizes of EDAIPDplasma diminish with concentration. Figire viiib shows that on diluting further, no EDAIPDplasma persist, i.e., there are too few solute particles to sustain EDAIPDplasma. At these concentrations, vigorous shaking just breaks up CDrot and creates new EDACDrot. These in turn stabilize new CDrot, as pictured in Figure viiib. Figures vb-viib illustrate that CDrot may align with their dipole moments parallel. Figure viiib illustrates that at certain concentrations their dipoles may be aligned anti-parallel. (Note that the sizes of the various domains, their broken pieces and the sizes of the solvated solutes with their hydration shells are not presented according to their realistic scale ratios.)
) CDrot get stabilized by EDAIPDplasma, i.e., the irregular shaped EDAIPDplasma are located within the elongated ovals representing CDrot. The dark blue arrows symbolize the dipole moment of CDrot. Figure vib shows that vigorous shaking excites or breaks up CDrot, thus creating EDACDrot. The chunk outlined with an irregular shaped broken curve and located at the bottom of one of the left CDrot represents the EDACDrot. Figures vib-viib show that at certain concentrations both EDAIPDplasma and EDACDrot are present within CDrot, though the sizes of EDAIPDplasma diminish with concentration. Figire viiib shows that on diluting further, no EDAIPDplasma persist, i.e., there are too few solute particles to sustain EDAIPDplasma. At these concentrations, vigorous shaking just breaks up CDrot and creates new EDACDrot. These in turn stabilize new CDrot, as pictured in Figure viiib. Figures vb-viib illustrate that CDrot may align with their dipole moments parallel. Figure viiib illustrates that at certain concentrations their dipoles may be aligned anti-parallel. (Note that the sizes of the various domains, their broken pieces and the sizes of the solvated solutes with their hydration shells are not presented according to their realistic scale ratios.)
SDVSPLwe-ne model
Figures 1ib-viiib present a schematic picture of the SDVSPLwe-ne model proposed by Yinnon and Yinnon (2011), i.e., the structure of SDVSPLwe-ne for different concentration ranges. Its details we discuss in the following paragraphs. We emphasize that the model only holds when the solvent molecules have a sufficiently strong electric dipole moment.
i. For C above the transition concentration ![]() , few or none weak- or non-electrolytic solutes solvate. Most solutes do not solvate. The few solvated solutes move randomly — see Figure 1ib. Aggregates of unsolvated solutes may stabilize
, few or none weak- or non-electrolytic solutes solvate. Most solutes do not solvate. The few solvated solutes move randomly — see Figure 1ib. Aggregates of unsolvated solutes may stabilize ![]() and may form supra-
and may form supra-![]() . For C in between the transitions concentrations, i.e.,
. For C in between the transitions concentrations, i.e., ![]() <C<
<C<![]() , solvated solutes organizing in CDplasma enhances (drives) the solvation process. At these C, part of the solvated weak electrolytes organize in CDplasma and supra-CDplasma (see Figure 1iib). As to solvated non-electrolytic compounds, only those having a sufficiently large electric dipole moment organize in such domains. CDplasma and supra-CDplasma may stabilize
, solvated solutes organizing in CDplasma enhances (drives) the solvation process. At these C, part of the solvated weak electrolytes organize in CDplasma and supra-CDplasma (see Figure 1iib). As to solvated non-electrolytic compounds, only those having a sufficiently large electric dipole moment organize in such domains. CDplasma and supra-CDplasma may stabilize ![]() and supra-
and supra-![]() . The aforesaid holds independent of the solutions’ preparation procedure, i.e., not just for SDVSPLwe-ne but also for solutions prepared without serial dilutions or vigorous shaking. Serial dilutions or vigorous shaking affect CDplasma, mainly causing their breakup. However, after perturbations are over CDplasma reform, as illustrated in Figure 1iib.
. The aforesaid holds independent of the solutions’ preparation procedure, i.e., not just for SDVSPLwe-ne but also for solutions prepared without serial dilutions or vigorous shaking. Serial dilutions or vigorous shaking affect CDplasma, mainly causing their breakup. However, after perturbations are over CDplasma reform, as illustrated in Figure 1iib.
ii. At C~![]() , CDplasma containing weak electrolytes transform into IPDplasma (see Figs.1iib-iiib). The transformation modifies electric conductivity and its dependence on C and time, because IPDplasma are superfluidic and crystalline structured while CDplasma are not superfluidic. As to solvated non-electrolytes, only those having a sufficiently large electric dipole moment organize in IPDplasma. Differences between the transition concentrations
, CDplasma containing weak electrolytes transform into IPDplasma (see Figs.1iib-iiib). The transformation modifies electric conductivity and its dependence on C and time, because IPDplasma are superfluidic and crystalline structured while CDplasma are not superfluidic. As to solvated non-electrolytes, only those having a sufficiently large electric dipole moment organize in IPDplasma. Differences between the transition concentrations ![]() and
and ![]() may be tiny, or solutes even might not organize in CDplasma, i.e., these only organize in IPDplasma. Incorporation of solvated solutes in IPDplasma may be the drive behind the solvation process of solutes with low solubility product constants. Critical micelle concentrations may equal
may be tiny, or solutes even might not organize in CDplasma, i.e., these only organize in IPDplasma. Incorporation of solvated solutes in IPDplasma may be the drive behind the solvation process of solutes with low solubility product constants. Critical micelle concentrations may equal ![]() or
or ![]() , but this has not yet been verified. IPDplasma may aggregate in supra-IPDplasma. These domains may stabilize
, but this has not yet been verified. IPDplasma may aggregate in supra-IPDplasma. These domains may stabilize ![]() and supra-
and supra-![]() (see Figure 1 iiib). Dilution below
(see Figure 1 iiib). Dilution below ![]() diminishes the number of randomly moving solvated solutes as well as the number of solutes incorporated in IPDplasma. At very low C the number of solvated solutes is too low for formation of IPDplasma. The aforesaid holds independent of the solutions’ preparation procedure, i.e., not just for SDVSPLwe-ne, but also for solutions prepared without serial dilutions or vigorous shaking.
diminishes the number of randomly moving solvated solutes as well as the number of solutes incorporated in IPDplasma. At very low C the number of solvated solutes is too low for formation of IPDplasma. The aforesaid holds independent of the solutions’ preparation procedure, i.e., not just for SDVSPLwe-ne, but also for solutions prepared without serial dilutions or vigorous shaking.
iii. For solutions containing IPDplasma, their vigorous shaking affects their properties. The effect is the same as that for SDVSASES containing IPDplasma. That is, vigorous shaking excites or breaks up IPDplasma, as pointed out by Yinnon and Yinnon (2011) and Yinnon and Liu (2015b) (see Fig.1ivb). Excitations induce years-long lasting vortices in the superfluidic IPDplasma (Yinnon and Elia, 2013). [For short discussion on these vortices see Yinnon and Liu (2015a).] The vortices partly destroy the spherical symmetric alignments of the dipole moments of the solvent molecules surrounding the crystalline ordered solvated solutes in IPDplasma. Hence excited IPDplasma and their broken pieces have electric dipoles, i.e., are electric dipole aggregates, which we denote as EDAIPDplasma (see Figure 1ivb). EDAIPDplasma have the remnant crystalline structure of their “mother” IPDplasma (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013). (As to CDplasma, only their solvation shells’ few solvent molecules are aligned, i.e., their perturbation, for example vigorous shaking, does not create 10-6 m-sized electric dipole aggregates.)
iv. For aqueous SDVSPLwe-ne, EDAIPDplasma induce electric dipoles in the quasi free electron clouds of ![]() . The interactions between the dipoles of these clouds, as well as between these and the dipole moments of EDAIPDplasma, may stabilize
. The interactions between the dipoles of these clouds, as well as between these and the dipole moments of EDAIPDplasma, may stabilize ![]() and supra-
and supra-![]() (see Figure 1 ivb).
(see Figure 1 ivb).
v. For concentrations less than the critical concentration for CDrot formation (i.e., C<![]() ), due to the interactions between the dipoles of EDAIPDplasma and the dipoles of the solvent molecules, EDAIPDplasma stabilize CDrot and supra-CDrot (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013) — see Fig.1vb. In other words, EDAIPDplasma, due to their significant asymmetric charge distributions, stabilize CDrot. Stabilization of CDrot also may be induced by solutes with sufficient large electric dipoles. Thus even when no IPDplasma and hence EDAplasma form in SDVSPLwe-ne, some types of non-electrolytic solutes still might induce CDrot stabilization for C<
), due to the interactions between the dipoles of EDAIPDplasma and the dipoles of the solvent molecules, EDAIPDplasma stabilize CDrot and supra-CDrot (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013) — see Fig.1vb. In other words, EDAIPDplasma, due to their significant asymmetric charge distributions, stabilize CDrot. Stabilization of CDrot also may be induced by solutes with sufficient large electric dipoles. Thus even when no IPDplasma and hence EDAplasma form in SDVSPLwe-ne, some types of non-electrolytic solutes still might induce CDrot stabilization for C<![]() , and subsequent vigorous shaking might create EDACDrot (and in aqueous solutions supra-CDrot<supra-
, and subsequent vigorous shaking might create EDACDrot (and in aqueous solutions supra-CDrot<supra-![]() >) (Yinnon et al., 2011, 2013).
>) (Yinnon et al., 2011, 2013).
Vigorous shaking excites or breaks up CDrot (see Figure 1vb). Excitations induce years-long vortices in the superfluidic CDrot. Due to ferroelectric ordering of the molecules constituting CDrot, excited or broken CDrot also are electric dipole aggregates, i.e., EDACDrot (see Figure 1vib). Unlike EDAIPDplasma, EDACDrot are not crystalline ordered. Due to interactions between the dipoles of EDACDrot and the polar solvent molecules, EDACDrot also stabilize CDrot. Therefore, serial dilutions with vigorous shaking at each dilution step diminish EDAIPDplasma but EDACDrot persist. These EDACDrot stabilize CDrot and supra-CDrot too. As a result CDrot persist up to ULC and beyond (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013) (see Figures 1vib-viiib). The aforementioned effects of vigorous shaking of SDVSPLwe-ne for C<![]() are the same as those for SDVSASES described in Yinnon and Liu (2015b).
are the same as those for SDVSASES described in Yinnon and Liu (2015b).
vi. In aqueous SDVSPLwe-ne, CDrot induce electric dipoles in the quasi free electron clouds of ![]() . The interactions between the dipole moments of these clouds, as well as between these and the dipole moments of EDACDrot, may stabilize
. The interactions between the dipole moments of these clouds, as well as between these and the dipole moments of EDACDrot, may stabilize ![]() and supra-
and supra-![]() , i.e., cause formation of [supra-CDrot <supra-
, i.e., cause formation of [supra-CDrot <supra-![]() >] (see Figure 1vb) (Del Giudice et al., 2010). The aforementioned also holds for aqueous SDVSASES — see Yinnon and Liu (2015b).
>] (see Figure 1vb) (Del Giudice et al., 2010). The aforementioned also holds for aqueous SDVSASES — see Yinnon and Liu (2015b).
vii. QED domains affect physicochemical properties, requiring adjustment in customary equations. For example adjustments in equations describing electric conductivity are required because the following molecules do not collide: solvent molecules incorporated in the superfluidic domains (i.e, CDelec, CDrot, IPDplasma), solvent molecules in the hydration shells of solvated solutes in CDplasma; solvated solutes incorporated in CDplasma or in IPDplasma. Hence in polar liquids and their solutions, the electric conductivity is an inverse function only of intermolecular collisions involving: the randomly moving solvent and solute molecules not included in the domains; solvent molecules incorporated in CDplasma but not part of the solvation shells of the solutes included in these domains. A decrease of the fractions of these colliding particles enhances the electric conductivity. Also the electric dipole moments of EDAIPDplasma, of CDrot, of EDACDrot and of the quasi free electron clouds of ![]() reduce intermolecular collisions of randomly moving molecules neighboring on these domains. The reductions raise electric conductivity (Yinnon and Yinnon 2011; Yinnon and Elia, 2013). Currently, we are investigating quantitative implications.
reduce intermolecular collisions of randomly moving molecules neighboring on these domains. The reductions raise electric conductivity (Yinnon and Yinnon 2011; Yinnon and Elia, 2013). Currently, we are investigating quantitative implications.
