 Properties and Size of Multiple Non-bulk Water Fractions on Proteins and in Cells
Properties and Size of Multiple Non-bulk Water Fractions on Proteins and in Cells
Cameron I1* and Fullerton G21
Department of Cellular and Structural Biology, University of Texas Health Science at San Antonio, San Antonio, Texas, 78229 USA
2Department of Radiology, University of Colorado, 12700 East 19th Avenue, Aurora, Colorado, 80045 USA
*Correspondence E-mail: cameron@uthscsa.edu
Key Words: Water, Proteins, Cells, Water-of-hydration, Non-bulk-water, Water properties
Received May 9th, 2014; Revised Sept 24th, 2014; Accepted Oct 5th, 2014; Published Oct 25th, 2014; Available online Nov 30th, 2014
doi: 10.14294/WATER.2014.5
Abbreviations: g/g grams water per gram dry mass, SHM stoichiometric hydration model, SASA solvent accessible surface area, QENS quasi-elastic neutron scattering spectroscopy, NMR nuclear magnetic resonance, CD circular dichroism spectroscopy, VW vicinal water, EZ exclusion zone water, PML polarized multilayered water, OUR osmotically unresponsize water, TEM transmission electron microscopy.
Abstract
This report reviews evidence on the physical properties and size, in g water per g dry mass (g/g) of multiple non-bulk water of hydration fractions on proteins and in cells. A molecular stoichiometric hydration model (SHM) is presented that explains the four observed and measured monolayer water of hydration fractions on tendon collagen (Fullerton et al. 2006a, Fullerton and Cameron 2007). This SHM has been shown applicable to globular proteins and to cells (Cameron et al. 2011). The extent of non-bulk water has been found to increase during protein unfolding and decrease during protein aggregation (Fullerton et al. 2006). This review also presents evidence that multilayers of water, with non-bulk water properties, extend out from the surface of proteins and biomacromolecules in cells. These facts have been largely overlooked or ignored by most protein chemists and cell biologists. In the conclusion it is recalled that a major fraction of cell water has physical and physiological properties that differ from those of bulk water. Thus studies that do not take the physical properties and size of non-bulk water fractions into account must be judged incomplete.
Article Outline
- Introduction
- Physical Measured Properties of Bulk and Non-bulk Water Fractions on Proteins and in Cells
- Terahertz Spectroscopy’s Contributions to Understanding the Extent of the Non-bulk Water of the Hydration Shell Around Proteins
- Conclusions
- References
- Discussion with Reviewers
Introduction
The presence and extent of multiple water of hydration fractions on proteins and in cells has been a subject of debate (Ball 2008, Pollack and Clegg 2008, Fullerton and Cameron 2007, Cameron and Fullerton 2008, Cameron, Lanctot and Fullerton 2011). The physical characteristics and size of multiple water of hydration fractions has been measured on tendon/collagen by water proton NMR spin-lattice relaxation at different levels of collagen hydration (Fullerton et al. 2006). Three distinct hydration compartments were identified and their size quantified. The sizes were defined by integral multiples of N = 1, 4 and 24 times the value of one water molecule bridge per three collagen amino acid residues (as proposed by Ramachandran in 1968). This single water bridge + 0.0658 g water / g dry. The results have led to a method for calculating the size in g water/g dry mass of each of four monolayer water of hydration fractions based on the known amino acid composition of tendon/collagen and of globular proteins. The findings led to development of the molecular stoichiometric hydration model (SHM) that explains the agreement between the four measured sizes of the water of hydration fractions in the monolayer of water covering the surfaces of native tendon/collagen and globular proteins (Fullerton and Cameron 2007, Cameron et al. 2011).
The names, sizes and location of four non-bulk water fractions are: 1) a single water bridge or Ramachardran bridge per every three amino acids. This single water bridge is located between covalent bound pairs of opposite partial charge on the protein backbone (0.066 g/g), 2) a double water bridge occurs between fixed opposite charge groups that are located a little further apart (0.20 g/g). Together these two fractions equal 0.26 g/g and is termed backbone hydration 3) a dielectric water clusters occurs over the remaining polar-hydrophilic surface 0.54 g/g and 4) water clusters occur over the remaining hydrophobic surface (0.8 g/g). These four water fractions constitute monolayer water coverage of the native collagen molecule for a total of 1.6 g/g on collagen. The same four fractions are calculated to occur in monolayer water coverage of a number of globular proteins (Fullerton and Cameron 2007, Cameron et al. 2011).
Protein chemist and cell physiologist generally acknowledge the presence and size of the first two water of hydration fractions listed above and commonly refer to this as “bound water” amounting to 0.2 to 0.4 g/g. In a review of this subject Ball (2008) points out that this “bound water” is indicated by x-ray scattering crystallography and is hydrogen bonded to specific sites on the protein surface and remains at these sites even when exposed to a vacuum at ambient temperatures. The motional correlation time of this bound water is reported to be between 100 to 1000 times slower than bulk water (Cameron and Fullerton 2008). The general assumption or claim by chemists and physiologists is that all water greater than this “bound water” amount has the physical properties of water in bulk or has bulk-like water dynamics. However there is growing experimental evidence to prove that the extent of water with non-bulk physical properties on proteins and in cells is much greater than the size of this well documented “bound water” fraction.
Physical Measured Properties of Bulk and Non-bulk Water Fractions on Proteins and in Cells
What are the measurable physical properties that distinguish between bulk and non-bulk water fractions? Table 1 list a number of physical properties. The list includes twelve properties but this list is not all-inclusive. The method of measurement of each listed physical property in Table 1 is referenced to published measurement reports on each of the properties.
Table 2 summarizes results on the measured sizes of bulk and non-bulk water fractions on proteins and in eukaryotic cells and tissue of vertebrates. The presence and size of multiple non-bulk water fractions is indicated. The size and name of four monolayer fractions are listed for the mean value of eight proteins and for five vertebrate tissue cell types. The individual size values measured for the four monolayer water subcompartments closely match the size predicted by the SHM. A variable sized multilayer water fraction is also listed in Table 2.
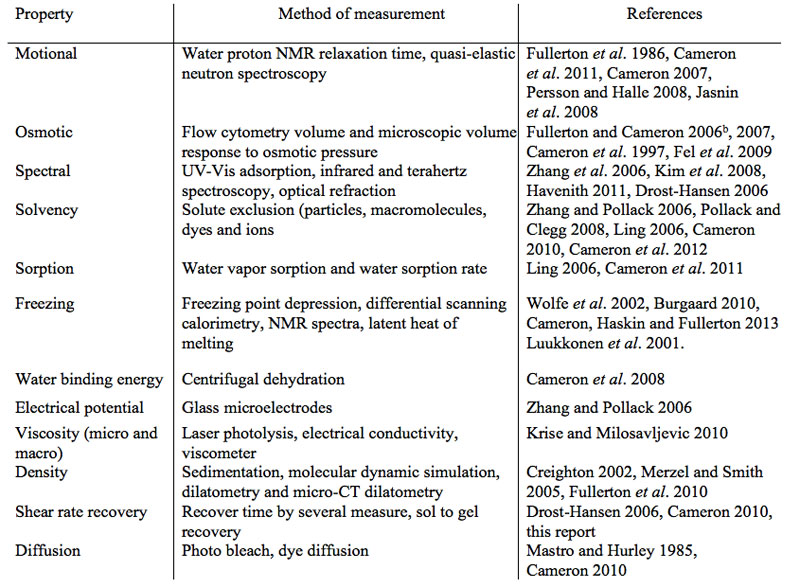
Table 1: Methods used to measure the physical properties of bulk and non-bulk water fractions on proteins and in cells.