Rendering our above detailed qualitative SDVSPLwe-ne model into quantitative one requires numerous computations, which are beyond this paper’s scope.
Discussion
Correspondence between SDVSPLwe-ne properties predicted by QED and observed ones
Recently published experimental data, which evidence the characteristics of associates present in a SDVSPLwe-ne as predicted by its QED model (summarized in paragraphs i-viii above), we cite and discuss in paragraphs i-viii below.
i. For many weak electrolytes and non-electrolytes in various polar solvents, below a solute type dependent transition concentration of the order of 1 – 10-3 M, part of the solvated solutes and numerous solvent molecules were observed to organize in ~10-7 – 10-6 m sized groupings. These groupings are not micelles. The groupings were observed with techniques like DLS, laser light scattering, static light scattering, nanoparticle tracking analysis, scanning electron microscopy and nuclear magnetic resonance (Li and Ogawa, 2000; Samal and Geckeler, 2001; Kononov et al., 2002; Sedlak, 2006; Sedlak and Rak, 2013; Hagmeyer et al., 2012; Konovalov, 2013; Ryzhkina et al., 2013). These groupings are present in serial diluted vigorously shaken solutions, as well as in solutions not perturbed by these techniques. These groupings have characteristics conforming to those of CDplasma and ![]() (Yinnon and Yinnon, 2009). It should be stressed, however, that for specific concentration ranges, numerous solutes form other cluster types which are not CDplasma.
(Yinnon and Yinnon, 2009). It should be stressed, however, that for specific concentration ranges, numerous solutes form other cluster types which are not CDplasma.
Identifying CDplasma is difficult, as holds for ![]() stabilized by CDplasma. (
stabilized by CDplasma. (![]() may also be stabilized by other cluster types.) EMF screening induced disappearance of groupings promises to be an effective method for exposing their QED nature. Radio frequency screening of tetra Hz to mega Hz EMF destroying ~10-6 m domains and UV EMF screening by Permalloy destroying ~10-7 m domains we expect to be optimal probes for identifying, respectively, CDplasma and
may also be stabilized by other cluster types.) EMF screening induced disappearance of groupings promises to be an effective method for exposing their QED nature. Radio frequency screening of tetra Hz to mega Hz EMF destroying ~10-6 m domains and UV EMF screening by Permalloy destroying ~10-7 m domains we expect to be optimal probes for identifying, respectively, CDplasma and ![]() (Yinnon and Liu, 2015b). An exemplary study partly corroborating this expectation is that of aqueous SDVSPLwe-ne of 2,4,6,8-tetramethyl-2,4,6,8-tetraazabicyclo [3.3.0] octane-3,7-dione (the active ingredient in the tranquilizer Mebicar). For this SDVSPLwe-ne, Ryzhkina et al. (2013) measured its groupings’ diameter under normal conditions (Dlb), i.e., for samples kept at the laboratory bench. Moreover, they measured the groupings’ diameter (Dp) for samples kept in Permalloy containers. In samples kept at the laboratory bench, at ~10-2 M<C<~10-1 M, clusters with Dlb≈ 1.5×10-9 m (hydrated solute molecules) and domains with Dlb≈ 7×10-6 m are present; on diluting to ~10-3 M, the Dlb≈1.5×10-9 m clusters disappear; at ~10-5 M<C<~10-3 M, Dlb≈8×10-8 m and Dlb≈2×10-7 m domains are dominant. Screening samples by Permalloy does not significantly affect the domains present at ~10-2 M<C<~10-1 M, evoking the Dp=Dlb≈7×10-6 m domains are CDplasma. (Recall that the diameter of CDplasma is of the order of 10-6 m.) Radio frequency screening of samples is called for to verify this evocation. Screening by Permalloy reduces prevalence and dominance of the ~8×10-8 m and ~2×10-7 m domains present at ~10-5 M<C<~10-3 M; due to their diminished dominance, the Dp≈7×10-6 m domains again become observable. Available electric conductivity data is insufficient for exposing a
(Yinnon and Liu, 2015b). An exemplary study partly corroborating this expectation is that of aqueous SDVSPLwe-ne of 2,4,6,8-tetramethyl-2,4,6,8-tetraazabicyclo [3.3.0] octane-3,7-dione (the active ingredient in the tranquilizer Mebicar). For this SDVSPLwe-ne, Ryzhkina et al. (2013) measured its groupings’ diameter under normal conditions (Dlb), i.e., for samples kept at the laboratory bench. Moreover, they measured the groupings’ diameter (Dp) for samples kept in Permalloy containers. In samples kept at the laboratory bench, at ~10-2 M<C<~10-1 M, clusters with Dlb≈ 1.5×10-9 m (hydrated solute molecules) and domains with Dlb≈ 7×10-6 m are present; on diluting to ~10-3 M, the Dlb≈1.5×10-9 m clusters disappear; at ~10-5 M<C<~10-3 M, Dlb≈8×10-8 m and Dlb≈2×10-7 m domains are dominant. Screening samples by Permalloy does not significantly affect the domains present at ~10-2 M<C<~10-1 M, evoking the Dp=Dlb≈7×10-6 m domains are CDplasma. (Recall that the diameter of CDplasma is of the order of 10-6 m.) Radio frequency screening of samples is called for to verify this evocation. Screening by Permalloy reduces prevalence and dominance of the ~8×10-8 m and ~2×10-7 m domains present at ~10-5 M<C<~10-3 M; due to their diminished dominance, the Dp≈7×10-6 m domains again become observable. Available electric conductivity data is insufficient for exposing a ![]() , i.e., currently it is impossible to designate the Dlb=Dp≈7×10-6 m domains present at 10-2 M<C<~10-1 M and at ~10-5 M<C<~10-3 M, respectively, as CDplasma or IPDplasma. The fact that screening by Permalloy does destroy part but not all ~8×10-8 m and ~2×10-7 m domains implies some of these might be
, i.e., currently it is impossible to designate the Dlb=Dp≈7×10-6 m domains present at 10-2 M<C<~10-1 M and at ~10-5 M<C<~10-3 M, respectively, as CDplasma or IPDplasma. The fact that screening by Permalloy does destroy part but not all ~8×10-8 m and ~2×10-7 m domains implies some of these might be ![]() . (Recall that the diameters of
. (Recall that the diameters of ![]() and supra-
and supra-![]() are of the order of 10-7 M.) Those ~8×10-8 m and ~2×10-7 m domains which were not destroyed by the Permalloy screening might be EDAplasma — as perhaps radio frequency screening can reveal. ζ-potential data provides additional evidence for presence of
are of the order of 10-7 M.) Those ~8×10-8 m and ~2×10-7 m domains which were not destroyed by the Permalloy screening might be EDAplasma — as perhaps radio frequency screening can reveal. ζ-potential data provides additional evidence for presence of ![]() . At ~10-5 M <C<~10-3 M, the ζ-potential varies between -11.5 mV and -13 mV, which is compatible with the ζ-potential of
. At ~10-5 M <C<~10-3 M, the ζ-potential varies between -11.5 mV and -13 mV, which is compatible with the ζ-potential of ![]() , as discussed below in paragraph vi.
, as discussed below in paragraph vi.
For C<~10-6 M , 10-7 m and 10-6 m domains are present, but these disappear on screening by Permalloy, i.e., these are not CDplasma or IPDplasma. A conclusion commensurate with the QED model, which predicts that at such low C the number of solutes is too small for forming a significant number of these domains. The domains present at C<~10-6 M we discuss below in paragraph v.d.
ii. The domains observed in numerous ~10-6 M<C<~10-4 M weak- and non-electrolytes solutions, with properties differing from those of the domains present for C>~10-4 M, might be IPDplasma . Albeit, these could be other cluster types, e.g., micelles. Radio frequency screening destroying the domains will be required for identifying their IPDplasma nature. While we await such screening outcomes, for some exemplary solutions we present physicochemical data attributable to IPDplasma presence and ![]() stabilized by these domains:
stabilized by these domains:
(a) For aqueous SDVSPLwe-ne of Melafen [the melamine salt of bis(hydroxymethyl)phosphinic acid dihydrate] in water (with 1% D2O) Konovalov et al. (2008) found: their dilution from ~1×10-3 M to ~1×10-4 M reduces the UV absorption coefficient from 31900 to 30000, but on further dilution it increases to 34700 and 43500, respectively, for ~1×10-5 M and ~1×10-6 M; other UV spectral features indicate at ~2×10-3 M the solution has a salt character; Infra Red (IR) spectra indicate for ~8×10-2 M<C<~1.6×10-1 M Melafen dissociates into anions and cations with their hydration shell surrounded by a mobile H2O network; with the ionic nature of aqueous Melafen for C>~10-3 M, the reduction in UV absorption observed on diluting from ~1×10-3 M to ~1×10-4 M corresponds to that typical for salt solutions, while the increase in UV absorption for C<~10-4 M is attributable to association of ions. The aforementioned concurs with the ionic associates present at C<~10-4 M being IPDplasma, which stabilize ![]() interacting with UV EMF. Also for SDVSPLwe-ne of hemin derivatives at ~1×10-6 M<C<~1×10-4 M Ryzhkina et al. (2011c) measured UV absorbance. They concluded its enhancements with dilution are ascribable to various types of aggregates. As to their nature, e.g., are these IPDplasma which stabilize
interacting with UV EMF. Also for SDVSPLwe-ne of hemin derivatives at ~1×10-6 M<C<~1×10-4 M Ryzhkina et al. (2011c) measured UV absorbance. They concluded its enhancements with dilution are ascribable to various types of aggregates. As to their nature, e.g., are these IPDplasma which stabilize ![]() , the current available data is insufficient for reaching at conclusions and EMF screening is called for.
, the current available data is insufficient for reaching at conclusions and EMF screening is called for.
(b) For aqueous SDVSPLwe-ne of the antioxidant Ichfan C-10 (a 2,6-dialkylphenol derivative), Ryzhkina et al. (2009a, 2011d) showed:
1. For C above the critical micelle concentration (~1×10-3 M) , surface tension of samples kept at the laboratory bench (σlb) or in Permalloy containers (σp) indicate it is a functional cationic surfactant. Electrophoresis evidence their micelles’ ζ-potential varies from 40 to 15 mV.
2. On diluting from ~1×10-3 M to ~5×10-7 M, Dlb increases from ~1.0×10-7 m to ~1.6×10-7 m, the ζ-potential of associates in samples kept at the laboratory bench (ζlb) drops to -12 mV, and σlb strongly increases approaching that of double distilled water thus indicating the domains are not micelles or pre-micelle aggregates.
3. For samples kept on the laboratory bench, domain size distributions are unimodal for ~10-6 M<C<~10-3 M. However, for samples kept for 18 hours in Permalloy containers the distributions are not unimodal for ~10-9 M<C<~10-5 M. Clusters with ~10-9 m <Dp<~10-8 m and domains with ~10-7 m <Dp<~10-6 m persist.
4. At ~10-7 M<C<~10-5 M, the electric conductivity of samples kept at the laboratory bench (χlb) are slightly larger than the electric conductivity of samples kept in Permalloy containers (χP). For this concentration range also σlb is slightly larger than σp.
Paragraphs 2-4 mentioned changes in physicochemical variables observed on diluting from ~10-3 M to ~10-7 M indeed are attributable to micelles transforming to IPDplasma which in turn stabilize ![]() , e.g.: Dp≈10-6 m conforms to the ~10-6 m diameter of IPDplasma; Dlb≈10-7 m conforms to the ~10-7 m diameter of
, e.g.: Dp≈10-6 m conforms to the ~10-6 m diameter of IPDplasma; Dlb≈10-7 m conforms to the ~10-7 m diameter of ![]() ; ζlb= -12 mV is ascribable to the quasi free electrons of
; ζlb= -12 mV is ascribable to the quasi free electrons of ![]() ; χlb versus χP and σlb versus σP discrepancies are attributable to the ζ-potential and the dipole moment of the distorted clouds of quasi free electrons of
; χlb versus χP and σlb versus σP discrepancies are attributable to the ζ-potential and the dipole moment of the distorted clouds of quasi free electrons of ![]() . The χlb versus χP discrepancies are also attributable to the superfluidity of
. The χlb versus χP discrepancies are also attributable to the superfluidity of ![]() . Studying the effects of radio frequency screening is required for evidencing the IPDplasma nature of the domains. Such screening is also desirable for other solutions, e.g., aqueous SDVSPLwe-ne of potasium phenosan, α-tocopherol or membranotropic amphiphilic calix[4]resorcinarene with tris(hydroxymethyl)methylamide functional groups, because Ryzhkina et al. (2012a,c) observed these solutions have physicochemical properties similar to aforementioned ones for Ichfan C-10.
. Studying the effects of radio frequency screening is required for evidencing the IPDplasma nature of the domains. Such screening is also desirable for other solutions, e.g., aqueous SDVSPLwe-ne of potasium phenosan, α-tocopherol or membranotropic amphiphilic calix[4]resorcinarene with tris(hydroxymethyl)methylamide functional groups, because Ryzhkina et al. (2012a,c) observed these solutions have physicochemical properties similar to aforementioned ones for Ichfan C-10.
iii. Creation of EDAIPDplasma by vigorous shaking of solutions requires IPDplasma presence, which as discussed above, still has to be unambiguous evidenced for SDVSPLwe-ne. Radio frequency screening and Fourier transform analyses of the molar conductivity dependence on time for assessing EDAIPDplasma presence are called for. The benefits of such Fourier transform analyses is discussed in Yinnon and Liu (2015b).
iv. As to ![]() stabilized by EDAplasma, their presence in aqueous SDVSPLwe-ne can be revealed by Dlb, Dp, χlb and χP data. However, clusters other than EDAIPDplasma, e.g., micelles or pre-micelle aggregates, might also underlie
stabilized by EDAplasma, their presence in aqueous SDVSPLwe-ne can be revealed by Dlb, Dp, χlb and χP data. However, clusters other than EDAIPDplasma, e.g., micelles or pre-micelle aggregates, might also underlie ![]() stabilization. While we wait for verification of presence of EDAIPDplasma by the techniques mentioned in paragraph iii, in the meanwhile for some exemplary SDVSPLwe-ne we present physicochemical data consistent with
stabilization. While we wait for verification of presence of EDAIPDplasma by the techniques mentioned in paragraph iii, in the meanwhile for some exemplary SDVSPLwe-ne we present physicochemical data consistent with ![]() presence. For aqueous SDVSPLwe-ne of Ichfan C-10, potasium phenosan, and α-tocopherol, Ryzhkina et al. (2011d, 2012a, 2012c) showed EMF affect these SDVSPLwe-ne differently at ~10-20 M<C<Cthr versus at Cthr<C<~10-3 M with ~10-10 M<Cthr< ~10-6 M:
presence. For aqueous SDVSPLwe-ne of Ichfan C-10, potasium phenosan, and α-tocopherol, Ryzhkina et al. (2011d, 2012a, 2012c) showed EMF affect these SDVSPLwe-ne differently at ~10-20 M<C<Cthr versus at Cthr<C<~10-3 M with ~10-10 M<Cthr< ~10-6 M:
(a) For Cthr<C<~10-3M, Dlb distributions are unimodal with ~10-7 m <Dlb<~3.5×10-7 m. However, Dp distributions are not unimodal: clusters with 10-9 m <Dp <10-8 m and domains with 5×10-8 m <Dp < – 4×10-6 m are distinguishable. For C<Cthr, no domains are present in samples kept for 18 hours in Permalloy containers and the liquid loses its typical SDVSPLwe-ne physicochemical properties. The aforementioned unimodality and values of Dlb are commensurate with supra-![]() dominating the domain distribution and mainly determining Dlb. The 5×10-8 <Dp < – 4×10-6 m domains might be EDAIPDplasma and the 10-9 m <Dp <10-8 m clusters might be hydrated ions, which become distinguishable with DLS when the more abundant
dominating the domain distribution and mainly determining Dlb. The 5×10-8 <Dp < – 4×10-6 m domains might be EDAIPDplasma and the 10-9 m <Dp <10-8 m clusters might be hydrated ions, which become distinguishable with DLS when the more abundant ![]() are eliminated by the Permalloy container screening UV EMF.
are eliminated by the Permalloy container screening UV EMF.
(b) For Cthr<C<~10-5 M χP is about 10% lower than χlb. The difference is solute type dependent. It is attributable to ![]() and supra-
and supra-![]() stabilized by EDAIPDplasma or other cluster types in samples kept at the laboratory bench. Recall that
stabilized by EDAIPDplasma or other cluster types in samples kept at the laboratory bench. Recall that ![]() are superfluidic. Therefore on their disappearance the number of intermolecular collisions increases and χ decreases.
are superfluidic. Therefore on their disappearance the number of intermolecular collisions increases and χ decreases.
v. For C<Ccrit, fingerprints of ferroelectric orderings participating in dissipative dynamics were identified in χ, heat of mixing and pH data of SDVSPLwe-ne (Elia and Niccoli, 1999, 2004a). These fingerprints were attributed to CDrot and EDACDrot by Yinnon and Yinnon (2011) and Yinnon and Elia (2013). In serial diluted solutions which were not vigorously shaken after each dilution step, no such fingerprints were observed. Also IR spectra pass band coefficient fluctuations (IR-SPBCF) of several aqueous SDVSPLwe-ne , observed by Zubareva et al. (2003), are attributable to the dissipative dynamics of CDrot and EDACDrot. These IR-SPBCF resemble those of SDVSASES. The latter were shown to conform with CDrot and EDACDrot agglomeration and reorganization in supra-domains (Yinnon and Liu, 2015b).
Dielcometric titrationsd provided the first unambiguous evidence for ferroelectric ordering in non-aqueous and aqueous SDVSPLwe-ne and showed the type of ordering depends on concentration (Konovalov et al., 2008):
dDielcometric titrations provide the dependence of the dielectric permittivity (ε) on C, i.e. ε(C). As to ε(C), its deviation from linearity is a measure of intermolecular interactions of the mixture’s components, with slope ratios characterizing its polarization affinity. Usually, the slope ratio of ε(C) changes upon formation of associates. A sharp positively sloped ε(C) leveling off in a plateau is characteristic of linear ordering of subunits and formation of “chain associates” in which the dipole moments are more or less parallel oriented. On increasing C above that of the plateau, ε(C) may become negatively sloped and reach a new plateau due to additional association leading to multipole aggregates with associated particles’ dipoles “inverse-parallel” orientated leading to formation of non-polar aggregates. The non-polar aggregates with the “inverse parallel orientation” can also form at the first step of aggregation, omitting the “chain aggregate” phase. The transition point at which the slope of ε(C) changes corresponds to the critical aggregate concentration e.g., critical micelle concentration, if the solute is a surfactant.
(a) For SDVSPLwe-ne of Melafen with the low-polarity chloroform as solvent, the dielectric permittivity (ε) dependence on C, i.e., ε(C) has two inflection points in the ~10-10 M<C<~10-4 M range: for ~10-10 M<C<~3.3×10-8 M ε(C) is linear with a steep slope; for ~3.3×10-8 M<C<~6.6×10-6 M ε(C) is linear too but the slope is about half that one for ~10-10 M<C<~3.3×10-8 M; at ~6.6×10-6 M ε(C) reaches a plateau which continues up to C≈1×10-4 M (Konovalov et al., 2008). Based on the well known ε(C) characteristics: the steeply sloped ε(C) is attributable to chain associates of CDrot and EDACDrot; the moderately sloped ε(C) is attributable to these domains being organized in multipole aggregates; the plateau at ~6.6×10-6 M<C<~1×10-4 M is attributable to EDAIPDplasma organized with their dipoles “inverse-parallel” orientated or to other non-polar groupings. The aforementioned hints![]() that ≈10-6 M. To verify this paragraph’s attributions, EMF screening of SDVSPLwe-ne of Melafen is called for, because association of the molecules constituting CDrot is mediated by FIR EMF.
that ≈10-6 M. To verify this paragraph’s attributions, EMF screening of SDVSPLwe-ne of Melafen is called for, because association of the molecules constituting CDrot is mediated by FIR EMF.
(b) Our model’s assumption that domain formation in SDVSPLwe-ne at low and ultralow concentrations involves the dipoles of the polar solvent molecules is supported by ε(C) of SDVSPLwe-ne of α-tocopherol in the non-polar carbon tetrachloride versus that in chloroform (Ryzhkina et al., 2011b). For the former at ~10-24 M<C<~10-3 M, ε(C) is linear and only slightly changes, indicating absence of association. For the latter at ~10-20 M<C<~10-3 M, ε(C) is non-linear and has kinks at C≈1×10-20 M, C≈1×10-15 M and C≈1×10-10 M. The kinks are related to formation and rearrangement of domains. For ~10-20 M<C<~10-15 M, growth of ε with C is steepest, but lessens when C increases. For ~10-4 M<C<~10-3 M, ε(C) is flat. The aforementioned ε(C) features indicate domains only form in SDVSPLwe-ne with polar solvent molecules.