The extent of non-bulk water over the total tissue water content (less extracellular water assumed to be bulk) was used to calculate the total extent of non-bulk water in the listed tissues. The mean of the eight values for percent non-bulk water in Table 2, is 85%.
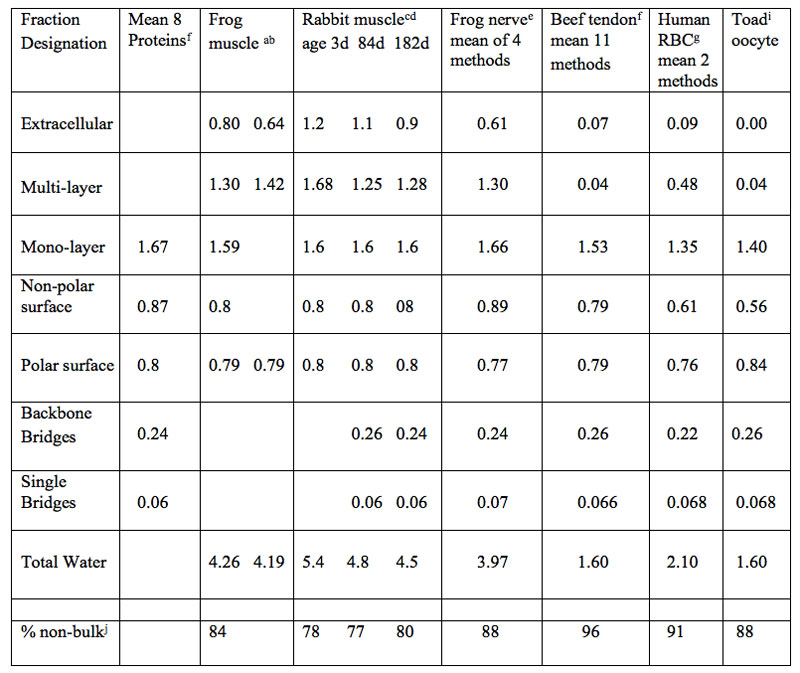
Table 2: Measured size of protein and vertebrate tissue water of hydration fractions (g water/g dry mass), d=days of age.
aKimura et al. 2005
bBelton et al. 1972
cBerenyi et al. 1996
dCameron et al. 2008
eKalona and Vaslescu 1985
fCameron et al. 2011
gCameron et al. 1988, Fullerton and Cameron 2006
fFullerton and Cameron 2007
iCameron et al. 1996
jAssuming extracellular fraction is bulk water this fraction was subtracted from the total tissue water value.
Table 3 summarizes data from four recent reports on the extent of non-bulk water in g water/g dry mass (g/g) in growing and dormant (spore core) bacteria. Two bacterial species, Escherichia coli and the extreme halophile Haloarcula marismortui, were reported to have 85% of their water molecules with bulk-like water dynamics while the remaining 15% was motionally retarded by a factor of 15 on average with an overall correlation time of 27 ps vs. bulk water of 1.74 ps. This slower correlation time is attributed to slower motion of water molecules in direct contact with the macromolecular surface (monolayer) and to surface hydration sites secluded (buried) in supermolecular assemblies (Persson and Halle 2008, Qvist et al. 2009).
Given a total water content of 2.58 g/g for E. coli the 15% non-bulk water would amount to 0.39 g/2.58 g for E. coli (Table 3). This non-bulk water value was calculated based on the solvent (water molecule) accessible surface area (SASA) in the first monolayer coverage of proteins and nucleic acids. Given a total water content of 1.09 g/g for H. marismortui, also with 15% non-bulk water, would amount to 0.16 g/g in direct contact with the macromolecular surface (Table 3).