(c) For aqueous SDVSPLwe-ne of membranotropic amphiphilic calix[4] resorcinarene with tris(hydroxymethyl)methylamide functional groups (a compound with structure and properties rendering it a simple synthetic model of natural polypeptide cluster glycoconjugates inducing cascades of physiological reactions when binding to cell membranes), Ryzhkina et al. (2012c) also employed dielcometry to evidence ferroelectric ordering. They found for the concentration dependence of Δε [i.e. Δε(C) with Δε the difference between ε of this SDVSPLwe-ne and ε of double distilled water]: on diluting from ~10-4 M to ~10-5 M Δε decreases from 7.5 to 0, on diluting from ~10-5 M to ~5×10-7 M Δε stays 0, on diluting from ~5×10-7 M to ~5×10-8 M Δε increases from 0 to ~1, on diluting from ~5×10-8 M to ~3×10-9 M Δε increases to ~1.5, on diluting from ~3×10-9 M to ~10-10 M Δε decreases to ~0.75, on diluting from ~10-10 M to ~5×10-11 M Δε increases to ~1 and on diluting from ~5×10-11 M to ~10-12 M Δε decreases to ~0.5 (see Ryzhkina et al. 2012c Figure 2). In addition they demonstrated the orderings induce optical activity. By combining their Δε, optical activity and Dlb data, they evidenced different types of groupings containing ferroelectric-ordered molecules cause this Δε(C): for ~10-6 M<C<~10-3 M the Dlb distribution is bimodal with Dlb≈4×10-9 m clusters and ~1.2×10-7 m<Dlb<1.8×10-7 m domains (on dilution from ~1×10-3 M to ~1×10-5 M the fraction of the Dlb≈4×10-9 m clusters decreases from 0.3 to 0.1 while that of the Dlb≈10-7 m domains increases to 0.9); for ~10-12M<C<~10-7 M the Dlb distribution is bimodal with Dlb of the order of 10-7 m and 10-6 m and their fractions respectively 0.7 and 0.3; for C<~10-12 M the domain distribution is polymodal preventing assessment of Dlb. Their σlb data evidence the solute is not a surfactant at ~10-12 M<C<~10-3 M. They supplemented their Dlb data with atomic force microscopy (AFM) imaging. AFM in contact mode for ~10-6 M<C<~10-3 M revealed 1×10-7 m – 6×10-7 m wide up to 3.5×10-8 m high hemispherical discrete relief particles, with the largest particles composed of smaller ones and all particles randomly distributed on the substrate — their prevalence strongly diminishes with concentration. At C≈10-7 M the discrete particles are still present, but AFM in tapping mode revealed another type of particles appears: ~8×10-8 m wide 1.3 x10-9 m – 1.7 x10-9 m high chain oriented soft particles aggregated in a thin film covering the substrate (similar soft particles were observed for aqueous NaCl SDVSASES at C≈1.7×10-7 M (Lo et al., 2009). For ~10-11 M<C<~10-8 M, AFM in tapping mode exposed ~10-6 m sized soft particles forming different complex structures, e.g., ribbon or rounded configurations (similar ribbons were observed with Transmission Electron Microscopy of aqueous NaCl SDVSASES at C≈1.7×10-11 M (Lo, 1996). The aforementioned Dlb and AFM analyses of Ryzhkina et al. (2012c) expounded the groupings present at ~10-6 M<C<~10-3 M are associates composed of solute and solvent molecules, while the domains present for C<~10-7 M are mainly composed of solvent molecules. Placing samples for 18 hours in Permalloy containers also enabled them to unambiguously demonstrate the different nature of the groupings present at C below and above 10-7 M: for C<~10-7 M no groupings are distinguishable, i.e., Cthr≈10-7 M; at ~10-6 M<C<~10-3 M samples’ physicochemical properties are slightly altered. While the alterations are attributable to ![]() stabilized by other groupings types, radio frequency screening and examining its effect on Δε(C) are called for to investigate whether these groupings include IPDplasma and EDAIPDplasma. With the 10-7 m and 10-6 m groupings present at C<~10-7 M mainly being composed of H2O, the disappearance of these on screening by Permalloy and the ferroelectric ordering of their molecules exposed by above cited Δε(C) data, the properties of these groupings match those of CDrot, EDACDrot,
stabilized by other groupings types, radio frequency screening and examining its effect on Δε(C) are called for to investigate whether these groupings include IPDplasma and EDAIPDplasma. With the 10-7 m and 10-6 m groupings present at C<~10-7 M mainly being composed of H2O, the disappearance of these on screening by Permalloy and the ferroelectric ordering of their molecules exposed by above cited Δε(C) data, the properties of these groupings match those of CDrot, EDACDrot, ![]() and [supra-CDrot <supra-
and [supra-CDrot <supra-![]() >], implying
>], implying![]() ≈Cthr≈10-7 M. As to the AFM data and the detailed Δε values, according to theoretical aspects of Δε(C) summarized in footnote d: for ~3×10-8M<C<~5×10-7 M these are ascribable to chain structures of CDrot and EDACDrot, with these domains’ electric dipole moments parallel oriented; for ~1×10-10 M<C<~3×10-8 M the kinks in Δε(C) signifies changes in the arrangement of these electric dipole moments, e.g., these are attributable to polar chain as well as less polar multi-pole structures; the maximum of Δε(C) at C≈10-11 M also is ascribable to polar chain structures dominating the groupings’ distribution. This attribution is supported by the non-zero optical activity observed for this C range.
≈Cthr≈10-7 M. As to the AFM data and the detailed Δε values, according to theoretical aspects of Δε(C) summarized in footnote d: for ~3×10-8M<C<~5×10-7 M these are ascribable to chain structures of CDrot and EDACDrot, with these domains’ electric dipole moments parallel oriented; for ~1×10-10 M<C<~3×10-8 M the kinks in Δε(C) signifies changes in the arrangement of these electric dipole moments, e.g., these are attributable to polar chain as well as less polar multi-pole structures; the maximum of Δε(C) at C≈10-11 M also is ascribable to polar chain structures dominating the groupings’ distribution. This attribution is supported by the non-zero optical activity observed for this C range.
(d) For ~10-18 M ≤C≤Cthr≈10-6 M aqueous SDVSPLwe-ne of 2,4,6,8-tetramethyl-2,4,6,8-tetraazabicyclo[3.3.0] octane-3,7-dione (Mebicar), Ryzhkina et al. (2013) also demonstrated ferroelectric ordering. It disappears on screening by Permalloy. With DLS they observed Dlb≈10-7 m and Dlb≈10-6 m domains, which disappear on screening by Permalloy. With dielcometry they evidenced Δε of samples kept at the laboratory bench (Δεlb) strongly depends on concentration. It varies between about -1.5 and +1, indicating ferroelectric ordering of the domains depends on concentration. Negative Δεlb values signifying domains form low-polar multipole structures, thus decreasing ε of the solution below that of double distilled water. The maximal positive Δεlb at ~1×10-10 M is attributable to domains forming chain (ribbon) structures wherein the dipole moments are arranged uni-directionally. Δε(C) for samples screened by Permalloy, i.e., Δεp(C), equals 0. With Dlb≈10-6 m domains present at C<~10-6 M, these mainly being composed of H2O, their disappearance on screening by Permalloy and the ferroelectric ordering of their molecules exposed by dielcometry, the properties of these domains match those of CDrot and EDACDrot, implying ![]() ≈Cthr≈10-6 M. Characteristics of the ~10-7 m domains, which we discuss below in paragraph vi, indicate these are .
≈Cthr≈10-6 M. Characteristics of the ~10-7 m domains, which we discuss below in paragraph vi, indicate these are .
(e) σlb dependence on C [i.e. σlb(C) ] supplements the Δεlb(C) reflecting the inverse-parallel oriented polar domains versus polar chain domains orderings for 10-18 M<C<![]() ≈Cthr aqueous SDVSPLwe-ne: Ryzhkina et al. (2009, 2011c-d) for various aqueous SDVSPLwe-ne observed in this C range (which is below these solutions’ critical micelle concentration), that σlb equals the surface tension of double distilled water, with the exception of two broad minima, one near C≈
≈Cthr aqueous SDVSPLwe-ne: Ryzhkina et al. (2009, 2011c-d) for various aqueous SDVSPLwe-ne observed in this C range (which is below these solutions’ critical micelle concentration), that σlb equals the surface tension of double distilled water, with the exception of two broad minima, one near C≈![]() and the other near C≈10-13 M for aqueous SDVSPLwe-ne of Ichfan C-10 or near C≈10-16 M for a Hemin derivative.
and the other near C≈10-13 M for aqueous SDVSPLwe-ne of Ichfan C-10 or near C≈10-16 M for a Hemin derivative.
This section’s cited experimental data indicate: domains are present for C<Ccrit for SDVSPLwe-ne of certain solute and solvent types only when during their preparation after each dilution step the liquid is vigorously shaken; for C<Ccrit domains only are present in polar solvents; the numerous solvent molecules constituting these domains are ferroelectric ordered; these domains aggregate into chain or multipole structures; these domains disappear on screening by Permalloy. These indications evoke the domains include CDrot and EDACDrot and Ccrit=![]() . (Our discussion as to these domains also including
. (Our discussion as to these domains also including ![]() at C<Ccrit, we present in paragraph vi.) Aforementioned evocation replaces the enigma “Why does SDVSPLwe-ne of some solutes at ULC contain domains while others have the customary infinite diluted solutions characteristics?” to the query “Which entity stabilize CDrot at
at C<Ccrit, we present in paragraph vi.) Aforementioned evocation replaces the enigma “Why does SDVSPLwe-ne of some solutes at ULC contain domains while others have the customary infinite diluted solutions characteristics?” to the query “Which entity stabilize CDrot at ![]() ?”. With the current fuzziness concerning IPDplasma and EDAIPDplasma presence in SDVSPLwe-ne, we cannot assess whether solvated solutes with a sufficiently large asymmetric charge distribution or only EDAIPDplasma stabilize CDrot at
?”. With the current fuzziness concerning IPDplasma and EDAIPDplasma presence in SDVSPLwe-ne, we cannot assess whether solvated solutes with a sufficiently large asymmetric charge distribution or only EDAIPDplasma stabilize CDrot at ![]() . Since IPDplasma and EDAIPDplasma formations both require solvated solutes with a sufficiently large asymmetric charge distribution, we contend that only SDVSPLwe-ne of such solutes contain CDrot at ULC. As a first step towards verifying our contention, in Table 1 we present a variety of bioactive compounds and their measured or calculated electric dipole moment reported in the literature. We randomly choose these compounds from those listed by Konovalov (2013). Based on our SDVSPLwe-ne model, we predict that only in ULC SDVSPLwe-ne of bioactive compounds which have a dipole moment larger than the dipole moment of the solvent molecules, CDrot and EDACDrot are present for C<
. Since IPDplasma and EDAIPDplasma formations both require solvated solutes with a sufficiently large asymmetric charge distribution, we contend that only SDVSPLwe-ne of such solutes contain CDrot at ULC. As a first step towards verifying our contention, in Table 1 we present a variety of bioactive compounds and their measured or calculated electric dipole moment reported in the literature. We randomly choose these compounds from those listed by Konovalov (2013). Based on our SDVSPLwe-ne model, we predict that only in ULC SDVSPLwe-ne of bioactive compounds which have a dipole moment larger than the dipole moment of the solvent molecules, CDrot and EDACDrot are present for C<![]() =Cthr. Since for H2O in its gas phase, the electric dipole moment is 1.85 D and in liquid phase its values has been estimated as 2.9±0.6D (Kemp et al., 2008), we predict that only when the dipole moment of the bioactive compound is above ~3D, their ULC aqueous SDVSPLwe-ne contain CDrot and EDACDrot. Table 1 presents our predictions. Measurements confirming these predictions are called for.
=Cthr. Since for H2O in its gas phase, the electric dipole moment is 1.85 D and in liquid phase its values has been estimated as 2.9±0.6D (Kemp et al., 2008), we predict that only when the dipole moment of the bioactive compound is above ~3D, their ULC aqueous SDVSPLwe-ne contain CDrot and EDACDrot. Table 1 presents our predictions. Measurements confirming these predictions are called for.
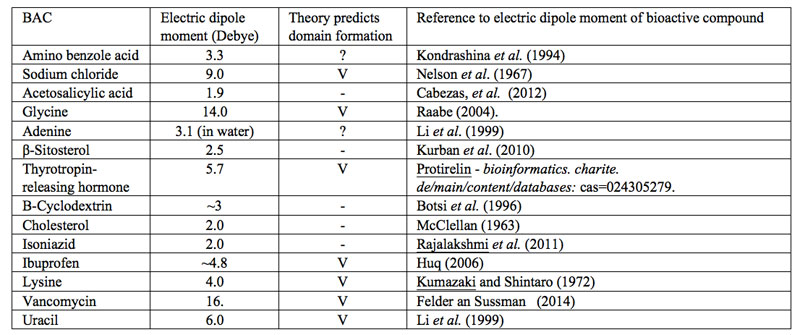
Table 1: SDVSPLwe-ne of bioactive compounds and predictions concerning these liquids containing domains at ULC. The symbol “V” indicates that our model predicts presence of domains in ULC SDVSPLwe-ne. The symbol “?” indicates that according to our model it is doubtful that domains are present in ULC SDVSPLwe-ne. The symbol “-” indicates that our model predicts no domains are present in ULC SDVSPLwe-ne.
vi. ![]() stabilized by CDrot cannot be evidenced by examining differences between physicochemical properties of samples kept at the laboratory bench or in Permalloy containers. Such differences we analyzed in paragraphs i, ii and iv for uncovering
stabilized by CDrot cannot be evidenced by examining differences between physicochemical properties of samples kept at the laboratory bench or in Permalloy containers. Such differences we analyzed in paragraphs i, ii and iv for uncovering ![]() stabilization by CDplasma, IPDplasma or EDAplasma. However, screening by Permalloy destroys both and CDrot. With UV absorbance sensitive to
stabilization by CDplasma, IPDplasma or EDAplasma. However, screening by Permalloy destroys both and CDrot. With UV absorbance sensitive to ![]() (Del Giudice et al., 2010; Yinnon et al., 2015c), gathering UV absorbance data for various SDVSPLwe-ne with C<Cthr≈
(Del Giudice et al., 2010; Yinnon et al., 2015c), gathering UV absorbance data for various SDVSPLwe-ne with C<Cthr≈![]() is called for. While awaiting such measurements, we focus on
is called for. While awaiting such measurements, we focus on ![]() fingerprints in ζ-potential data, because many physicochemical properties of dispersed systems are determined by their dispersed particles’ surface charge. Ryzhkina et al. (2010a, 2011d, 2012a-d, 2013) observed: ζlb of the Dlb≈1×10-7 m to Dlb≈4×10-7 m sized domains, present in aqueous SDVSPLwe-ne at ~10-20 M<C<Cthr (which we denote ) typically varies from -2 to -20 mV, non-linear changes with concentration and is related to their Dlb (which we denote D); also is related to the Δεlb and pH of samples kept at the laboratory bench (pHlb); correlates with χlb at solute type dependent concentration ranges. (In the aforementioned and the rest of this paragraph, we only cited data for ~10-20 M<C<Cthr samples kept at the laboratory bench, because at these concentrations no domains are present in samples screened by Permalloy.) With the diameter of
fingerprints in ζ-potential data, because many physicochemical properties of dispersed systems are determined by their dispersed particles’ surface charge. Ryzhkina et al. (2010a, 2011d, 2012a-d, 2013) observed: ζlb of the Dlb≈1×10-7 m to Dlb≈4×10-7 m sized domains, present in aqueous SDVSPLwe-ne at ~10-20 M<C<Cthr (which we denote ) typically varies from -2 to -20 mV, non-linear changes with concentration and is related to their Dlb (which we denote D); also is related to the Δεlb and pH of samples kept at the laboratory bench (pHlb); correlates with χlb at solute type dependent concentration ranges. (In the aforementioned and the rest of this paragraph, we only cited data for ~10-20 M<C<Cthr samples kept at the laboratory bench, because at these concentrations no domains are present in samples screened by Permalloy.) With the diameter of ![]() and the size of supra-
and the size of supra-![]() of the order of 10-7 m (similar to D), we conjecture: the negative charged domains are
of the order of 10-7 m (similar to D), we conjecture: the negative charged domains are ![]() and supra
and supra![]() -; is due to the quasi free electrons located at their boundary; pHlb (typically varying from ~5.8 to 7) result from the quasi free protons (which are the partners of the quasi free electrons) and are dispersed in the solvent surrounding CDrot. As to the reasoning underlying this conjecture, for an H2O ensemble with finite dimensions, ferroelectric ordering implies addition of an electrostatic term to its potential energy [the 2nd term in Eq. 4 of Iitaka (2010)], representing the effect of the macroscopic electric field due to the charge distribution at the boundary. This term’s contribution to the potential energy is about hundred times larger than the energetic differences of various hydrogen bond configurations. Alike charged particles, dispersed within the ensemble, screen the boundary polarization and reduce this term. Hence accumulation of alike charged particles in CDrot and expulsion of opposite charged ones reduce polarization at the CDrot boundary and stabilize these domains. Apparently, the energy gained from CDrot stabilization is higher than that required for separating the quasi free electrons of
-; is due to the quasi free electrons located at their boundary; pHlb (typically varying from ~5.8 to 7) result from the quasi free protons (which are the partners of the quasi free electrons) and are dispersed in the solvent surrounding CDrot. As to the reasoning underlying this conjecture, for an H2O ensemble with finite dimensions, ferroelectric ordering implies addition of an electrostatic term to its potential energy [the 2nd term in Eq. 4 of Iitaka (2010)], representing the effect of the macroscopic electric field due to the charge distribution at the boundary. This term’s contribution to the potential energy is about hundred times larger than the energetic differences of various hydrogen bond configurations. Alike charged particles, dispersed within the ensemble, screen the boundary polarization and reduce this term. Hence accumulation of alike charged particles in CDrot and expulsion of opposite charged ones reduce polarization at the CDrot boundary and stabilize these domains. Apparently, the energy gained from CDrot stabilization is higher than that required for separating the quasi free electrons of ![]() from their quasi free protons. Thus on CDrot stabilization: some H2O incorporated in
from their quasi free protons. Thus on CDrot stabilization: some H2O incorporated in ![]() dissociate (i.e., their dissociation constant shifts); those quasi free protons becoming hydroxonium ions (H3O+) disperse in between the CDrot causing the solvent’s slightly acid character; their counter-ions, the negatively charged
dissociate (i.e., their dissociation constant shifts); those quasi free protons becoming hydroxonium ions (H3O+) disperse in between the CDrot causing the solvent’s slightly acid character; their counter-ions, the negatively charged ![]() , locate within the CDrot and screen their boundary polarization. With calculation of the energetic changes involved in the mechanisms outside the scope of this paper, we turn to other data supporting our conjecture.
, locate within the CDrot and screen their boundary polarization. With calculation of the energetic changes involved in the mechanisms outside the scope of this paper, we turn to other data supporting our conjecture.
For aqueous SDVSPLwe-ne of 2,4,6,8-tetra-methyl-2,4,6,8-tetraazabicyclo [3.3.0] octane-3,7-dione at ~10-17 M<C<Cthr (Mebicar), Ryzhkina et al. (2013) found: the molarities at which the dependence of pHlb and D on concentration have maximum coincide; for ~10-16 M<C<~10-10 M, pHlb and D statistically significantly correlate. The findings indicate domains reorganization involves H3O+. Indeed according to QED,![]() organizing in supra-
organizing in supra-![]() affects the energy levels of their quasi free electrons and quasi free protons (Preparata, 1995 chapters 3 and 10), altering the dissociation energy of their H2O. To the best of our knowledge, the aforementioned correlation is its first identified manifestation. However, such correlation was not observed for all SDVSPLwe-ne for which pHlb data were collected (Ryzhkina et al., 2012c). With the still rudimentary understanding of the effects of
affects the energy levels of their quasi free electrons and quasi free protons (Preparata, 1995 chapters 3 and 10), altering the dissociation energy of their H2O. To the best of our knowledge, the aforementioned correlation is its first identified manifestation. However, such correlation was not observed for all SDVSPLwe-ne for which pHlb data were collected (Ryzhkina et al., 2012c). With the still rudimentary understanding of the effects of ![]() agglomeration on the quasi free electrons and the quasi free protons, detailed calculations and comparing their results with pHlb and D data analyses are called for.
agglomeration on the quasi free electrons and the quasi free protons, detailed calculations and comparing their results with pHlb and D data analyses are called for.
With ![]() and D
and D![]() for many SDVSPLwe-ne at C<Cthr related but not statistically significantly correlated, the effects of
for many SDVSPLwe-ne at C<Cthr related but not statistically significantly correlated, the effects of ![]() organizing in supra-
organizing in supra-![]() seems to be influenced by quasi free electrons as well as by other factors, e.g., the polarization density in their neighborhood. Indeed, the extremums in the absolute values of
seems to be influenced by quasi free electrons as well as by other factors, e.g., the polarization density in their neighborhood. Indeed, the extremums in the absolute values of ![]() (i.e. |
(i.e. |![]() |) coincide with those of Δεlb for aqueous SDVSPLwe-ne of Mebicar at C<Cthr: the local minimum of Δεlb ≈ -0.05 at C≈10-7 M coincides with a local minimum of |
|) coincide with those of Δεlb for aqueous SDVSPLwe-ne of Mebicar at C<Cthr: the local minimum of Δεlb ≈ -0.05 at C≈10-7 M coincides with a local minimum of |![]() |– according to dielcometry theory, for concentration ranges at which Δε<0, polar domains likely are lined up in low polar multipole structures, thus decreasing ε of the solution compared to that of the solvent; the global maximum of Δεlb≈1 at C≈10-10M coincides with a global maximum of |
|– according to dielcometry theory, for concentration ranges at which Δε<0, polar domains likely are lined up in low polar multipole structures, thus decreasing ε of the solution compared to that of the solvent; the global maximum of Δεlb≈1 at C≈10-10M coincides with a global maximum of |![]() |≈12 mV, which is attributable to polar domains organized in chain (ribbon) structures with the dipole moments arranged unidirectionally; the global minimum of Δεlb ≈ -1.5 at C≈10-14 M coincides with a local minimum in |
|≈12 mV, which is attributable to polar domains organized in chain (ribbon) structures with the dipole moments arranged unidirectionally; the global minimum of Δεlb ≈ -1.5 at C≈10-14 M coincides with a local minimum in |![]() |≈4 mV — the global nature of this minimum of Δεlb indicates inverse-parallel orientation of the polar domains at this concentration range. As to the agglomeration of
|≈4 mV — the global nature of this minimum of Δεlb indicates inverse-parallel orientation of the polar domains at this concentration range. As to the agglomeration of ![]() into supra-
into supra-![]() at these extremums, we cite the local minimum of D
at these extremums, we cite the local minimum of D![]() ≈2.5×10-7 m at C≈10-7 M, the local minimum of D
≈2.5×10-7 m at C≈10-7 M, the local minimum of D![]() ≈2.7×10-7 m at C≈10-10 M and the global maximum of D≈4.2×10-7 m at C≈10-14 M. The aforementioned Δεlb and D
≈2.7×10-7 m at C≈10-10 M and the global maximum of D≈4.2×10-7 m at C≈10-14 M. The aforementioned Δεlb and D![]() values suggest that for inverse-parallel oriented CDrot, supra-
values suggest that for inverse-parallel oriented CDrot, supra-![]() sizes are maximal. However, the opposite was observed for aqueous SDVSPLwe-ne of membranotropic amphiphilic calix[4]resorcinarene: maxima in its D coincide with the polar domains mainly organizing in chain structures (Ryzhkina et al., 2012c). Hitherto, details of Δε(C) only were gathered for the SDVSPLwe-ne cited in this paragraph and theoretical aspects of CDrot orderings, agglomerations, their dependence on concentration and solute type has not been investigated; therefore research directed at studying these concentration dependencies is called for.
sizes are maximal. However, the opposite was observed for aqueous SDVSPLwe-ne of membranotropic amphiphilic calix[4]resorcinarene: maxima in its D coincide with the polar domains mainly organizing in chain structures (Ryzhkina et al., 2012c). Hitherto, details of Δε(C) only were gathered for the SDVSPLwe-ne cited in this paragraph and theoretical aspects of CDrot orderings, agglomerations, their dependence on concentration and solute type has not been investigated; therefore research directed at studying these concentration dependencies is called for.
vii. As to the observed effects of the various QED domains on the electric conductivity (χ) of SDVSPLwe-ne:
(a) For C>Cthr, we cannot yet explain the dependence of χ on C, i.e., χ(C). As noted above existing data is insufficient for assigning domains to CDplasma, IPDplasma or EDAIPDplasma, micelles or other aggregate types. Hence attributing χ values to any of these is not yet doable.
(b) For ~10-18 M<C<Cthr, Ryzhkina et al. (2011c, 2011d, 2012a, 2012c, 2012d, 2013) found: χ(C) for samples kept on the laboratory bench [χlb(C)] is much larger than χ(C) of samples kept in Permalloy containers [χP(C)]. For example for aqueous potassium phenosan SDVSPLwe-ne at C≈10-14 M χlb≈25 µS/cm while χP is an order of magnitude smaller. χP very slowly decreases with concentration and typically is ~2.5 μS/cm (slightly larger than χ of double distilled water). Moreover, |![]() | and χlb are significant statistical correlated — an enhancement in |
| and χlb are significant statistical correlated — an enhancement in |![]() | causes larger χlb . Also χlb(C) is related to D
| causes larger χlb . Also χlb(C) is related to D![]() (C), Δεlb(C) and σlb(C) . As to explanations for these phenomena:
(C), Δεlb(C) and σlb(C) . As to explanations for these phenomena:
1. χlb(C)>>χP(C), together with paragraphs v-vi, evoke the inequality results from Permalloy destroying CDrot and ![]() . χ is an inverse function of intermolecular collisions. For C<Cthr intermolecular collisions solely involve randomly moving H2O and solvated solutes. Recall that the H2O in the superfluidic CDrot or
. χ is an inverse function of intermolecular collisions. For C<Cthr intermolecular collisions solely involve randomly moving H2O and solvated solutes. Recall that the H2O in the superfluidic CDrot or ![]() do not collide. Thus the transformation of these domains’ coherent oscillating H2O into randomly moving H2O (induced by the EMF screening) enhances the number of intermolecular collisions and thus reduces χ. Also the electric dipoles of CDrot and the clouds of quasi free electrons of
do not collide. Thus the transformation of these domains’ coherent oscillating H2O into randomly moving H2O (induced by the EMF screening) enhances the number of intermolecular collisions and thus reduces χ. Also the electric dipoles of CDrot and the clouds of quasi free electrons of ![]() affect collisions of randomly moving H2O neighboring on CDrot (Yinnon and Yinnon, 2011, 2012; Yinnon and Elia, 2013).
affect collisions of randomly moving H2O neighboring on CDrot (Yinnon and Yinnon, 2011, 2012; Yinnon and Elia, 2013).