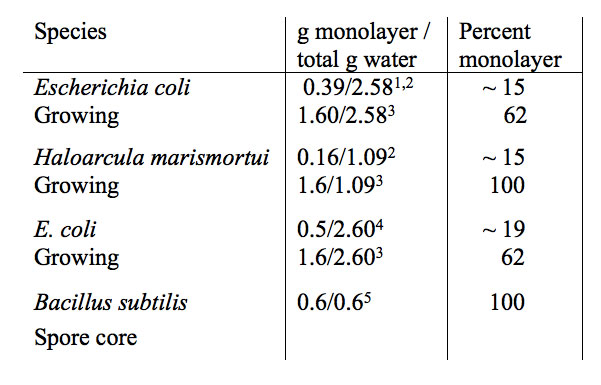
Table 3: Extent of monolayer and bulk water (g water/g dry mass) in growing and dormant (spore core) bacteria. SASA=solvent accessible surface area.
- After subtraction of extracellular water.
- Persson and Halle 2008 and, Qvist et al. 2008, concluded that water outside the first hydration monolayer was practically indistinguishable from bulk water using proton NMR spin relaxation rate data. Bacteria were pelleted using a 10,000 g force prior to measurements. Water solvent accessibly surface area (SASA) calculation was used to determine g non-bulk water on proteins and nucleic acids.
- Fullerton and Cameron 2007 used the SASA method of Miller et al. 1987a,b on numerous native protein types and report a consistent value in the 1.5 to 1.7 g/g range. The SASA value of 1.6 g/g was used to re-evaluate the extent of monolayer water in these bacterial cells.
- Jasnin et al. 2008 using QENS report that the viscosity of cell water was essentially the same as bulk water. Bacteria were pelleted using a 5,000 g force prior to measurements. The total water residence time was two times longer than that of bulk water. This slowing was attributed to a monolayer water fraction on the cells macromolecular surface. The 0.5 g/g monolayer value used by Jasnin et al. was obtained from Record et al. 1998.
- Sunde et al. 2009 using dynamic perturbation proton spin relaxation reported that the average spore core water rotational correlation time was 34 times slower than bulk water but not to that expected where it is in a glass-like state. Spore cores were pelleted using a 6,800 x g force prior to measurement.
There are two concerns with the studies and reports of Persson and Halle (2008) and Qvist et al. (2009) and Jasnin et al. (2008).
The first concern is with their estimates of the extent of monolayer water coverage on intracellular proteins. Persson and Halle, (2008) and Qvist et al. 2009) calculate, based on the SASA, that about 15% of cell water interacts directly as monolayer coverage on the macromolecular surfaces (about 0.39 g/g for E. coli). However, reports of Fullerton and Rahal 2007, Fullerton and Cameron 2007, Cameron and Fullerton 2011 indicate a much greater calculated and measured monolayer coverage of about 1.6 g/g on native proteins (Table 2). Thus given the total water content of E. coli at 2.58 g/g, then a water coverage over the surface monolayer in E. coli of 1.6 g/g (Table 3) would decrease to about one water layer above this first monolayer.
What this indicates is that the measured water motional retardation by a factor of 15 (as reported by Persson and Halle 2006 and Qvist et al. 2009), that was attributed to just a 15% of the intracellular water in their calculated monolayer is questionable. The calculation of a much larger monolayer value of 1.6 g/g suggests that water in the outer layers of interfacial water may be more slowed in motion than water in the bulk water state. The average correlation time of cell water would be expected to decrease from 27 ps to several times slower than the correlation time of bulk water (1.74 ps). This degree of slowing is in the reported measured range for monolayer water coverage of tendon/collagen (Fullerton and Rahal 2007 and Cameron and Fullerton 2008).