2. χlb(C) being correlated to |![]() |(C)
|(C)
and related to D![]() (C), Δεlb(C) and σlb(C), together with the strong concentration dependence of these variables, indicate domains rearrangements are reflected in χ. However, the current fuzziness concerning the effects of CDrot orderings on D
(C), Δεlb(C) and σlb(C), together with the strong concentration dependence of these variables, indicate domains rearrangements are reflected in χ. However, the current fuzziness concerning the effects of CDrot orderings on D![]() (C) and |
(C) and |![]() |(C) (see paragraph vi), hinders explaining these relations.
|(C) (see paragraph vi), hinders explaining these relations.
3. Paragraphs (1-2) imply modeling χ(C) for C<Cthr SDVSPLwe-ne requires adequate description of alternating electric field interactions with: randomly moving solvent and solvated solute molecules; as well as with the dipoles of CDrot, EDACDrot and the quasi free electron clouds of ![]() .
.
Experimental data cited in the above paragraphs i-vii and their analyses support most of the QED model of SDVSPLwe-ne’s aspects i-vii, respectively.
Interactions between bio-systems and SDVSPLwe-ne or SDVSASES
~10-20 M<C<~10-10 M laboratory-bench-kept serial diluted vigorously shaken polar liquids of a wide variety (thousands) of bioactive compounds, in a reproducible quantified manner, affect bio-systems, e.g., bio-macromolecules, cells, organs, organisms or populations (Palmina et al., 2009; Konovalov et al., 2014a-c). The bioactivity of these solutions persists even when their concentrations are much lower than those of the bio-systems. Rhyzkina et al. (2010a, 2011b, 2013), and Konovalov et al. (2014a-c) observed: the bioactivity of these solutions disappears on screening by Permalloy; the concentration dependence of the bioactivity is related to the solutions’ electric conductivity dependence on concentration χlb(C); it also is related to the absolute value of the ζ-potential dependence on concentration |![]() |(C) of the Dlb≈1×10-7 m to Dlb≈4×10-7 m sized domains present in these solutions. Thus evidently these domains play a role in the bioactivity. Contemplating the effects of SDVSPLwe-ne and SDVSASES on biosystems is hampered by the many unsolved puzzles pertaining to biochemical reactions. However, |
|(C) of the Dlb≈1×10-7 m to Dlb≈4×10-7 m sized domains present in these solutions. Thus evidently these domains play a role in the bioactivity. Contemplating the effects of SDVSPLwe-ne and SDVSASES on biosystems is hampered by the many unsolved puzzles pertaining to biochemical reactions. However, |![]() |(C) provides a clue.
|(C) provides a clue.
Most biochemical reactions are redox electron transfer reactions, involving bio-molecules with high electronic excitation energies. Electron transfer occurs over distances beyond van der Waals contact, i.e., direct donor-to-acceptor electronic interactions are negligible and customarily water is assumed to act as a go-between (Balabin et al., 2008). Since water molecules have high ionization energy (12.6 eV), as a matter of course these are not regarded as a source of nearly free electrons. Their usual conjectured roles include electron tunneling facilitation, solvation of counter-ions, dielectric screening, proton coupling.
Supra-CDrot<supra-![]() > stabilized at biological interfaces playing a central role in electron transfer was conjectured by Del Giudice et al. (2010, 2013). Water in biosystems can be considered interfacial water — it is but a fraction of a micron from an interface (cell membranes, macromolecules, etc). The quasi free electrons of the supra-
> stabilized at biological interfaces playing a central role in electron transfer was conjectured by Del Giudice et al. (2010, 2013). Water in biosystems can be considered interfacial water — it is but a fraction of a micron from an interface (cell membranes, macromolecules, etc). The quasi free electrons of the supra-![]() in interfacial water constitute a pool of nearly free electrons. The superfluidity of
in interfacial water constitute a pool of nearly free electrons. The superfluidity of ![]() entails its quasi free electrons reside in a collective state with a narrowly (peV) spaced spectrum. The pool’s excited states persist for macroscopic times, i.e., a supra-
entails its quasi free electrons reside in a collective state with a narrowly (peV) spaced spectrum. The pool’s excited states persist for macroscopic times, i.e., a supra-![]() is an efficient energy storage device. A
is an efficient energy storage device. A ![]() attracts molecules containing in their spectrum a frequency close to one of its own, e.g., a bioactive molecule. This bio-particle becomes a guest participant in the coherent dynamics of the H2O constituting
attracts molecules containing in their spectrum a frequency close to one of its own, e.g., a bioactive molecule. This bio-particle becomes a guest participant in the coherent dynamics of the H2O constituting ![]() . It settles on the boundary of the
. It settles on the boundary of the ![]() . The difference in frequencies of the guest and the
. The difference in frequencies of the guest and the ![]() perturbs the domain’s dynamics. Few guests do not harm their
perturbs the domain’s dynamics. Few guests do not harm their ![]() , but numerous guests wreck their host. Whenever the energy stored in supra-
, but numerous guests wreck their host. Whenever the energy stored in supra-![]() matches the guest’s activation energy, energy discharge of the supra-
matches the guest’s activation energy, energy discharge of the supra-![]() and onset of the chemical array occur simultaneously. With the ~10-7 m diameter of
and onset of the chemical array occur simultaneously. With the ~10-7 m diameter of ![]() , the H2O and quasi free electrons in supra-
, the H2O and quasi free electrons in supra-![]() all residing in collective states, QED possibly may elucidate biosystems’ fast electron transfer over long distances.
all residing in collective states, QED possibly may elucidate biosystems’ fast electron transfer over long distances.
Experimental data supporting the last paragraph’s conjecture of Del Giudice et al. (2010, 2013) has not yet been presented. Our analyses of SDVSPLwe-ne strengthen it. The relation between the concentration dependency of SDVSPLwe-ne’s bioactivity and |![]() |(C), together with paragraph’s vi’s attribution that quasi free electrons underlie , evokes SDVSPLwe-ne’s
|(C), together with paragraph’s vi’s attribution that quasi free electrons underlie , evokes SDVSPLwe-ne’s ![]() with their pool of superfluidic quasi free electrons and their guest bioactive molecules influence bio-systems. For example, the
with their pool of superfluidic quasi free electrons and their guest bioactive molecules influence bio-systems. For example, the ![]() in SDVSPLwe-ne together with their bioactive guests molecules might directly affect membranes or interact with their interfacial water’s
in SDVSPLwe-ne together with their bioactive guests molecules might directly affect membranes or interact with their interfacial water’s ![]() . Indeed enhancement of bio-systems’ biological activity or plasmatic membranes’ lipid order parameter, induced by SDVSPLwe-ne, was observed to depend on these liquids’ concentration in a manner similar to that of their χlb(C) and |
. Indeed enhancement of bio-systems’ biological activity or plasmatic membranes’ lipid order parameter, induced by SDVSPLwe-ne, was observed to depend on these liquids’ concentration in a manner similar to that of their χlb(C) and |![]() |(C) (Palmina et al., 2009; Konovlov, 2013; Konovalov et al., 2014a-c). Moreover as mentioned above, the concentration at which numerous SDVSPLwe-ne’s bioactivity, |
|(C) (Palmina et al., 2009; Konovlov, 2013; Konovalov et al., 2014a-c). Moreover as mentioned above, the concentration at which numerous SDVSPLwe-ne’s bioactivity, |![]() |, χlb and IR-SPBCF have extremums fall in small ranges (~10-9 M – 10-10 M; ~10-13 M – 10-15 M). Guest bioactive molecules’ oscillations resonating with the coherent oscillations of H2O in
|, χlb and IR-SPBCF have extremums fall in small ranges (~10-9 M – 10-10 M; ~10-13 M – 10-15 M). Guest bioactive molecules’ oscillations resonating with the coherent oscillations of H2O in ![]() , CDrot and [supra-CDrot <supra-
, CDrot and [supra-CDrot <supra-![]() >], and these bioactive molecules’ prevalence being low enough not to wreck their host, might underlie the extremums. As a first step for researching aforementioned conjecture, we study characteristics of
>], and these bioactive molecules’ prevalence being low enough not to wreck their host, might underlie the extremums. As a first step for researching aforementioned conjecture, we study characteristics of ![]() and [supra-CDrot <supra-
and [supra-CDrot <supra-![]() >] adjacent to membranes (Yinnon et al., 2015c WATER Journal in press; Liu et al., 2015). In the current absence of detailed knowledge on interactions between bio-molecules and
>] adjacent to membranes (Yinnon et al., 2015c WATER Journal in press; Liu et al., 2015). In the current absence of detailed knowledge on interactions between bio-molecules and ![]() or CDrot their study too is called for.
or CDrot their study too is called for.
Another conjecture for the bioactivity of SDVSPLwe-ne and SDVSASES, forwarded by Pershin (2014), bases on the quantum differences between ortho- and para-H2O spin isomers. Pershin’s approach seemingly complements ours, i.e., magnetic moments of vortices in CDrot and quasi free electrons interacting with the H2O spin isomers likely affect these solutions’ properties. Hitherto, interactions between spin isomers and QED domains have not been studied and such studies are called for. While such interactions likely affect domains in SDVSPLwe-ne and in SDVSASES, at least these cannot play a decisive role in CDrot formation, because these also form in non-aqueous serial diluted vigorously shaken solutions.
Conclusions
This paper shows that the in 2011 presented QED model for serial diluted solutions which were vigorously shaken after each dilution step enables consistent explanations also for recently observed characteristics. For example for the characteristics of molecular associates in serial diluted vigorously shaken polar liquids containing weak electrolytes or non-electrolytic compounds (SDVSPLwe-ne) with concentrations down to 10-20 M. In the past the model explicated various physicochemical properties of SDVSPLwe-ne. The closely related QED model for serial diluted vigorously shaken aqueous solutions of strong electrolytes (SDVSASES) also has provided consistent explanations for many phenomena (Yinnon and Yinnon, 2011; Yinnon and Elia, 2013; Yinnon and Liu, 2015b). Our above presented analyses of experimental results, in particular those of Konovalov and Ryzhkina (2014a), for the first time enabled verification of the following central features of the SDVSPLwe-ne model:
a) Interactions between EMF and molecules mediate several types of association, resulting in presence of various types of domains in specific concentration regimes. Screening samples from EMF by placing them in Permalloy containers verified this feature.
b) The typical characteristics of three domain types, i.e., CDrot, CDplasma and , predicted by the model agree with observed ones. For example:
• Their sizes being of the order of 10-5 m, 10-6 m and 10-7 m, respectively, and their organization in supra-domains was demonstrated with DLS and AFM.
• The macroscopic times (1 – 18 h) required for their stabilization, which was demonstrated with kinetic measurements.
• The ferroelectric ordering of the solvent molecules constituting CDrot, endowing these domains with a permanent electric dipole moment, are reflected in dielcometry and surface tension data. These domains were observed to agglomerate into supra-domains. Chain associates in which the dipole moments are more or less parallel oriented may compose these supra-CDrot. Multipole aggregates with the dipoles of CDrot “inverse-parallel” orientated, leading to formation of non-polar aggregates, were also observed.
• The dissipative dynamics of CDrot and ![]() are reflected in the IR spectra pass band coefficient fluctuations of SDVSPLwe-ne.
are reflected in the IR spectra pass band coefficient fluctuations of SDVSPLwe-ne.
• The critical concentration (![]() ) below which 10-5 m sized CDrot can persist over macroscopic times in SDVSPLwe-ne was identified with a combination of techniques, e.g., dielcometry, DLS, screening samples from ambient EMF by placing them in Permalloy containers.
) below which 10-5 m sized CDrot can persist over macroscopic times in SDVSPLwe-ne was identified with a combination of techniques, e.g., dielcometry, DLS, screening samples from ambient EMF by placing them in Permalloy containers.
• ![]() being negatively charged has been substantiated by microelectrophoresic measurements of their electrokinetic potential (ζ-potential).
being negatively charged has been substantiated by microelectrophoresic measurements of their electrokinetic potential (ζ-potential).
• Stabilization of or CDrot has been demonstrated with electric conductivity data, which reflect their superfluidic properties.
For one domain type, i.e., the ~10-6 m sized IPDplasma predicted to be present at 10-6 M<C<10-4 M in solution of weak electrolytes and non-electrolytes, we pointed out the kind of experiments required for unambiguous distinguishing these among the various types of observed domains. [Formation of IPDplasma in aqueous strong electrolytes has been verified, as discussed by Yinnon and Yinnon (2012), Yinnon and Liu (2015b].
c) The QED model’s prediction that only in solutions with polar solvents, their preparation by serial dilutions and vigorous shaking after each dilutions step induces molecular association at very low concentrations is supported by the experimental findings of Ryzhkina et al. (2011b). In SDVSPLwe-ne of α-Tocopherol dissolved in chloroform or dissolved in water they observed domains at 10-20 M<C<10-3 M. However, for SDVSPLwe-ne of α-Tocopherol dissolved in the non-polar tetrachloride, they could not discern molecular association at such low concentrations.
d) Only in solutions of certain solute types, serial dilutions and vigorous shaking the liquid after each dilution step perpetuate presence of domains at very low concentrations, e.g., at 10-20 M<C<10-10 M. The model predicts that the required characteristic of the solute is that it has a sufficiently large permanent or induced electric dipole moment. Experiments indeed verified that only for certain solutes, their serial diluted vigorous shaken solutions contain molecular associates at very low concentrations (Konovalov, 2013). However, these solutes and their specific characteristics have not yet been listed in the literature.
All these verifications support an important result attained with the QED model of polar liquids. The model uncovered the dynamics causing serial diluted solutions which were vigorously shaken after each dilution step at ultra low concentrations to have characteristics differing from those predicted by the customary electrostatic theories. The features of the QED model of SDVSPLne-se substantiated in this paper and in previous ones, together with the corroborations of the QED model of SDVSASES published in previous articles, lead to the conclusion: “stabilization of CDrot at C≈ is crucial for inducing the distinctive characteristics of SDVSPLne-se and SDVSASES at ultra low concentrations.” According to QED, CDrot can get stabilized by entities with a sufficiently large asymmetric charge distribution, e.g., solvated solutes or molecular associates with a large permanent or induced electric dipole moment. Once such an entity stabilizes CDrot, subsequent vigorous shaking excites these domains or breaks them up. Due to the ferroelectric ordering of the solvent molecules constituting CDrot, excited CDrot as well as their broken pieces also have an electric dipole moment, i.e., are electric dipole aggregates (EDACDrot). Successive dilution, diminishes CDrot and EDACDrot. However, these domains are molecular associates with an electric dipole moment, and as such stabilize new CDrot. Thus serial dilutions with vigorous shaking after each dilution step perpetuate presence of CDrot after these first got stabilized at C≈![]() . In aqueous solutions CDrot also may stabilize
. In aqueous solutions CDrot also may stabilize![]() and supra-
and supra-![]() .
.
As to the characteristics of entities stabilizing CDrot in serial diluted solutions, hitherto only for SDVSASES is there some experimental evidence. Below a solute type dependent transition concentration (![]() ), ions and the polar H2O organize in IPDplasma. Typically these IPDplasma are present at ~10-6 M<C< ~10-4 M. These domains have no asymmetric charge distribution. However, excitation of IPDplasma or their break up induced by vigorous shaking transforms IPDplasma into molecular associates with an asymmetric charge distribution, i.e., these are electric dipole aggregates (EDAIPDplasma). Accordingly in SDVSASES, EDAIPDplasma stabilize CDrot at C≈
), ions and the polar H2O organize in IPDplasma. Typically these IPDplasma are present at ~10-6 M<C< ~10-4 M. These domains have no asymmetric charge distribution. However, excitation of IPDplasma or their break up induced by vigorous shaking transforms IPDplasma into molecular associates with an asymmetric charge distribution, i.e., these are electric dipole aggregates (EDAIPDplasma). Accordingly in SDVSASES, EDAIPDplasma stabilize CDrot at C≈![]() . Such stabilization requires that the electrolyte solution is first diluted below
. Such stabilization requires that the electrolyte solution is first diluted below ![]() and subsequently vigorously shaken after each additional dilution step. For SDVSPLwe-ne no sufficient experimental data exist for determining presence of IPDplasma in dilute solutions, and their transformation to EDAIPDplasma induced by vigorous shaking. Moreover, for SDVSPLwe-ne, experimental data unambiguously verifying solutes with sufficiently large electric dipole moments stabilize CDrot at C≈
and subsequently vigorously shaken after each additional dilution step. For SDVSPLwe-ne no sufficient experimental data exist for determining presence of IPDplasma in dilute solutions, and their transformation to EDAIPDplasma induced by vigorous shaking. Moreover, for SDVSPLwe-ne, experimental data unambiguously verifying solutes with sufficiently large electric dipole moments stabilize CDrot at C≈![]() is also lacking. The kinds of experiments required for revealing the presence of IPDplasma are detailed in the discussion section.
is also lacking. The kinds of experiments required for revealing the presence of IPDplasma are detailed in the discussion section.
The abovementioned implies that important qualitative aspects of the structure and physicochemical properties of SDVSPLne-se and SDVSASES have now been explained. As to future research, in addition to that detailed in the Discussion section, we point to the following desirable projects:
• Quantitative verification of the structural and physicochemical properties of SDVSPLne-se and SDVSASES predicted by QED.
• Research directed at elucidating the effects of SDVSPLne-se and SDVSASES on bio-systems;
• Deriving equations for physicochemical properties of liquids containing the various kinds of QED domains; computing the values of the physicochemical variables and showing these agree with measured ones.
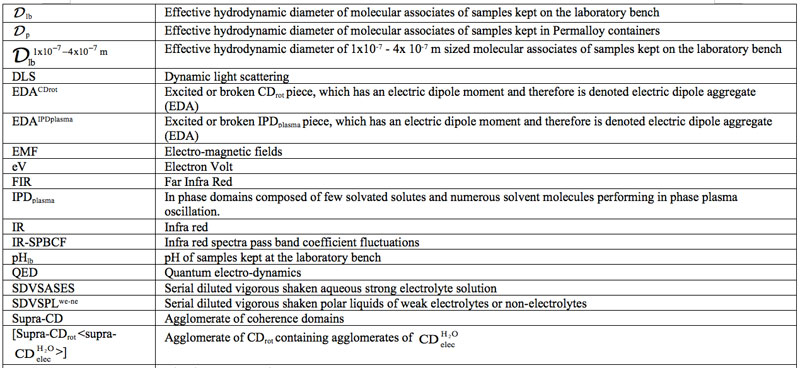
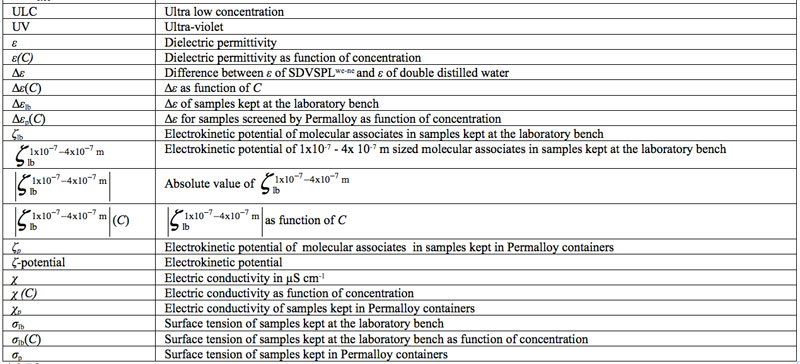
Table 2: List of abbreviations in alphabetic order, followed by Greek symbols abbreviations.
Acknowledgements
With much appreciation we thank Prof. A. I. Konovalov for his many helpful discussions, his careful reading of the manuscript and his detailed constructive comments. Tamar Yinnon expresses her gratitude to Prof. A. M. Yinnon for his continuous support and encouragement. She also gratefully acknowledges the valuable discussions with Prof. N.P. Pal’mina and her technical support. She also expresses her appreciation and thankfulness to Jesús Aguilar for his careful literature search. Z.-Q. Liu thankfully acknowledges the support of the National Natural Science Foundation of China (No. 11302118), Natural Science foundation of Shandong Province (No. ZR2013AQ015) and the Science Foundation of Qufu Normal University (No. BSQD2012053, xkj201305 ).
References
Arani R, Bono I, Del Giudice E; Preparata G (1995). QED coherence and the thermodynamics of water. Int J Mod Phys B 9: 1813-1841.
Balabin IA, Beratan DN, Skourtis SS (2008). Electron transfer: Chemical roles of water. Wiley Encyclopedia of Chemical Biology 1–9.
Bono I, Del Giudice E, Gamberale L, Henry M (2012). Emergence of the Coherent Structure of Liquid Water. Water 4: 510–532.
Botsi A, Yannakopoulou K, Hadjoudis E, Waite J (1996). AMI calculations on inclusions complexes of cyclomaltoheptaose (β-cyclodextrin) with 1,7-dioxaspiro [5.5] undecane and nonanal, and comparison with experimental results. Carbohydrate Res 283:1-16.
Cabezas C, Alonso JL, López JC, Mata S (2012). Unveiling the shape of aspirin in the gas phase. Angew Chem Int Ed 51: 1375 –1378.
Del Giudice E, Prepara G, Vitiello G (1988). Water as a free electric dipole laser. Phys Rev Lett 61: 1085-1088.
Del Giudice E, Preparata G, Fleischmann M (2000). QED coherence and electrolyte solutions. J Electroanal Chem 482: 110–116.
Del Giudice E, Vitiello G. (2006). Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transitions. Phys Rev A 74: 022105-1-022105-9.
Del Giudice E, Spinetti PR and Tedeschi A (2010). Water dynamics at the root of metamorphosis in living organisms. Water 2: 566-586.
Del Giudice E, Tedeschi A, Vitiello G, Voeikov VL (2013). Coherent structures in liquid water close to hydrophilic surfaces. J Phys: Conf Ser 442: 012028.
Elia V, Niccoli M (1999). Thermodynamics of Extremely Diluted Aqueous solutions. Ann New York Acad Sci 879: 241-248.
Elia V, Niccoli M (2000). New Physico-chemical properties of water induced by mechanical treatments: A calorimetric study at 25ºC. J Therm Anal Cal 61: 527-537.
Elia V, Niccoli M (2004). New Physico-chemical properties of extremely diluted aqueous solutions. J Therm Anal Cal 75: 815-836.
Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M (2013). Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with a Nafion® polymer membrane. WATER Journal 5: 16-26.
Elia V, Germano R, Napoli E (2015). Permanent Dissipative Structures in Water: The Matrix of Life? Experimental Evidences and their Quantum Origin. Curr Top Med Chem 15: 559-571.
Felder CE, Prilusky J, Sillman I, Sussman JL (2007). A server and database for dipole moments of proteins. Nucleic Acids Res 35: W512–W521.