The second concern with the studies and reports of Persson and Halle 2008, Qvist et al. 2009 and Jasnin et al. 2008, is with their use of high g force centrifugation to pellet their bacterial cells prior to measurement of cell water dynamics (Table 3). Persson and Halle (2008) tested the effect of their measurement procedure, including centrifugation at 10,000 x g for 25 minutes, on viability of E. coli and report that 70% of the cells survived the entire measurement procedure. It is not clear from this if centrifugation was or was not responsible for the lower than optimal cell survival value. Jasnin et al. (2008) pelleted E. coli using a g force of 5000 as part of their preparative procedure. It is therefore reasonable to ask if the centrifugation procedures used in the preparative cell dynamic measurement protocol may have changed the cell dynamic measurement results. Experiments have been designed to directly address the possible effect of centrifugation on viscosity and proton NMR spin-lattice (T1) relaxation time using thick egg white hydrogel and skeletal muscle. The viscous thick albumen hen egg white is a hydrogel fraction and can be separated from the thin egg white sol fraction by sieve filtration (Cameron and Fullerton 2010). The shaking of thick albumen gel caused the transition from a gel state to a sol state as demonstrated by flow through the sieve filter. Centrifugation of the thick gel, with a g force of 1500 for 30 minutes, caused a significant longer proton NMR longitudinal, spin-lattice (T1) relaxation time (Table 4). Notice however that allowing the sample in the centrifuge tube to rest for 4 hours following the centrifugation allowed the T1 time of the non-centrifuged thick albumen to recover. Centrifugation of the thick gel at 1500 x g for two hours allowed a fluid sol state to be poured off the top of the centrifuge tube. Proton NMR T1 times were measured in both the fluid sol fraction and the non-fluid gel fraction and the results demonstrated a significantly longer T1 relaxation time in the sol vs. the gel fraction (Table 4).

Table 4: Centrifugal force 1500 x g on water proton NMR spin-lattice (T1) relaxation time (m sec) of hen egg white hydrogela.
aAnalysis of variance followed by SNK multiple range test indicates that all means are significantly different except the mean of the 4 hr rest which is not significantly different than no centrifugation.
What these finding demonstrate is that, use of a centrifugal g force 3 to 6 times less than used in the studies of Jasnin et al. 2008, and of Persson and Halle 2008 is enough to change the water dynamic measurement values. The results reported in figure 1 show that centrifugation of fresh fish skeletal muscle with a g force of 14,000 (a somewhat higher g force than used to pellet bacterial cells) caused a significant time dependent lengthening of the T1 relaxation time. Clearly the use of g force in the preparative procedure used prior to measurement of water physical properties is a concern that should not be ignored and must be taken into account.

Figure 1: The extent of osmotically unresponsive water (OUR) on proteins and in cells.Proton NMR-T1 relaxation time in milliseconds of fresh fish skeletal muscle vs duration of centrifugation in minutes at a g force of 14,000 x g. The linear slope of increase in T1 relaxation time with minutes of centrifugation is significant (p<0.05).
When a known globular protein is dissolved in bulk water the colligative properties of the resulting bulk water solution changes its osmotic pressure, freezing point, boiling point and vapor pressure. The colligative properties of such a solution are predicted by the well-known ideal equation PV = nRT. But there is deviation from this ideality when adding a known number of globular protein particles to the solvent bulk water and this difference from ideal behavior has been attributed to an escape of some of the bulk water molecules onto and into the proteins. This is the simplest explanation but leads to questions of: 1) where have the escaped water molecules gone and 2) does the protein change its ability to interact with more or fewer water molecules by alteration of structural conformation and state of aggregation?
These two questions have been addressed by study of the globular protein, bovine serum albumin (BSA), by varying the salt concentration or the pH. The resulting experimental data were analyzed using the molecular model of Fullerton et al. (1992), to give both the extent of OUR water and the effective molecular weight of the membrane – impermeable BSA. The findings (Fullerton et al. 2006) show that change in salt concentration and of the pH, acting as membrane-penetrating cosolutes, markedly changes the BSA’s structural conformation and aggregation and in this way the extent of OUR water. The extent of change in amount of OUR water in BSA ranged from a minimum of 1.4 to a maximum of 11.7 g water per unit of dry mass of BSA. Changes in molecular weight values were measured and models of BSA conformation (i.e. protein folding and unfolding and aggregation and segmental motion) were offered to explain these results.