Georgalis Y, Kierzek AM, Saenger W (2000). Cluster Formation in Aqueous Electrolyte Solutions Observed by Dynamic Light Scattering. J Phys Chem B 104: 3405-3406.
Hagmeyer D, Ruesing J, Fenske T, Klein HW, Schmuck C, Schrader W, Minas da Piedade ME, Epple M (2012). Direct experimental observation of the aggregation of α-amino acids into 100-200nm clusters in aqueous solution. RSC Advances 2: 4690-4696.
Horne RA (1972). Water and Aqueous Solutions: Structure, Thermodynamic and Transport Processes, chapters 8-12. Wiley-Interscience New York.
Huq F (2006). Molecular Modelling Analysis of the Metabolism of Ibuprofen. J of Pharmacology and Toxicology 1: 456-463.
Iitaka T (2010). Stability of ferroelectric ice. arXiv: 1007.1792v1 cond-mat.mtrl-sci.
Kemp DD, Gordon MS (2008). An Interpretation of the Enhancement of the Water Dipole Moment Due to the Presence of Other Water Molecules. J Phys Chem A 112: 4885–4894.
Kondrashina YG, Vul’fson SG, Timosheva AP (1994). Kerr constants and dipole moment of aminibenzoic acids in dioxane. Russ Chem Bull 43: 803-805.
Kononov LO, Tsvetkov DE, Orlova AV (2002). Conceivably the first example of a phase transition in aqueous solutions of oligosaccharide glycosides. Evidence from variable temperature 1H NMR and optical rotation measurements for a solution of allyl lactoside. Russ Chem Bull Int Ed 51: 1337—1338.
Konovalov AI, Ryzhkina IS, Murtazina LI, Timosheva AP, Shagidullin RR, Chernova AV, Avvakumova LV, Fattakhov SG (2008). Supramolecular systems based on the melamine salt of bis(hydroxymethyl) phosphinic acid (melafen) dihydrate and surfactants : 11. Structure and self-association of melafen in water and chloroform. Izv Akad Nauk Ser Khim 1207–1214; Russ Chem Bull Int Ed. 57: 1231-1238.
Konovalov AI (2013). The formation of nanosized molecular ensembles in highly dilute aqueous solutions. Herald of the Russian Academy of Sciences 83: 513–519.
Konovalov AI, Ryzhkina IS (2014a). Formation of nanoassociates as a key to understanding of physicochemical and biological properties of highly dilute aqueous solutions. Russ Chem Bull Int Ed 63: 1-14.
Konovalov AI (2014b). Nano associates: Terra incognita. Science in Russia 199: 1-10.
Konovalov AI, Mal’tseva EL, Ryzhkina IS, Murtazina LI, Kiseleva YuV, Kasparov VV, Pal’mina NP (2014c). Formation of nanoassociates is a factor determining physicochemical and biological properties of highly diluted aqueous solutions. Dokl Phys Chem 456: 86-89.
Kumazaki M, Shintaro S (1972). Dielectric Studies of Electrolytic Poly-alpha-Amino Acids in Aqueous Solutions. J Phys Soc Jpn 32: 785-791.
Kurban S, Erkoç F, Erkoç S (2010). Quantum chemical treatment of β-sitosterol molecule. Pharm Bio 48: 637-642.
Li J, Cramer JJ, Truhlar DG (1999). Application of a universal solvation model to nucleic acid bases: Comparison of semiemperical molecular orbital theory, ab initio Hartree-Fock theory, and density functional theory. Biophys Chem 78:147-155.
Li L, Ogawa T (2000). Clusters and their properties in aqueous solutions of KDP, KCl and sugar. J Cryst Growth 211: 286-289.
Liu ZQ, Jiang SR, Yinnon TA, Kong XM, Li YJ (2015). Effects of interfaces on dynamics in micro-fluidic devices: slip-boundaries’ impact on rotation characteristics of polar liquid film motors. Submitted to Microfluid Nanofluid. arXiv: 1404.5136.
Lo S-Y (1996). Anomalous state of ice. Mod Phys Lett 10: 909-919.
Lo S-Y, Geng XU, Gann, D (2009). Evidence of stable-water-clusters at room temperature and normal pressure. Phys Lett A 373: 3872 -3876.
McClellan AL (1963). Tables of experimental dipole moments. W.H. Freeman and Company, San Fransisco.
Nelson RD, Lide DR, Maryott AA (1967). Selected values of electric dipole moments for molecules in the gas phase. National Standard Reference Data Series – National Bureau of Standards 10.
Palmina NP, Chasovskaya TE, Ryzhkina IS, Murtasina LI, Konovalov AI (2009). Water solutions of phenosan potassium salt: influence on biological membrane structure and conductivity. Dokl Biochem Biophys 429: 301–304.
Pershin SM (2014). Konovalov effect in low concentration aqueous solutions: The role of ortho/para spin isomers of water. Dokl Phys Chem 455: 37–40.
Preparata G (1995). QED Coherence in Matter. World Scientific, Singapore, New Jersey, London, Hong Kong.
Protirelin: http://bioinf.charite.de/superdrug/fullinfo.php?cas=024305279
Raabe G (2004). Semiempirical Calculations on the Dipole Moment Enhancement in the Solid State. Z Naturforsch 59a: 977 – 979.
Rajalakshmi G, Devipriya B, Parameswari AR, Stephen AD, Kumaradhas P (2011). Understanding the N-N bond cleavage and the electrostatic properties of isoniazid drug molecule via theoretical charge density study. Comp Theor Chem 966: 259-264.
Ryzhkina IS, Murtazina LI, Kiseleva YuV, Konovalov AI (2009a). Properties of Supramolecular nanoassociates formed in aqueous solutions of biologically active compounds in low or ultra-low concentrations. Dokl Phys Chem 428: 196–200.
Ryzhkina IS, Murtazina LI, Kiseleva YuV , Konovalov AI (2009b). Supramolecular Systems Based on Amphiphilic Derivatives of Biological Active Phenols: Self-Assembly and Reactivity over a Broad Concentration Range. Dokl Phys Chem 428: 201-205.
Ryzhkina IS, Murtazina LI, Sherman ED, Valitova YuN, Kataev EA, Konovalov AI (2010a). Low-Concentration Aqueous Solutions of Macrocyclic Pyridine-Pyrrole Compound: Relationship between the Parameters, Physicochemical Properties, and Physiological Activity of Supramolecular Nanosized Associates. Dokl Phys Chem 443: 142-146.
Ryzhkina IS, Murtazina LI, Nemtarev AV, Mironov VF, Academician Konovalov AI (2010b). Supramolecular water systems based on the new amphiphilic phosphacoumarins: synthesis, self-organization and reactivity. Mendeleev Commun 20: 148-150.
Ryzhkina IS, Murtazina LI, Sherman ED, Pantyukova ME, Masagutova EM, Pavlova TP, Fridland SV, Academician Konovalov AI (2011a). Physicochemical Substantiation of the Hormetic Response of Biosystems for Wastewater Treatment to the Action of Solutions of N,N_Diphenylguanidinium Bis(hydroxymethyl)phosphinate. Dokl Phys Chem 438: 98–102.
Ryzhkina IS, Kiseleva YuV, Murtazina LI, Pal’mina NP, Belov VV, Mal’tseva EL, Sherman ED, Timosheva AP, Academician Konovalov AI (2011b). Effect of α-Tocopherol concentrations on the self-organization, physicochemical properties of solutions, and the structure of biological membranes. Dokl Phys Chem 438:109–113.
Ryzhkina IS, Kiseleva YuV, Zheltukhina GA, Okorochenkov SA, Murtazina LI, Timosheva AP, Nebolsin VE, Academician Konovalov, AI (2011c). Relationship between self-organization, physicochemical properties, and biological activity of aqueous solutions of hemin derivatives. Dokl Phys Chem 440: 157-161.
Ryzhkina IS, Murtazina LI, Academician Konovalov AI (2011d). Action of the external electromagnetic Field is the condition of nanoassociate formation in highly diluted aqueous solutions. Dokl Phys Chem 440: 201–204.
Ryzhkina IS, Murtazina LI, Nemtarev AV, Mironov VF, Kataev EA, Academician Konovalov AI (2011e). Self-association of a phosphate receptor along and with a lipidomimetic in water: Effect of receptor low concentrations on the catalytic activity of mixed systems. Chem Phy Lett 511: 247-250.
Ryzhkina IS, Kiseleva YuV, Murtazina LI, Academician Konovalov AI (2012a) Effect of ultralow concentrations and electromagnetic fields. Dokl Phys Chem 446: 153–157.
Ryzhkina IS, Murtazina LI, Masagutova EM, Mishina OA, Pavlova TP, Fridland SV, Academician Konovalov AI (2012b). Self-Organization of Sodium Chloride Solutions in the Absence and Presence of a Biologically Active Substance of Low Concentration under Common and Hypoelectromagnetic Conditions. Dokl Phys Chem 446:184-189.
Ryzhkina IS, Kiseleva YuV, Timosheva AP, Safiullin RA, Kadirov MK, Valitova YuN, Academician Konovalov AI (2012c). Low-Concentration Aqueous Solutions of an Amphiphilic Calix[4]resorcinarene Derivative: Self-Organization, Physicochemical Pro-perties, and Biological Activity under Common and Hypoelectromagnetic Conditions. Dokl Phys Chem 447: 193-199.
Ryzhkina IS, Kiseleva YuV, Murtazina LI, Mishina OA, Sherman ED, Academician Konovalov AI (2012d). Comparative Study of Self-Organization and Physicochemical Properties of Highly Diluted Aqueous Solutions of Phenol Bioantioxidants. Dokl Phys Chem 447: 203-206.
Ryzhkina IS, Kiseleva YuV, Mishina OA, Timosheva AP, Sergeeva SY, Kravchenko AN, Academician Konovalov AI (2013). Correlations between the self-organization, physicochemical properties and biological activity of Mebicar in dilute aqueous solutions. Mendeleev Commun 23: 262-264.
Samal S, Geckeler KE (2001). Unexpected solute aggregation in water on dilution. Chem Commun 2224–2225.
Sedlak M (2006). Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: Light scattering characterization. J Phys Chem B 110: 4329-4338; 4339-4345; 13976-13984.
Sedlak M, Rak D (2013). Large-scale inhomogeneities in solutions of low molar mass compounds and mixtures of liquids: Supramolecular structures or nanobubles? J Phys Chem B 117: 2495-2504.
Sivasubramanian S, Widom A, Srivastava YN (2005). The Clausius–Mossotti phase transition in polar liquids. Physica A 345: 356 -366.
Yinnon CA, Yinnon TA (2009). Domains in aqueous solutions: theory and experimental evidence. Mod Phys Lett 23: 1959-1973.
Yinnon TA, Yinnon CA (2011). Electric dipole aggregates in very dilute polar liquids: Theory and experimental evidence. Int J Mod Phys B 25: 3707-3743.
Yinnon TA, Yinnon CA (2012). Domains of solvated ions in aqueous solutions, their characteristics and impact on electric conductivity: theory and experimental evidence. Mod Phys Lett 26: 1150006-1 to 1150006-14.
Yinnon TA, Elia V (2013). Dynamics in perturbed very dilute aqueous solutions: theory and experimental evidence. Int J Mod Phys B 27: 1350005-1 to 1350005-35.
Yinnon TA, Liu Z-Q (2015a). Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 1. Quantum Electrodynamic Aspects. WATER Journal 7: 33-47.
Yinnon TA, Liu Z-Q (2015b). Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 2. Quantum Electrodynamic Analyses of Experimental Data on Strong Electrolyte Solutions. WATER Journal 7: 48-69.
Yinnon TA, Elia V, Napoli E, Germano R, Liu ZQ (2015c). Water ordering induced by interfaces: an experimental and theoretical study. WATER Journal In Press, 2016.
Zubareva GM, Kargapolov AV, Yaguzhinsky LS (2003). Specific effect induced by subminute amounts of ascorbic acid on the fluctuation of transmission factor of water in the infrared spectral range. Dokl Biochem Biophys 388: 43 – 45.