The extent of OUR water in cells has been reported in five vertebrate cell types (Cameron et al. 1997, Fullerton et al. 2006b, Cameron and Fullerton 2014) and ranges from 1.1 to 2.2 g water per g dry mass. Data in a report by Maric et al. (2001) has been re-evaluated to give the OUR value of yet another cell type, that can now be added to the cell list of OUR water values. The cell type reported by Maric et al. is the primary cultured rat kidney inner medullary collecting duct (IMCD) cells. The method for measuring the cell volume change of the tissue cultured adherent IMCD cells was laser scanning reflection microscopy. Their cell swelling data fit a single exponential equation (r2 value 0.96) but a reanalysis of their data using a two phase exponential fit gives an even better data fit (r2 value 0.9874). Looking at the plot of the data in their figure 5 indicates that the data points of the higher osmotic pressure values fall below that of the single exponentional fit plot. A second indicator of a cells responses to the osmotic challenge is to plot the cell volume or water content versus the reciprocal of the osmotic pressure (one over the osmotic pressure of the bathing media). This procedure was done by using the data from Figure 5 of Maric et al. and the results are illustrated in Figure 2. This new figure reveals a linear slope with an Y- intercept of 64.75 area % (volume) at infinite osmotic pressure. This finding demonstrates that the IMCD cells are not responding as expected if the cells are acting as an ideal osmometer, because the linear slope of an ideal osmometer fit is expected to have a Y- intercept of zero. The difference between zero and the 64.75 area % value indicates that a volume of the water in the IMCD cell is OUR water. Also notice that the highest osmotic pressure area % values in this new figure fall below the best linear fit slope. This deviation from linearity indicates that at the higher osmotic pressures the cell is reducing the size of its OUR water fraction.

Figure 2: The correlation of the IMCD cell x-z-scan section area expressed in percent of control (cell volume measure) with change in bathing solution osmolarity (expressed as one over milliosmols). The data points were taken from Figure 5 of Maric et al. 2001. Notice the deviation from linearity of the highest osmolarity values and that the Y-intercept is not zero as expected were the cells acting as an ideal osmometer (see text for discussion).
Just how much of the IMCD cell water is OUR and at what extracellular osmotic pressure does the cell begin to deviate from the linear osmotic pressure slope? If one assumes that the water content of the IMCD cells at isosmotic pressure is 3.0 g water per g dry mass, then the data in Figure 2 can be replotted. The deviation of the highest osmotic pressure values from a linear fit occurred at about 600 m osm/kg dry mass.
If one eliminates the non-linear high osmotic pressure values and re-calculates the Y-intercept, one gets an OUR value of 2.41±0.216 g/g DM. Given an isosmotic IMCD total water content of 3.00 g/g the OUR water values of 2.41 g/g indicates that 80% of cells water is OUR at isosmotic and hypoosmotic environment conditions studied but that some of this OUR water becomes osmotically responsive under the hyperosmotic conditions in this study. This finding, taken together with the five previous OUW values reported for the other five vertebrate cell types (Fullerton et al. 2006), now ranges from 1.1 to 2.4 g/g.
A question posed above was: where has such a large amount of OUR water escaped or gone? Possibilities are: it has become slowed in its mobility by association with large slower moving molecules /proteins or it is encapsulated inside the proteins, or the cell hydrogel (Fels et al. 2009) or within other cellular compartments (Fullerton and Cameron 2007). The OUW is therefore excluded from thermodynamic solution expressions (like osmotic pressure). Encapsulated or motionally slowed water molecules are therefore restricted in their ability to interact with osmotic membranes.
Terahertz Spectroscopy’s Contributions to Understanding the Extent of the Non-bulk Water of the Hydration Shell Around Proteins
Terahertz spectroscopy techniques have become available for study of the collective motion of water molecules around biological molecules on a picosecond time scale (Heugen et al. 2006, Ebbinghaus et al. 2007, Born and Havenith 2009, Ding et al. 2010, Kim et al. 2008). By studying the change in absorbance with concentration of biological molecules the size of the hydration layer can be deduced. The studies reveal dynamic reorientation of water molecule outward from proteins surface for distances of up to 2 or more nanometers (7 or more water layers). The size of this shell of motionally perturbed water is larger than that found by NMR, neutron or x-ray crystallography measures.
Havenith (2011) and her group have developed a new method, called kinetic terahertz absorption (KITA), that enables study of changes in terahertz absorbance during the protein folding process on a m sec. time scale of resolution (Kim et al. 2008, Born and Havenith, 2009). The data indicates a rapid rearrangement of the protein hydration shell about 100 times faster than protein folding, as measured by CD and fluorescence spectroscopy. Thus water-protein rearrangement is a process in the protein folding process.
Such terahertz information has implications for cell biology because the proteins in cells are so closely packed that there is only about 2 to 3 nm between proteins. Thus there is just enough space for about 7 to 10 layers of water molecules between proteins. Terahertz measures reveal that the dynamic hydration layer around an individual protein extends out for 9-10 water molecules from the proteins surface (Born and Havenith 2009). The number of water molecules in the dynamic hydration shell of the protein ubiquitin extends up to 1.8 to 3 nm the protein surface amounting to a total hydration shell of 1000 water molecules. In cells a protein dynamic water shell of this size would extend so far as to overlap with the water shell of other proteins.
Two implications of these findings are: intracellular communication between dynamic water-protein networks seems possible, if not likely, and given the crowded cytoplasm of cells and the large hydration shell of proteins and other cellular molecules there would appear to be little space left for a bulk water fraction in cells. LeBard and Matyushov (2010) have recently reported results on the extent of the ferroelectric hydration shell around the protein (plastocyanin). This hydration shell extends 3-5 layers or 08.4 to 1.4 nm out from the proteins surface. This finding on the extent of the ferroelectric hydration shell around proteins and the results of the extend of the hydration shell of proteins, as measured by terahertz spectroscopy, reveal a multilayer water of hydration shell around proteins. Such large observed hydration shells on proteins describe the extent of the fast motional networks of water molecules that are affected by the protein surface. This level of hydration far exceeds that of the more static hydration layers of attached water molecules as measured by x-ray and neutron crystallography and NMR.
Exclusion Zone, Vicinal or Multilayers of Water Over Substrate Surfaces Has Physical Properties that Differ from Those of Bulk Water
The concept that multilayers of water adjacent to many hydrophilic surfaces differ from that of water in bulk has a long history (Pollack and Clegg 2008). Terms that have been used to describe non-bulk water multilayers are: unstirred layers (USLs), vicinal water (VW) and exclusion zone water (EZ). Exclusion zone water differs in its physical properties from that of bulk water (Drost-Hansen 2006, Zheng et al. 2006, Ling 2006) including: osmotic unresponsiveness, UV-Vis absorption spectrum, diffusion, infra-red spectrum, exclusion of latex microspheres and a host of other particles and dyes, viscosity, density, shorter proton NMR-T2 relaxation times, freezing properties, selective exclusion of hydrated ions of different diameter (Table 1).
That exclusion zone water has physical properties that differ significantly from those of bulk water has had little notice by most physiologists and cell biologists. As discussed above (Cameron and Fullerton, 2008) the prevailing dogma among physiologists and cell biologists is that all but a small fraction of cell water (estimated to be in the range of 0.2 to 0.4 g water/g dry mass) is bulk or bulk-like in its physical properties. Opposition to this dogma has come from a number of scientists including Pollack and Clegg 2008, Cameron and Fullerton 2011, Ling 2006, Mentré 2001, 2012).
Ultrastructural evidence for cell zones lacking observable structural elements adjacent to membranes and cytoskeletal structures is summarized next. Transmission electron microscopic (TEM) observations give evidence of a clear structureless zone of cytoplasm or hyaloplasm adjacent to: some plasma membranes, Golgi apparatus membranes, actin filaments and microtubules. Mollenhauer and Morre (1978) described such zones as lacking ribosomes and cytoplasmic organelles. A review of literature indicates that microtubules (Stebbings and Willison 1973, Stebbings and Hunt 1982), actin filaments (Kamitsubo 1972), region just inside cilia membrane and mitochondria (Trombitas et al. 1993) are surrounded by a zone where electron dense material is scarce or absent. One has only to look at transmission electron microscopic images to see such zones (Fawcett 1966). The following is a list of such ultrastructure zone examples found in Don Fawcett’s atlas, The Cell (1966, Saunders Philadelphia) by location and figure numbers:
1) nuclear envelope 1, 5-8, 223; 2) lamellar cisternae Golgi 74; 3) rough ER cisternae 85; 4) dense zymogen granule core and membrane 89; 5) protein crystal and surrounding membrane 90; 6) around microtubules 134; 7) under plasma membrane 135, 136, 199, 205, 230, 239; 8) halo around mucous droplets 142; 9) cilia membrane and microtubule doublets 230; 10) sperm mid piece: under cell membrane 235, mitochondria and outer fiber sheath 238.
Mayer et al. (2006) also report the presence of apparent structureless spaces adjacent to cellular membranes. Even the removal of most of the cells water, by osmosis, was not enough to totally deplete zones lacking EM observable structures (Albrecht-Buchler and Bushnell, 1982).
Some approximate sizes of ultrastructural zones are: clear space between the microvillus plasma membrane and the actin filaments 20-25 nm, space between the cilia plasma membrane and the outer microtubule doublet 21 nm and the space around microtubules > 20 nm. It may be that these structureless zone contain non-aqueous material not observable by EM. There is a note of warning to add to these EM observations, which is that tissue preparation for TEM sectioning normally involves dehydration that could have caused shrinking and the artifactual creation of the observed exclusion zones. It also seems that regions devoid of organelles can contain considerable macromolecules (Zierold 1986). Electron transparent zones in electron micrographs may contain macromolecules perhaps in a gel like network as described next.
Gallyas and Pal (2008) report that head injury causes some normal neuron axons to shrink and stain “dark”. They propose that the observed ultrastructural space between microtubules in normal nerve axons consist of a contractible intracellular gel that is continuous in all or most spaces among the visible ultrastructural elements. Shrinkage of this hypothesized gel was thought to be responsible for the ultrastructural compaction observed in dark neurons and dark axons. They also propose that the uncompacted undamaged axon gel is surrounded with multilayers of oriented water molecules that can lose its multilayer orientation resulting in gel compaction and cell water lose.
Support for their proposal is found by a morphometric analysis of cross sections of normal and dark axons from figure 5 of their report. In the “dark” compacted axon there is a 41% increase in number of microtubules per unit area and a 44.5% decrease in average distance between microtubules. This suggests a large percent water loss value which appears to agree with the cross sectional area decrease observed between the uncompacted normal axon and the compacted “dark” axon in figure 5 of Gallyas and Pal (2008). These authors suggest that membrane derived and gel derived cell function can coexist. Don’t confuse the exclusion zones demonstrated by Pollack at the surface of various objects and the clearest zones observed by EM in cells. The first ones are made of pure water. The second ones may indeed by gels that exploit the properties of interfacial water.
Scientists have proposed ideas to explain existence of multilayers of water with non-bulk properties over biological and non-biological surfaces (Klotz 1962, Ling 2006, Chaplin 2006 and Drost-Hansen 2006). Next is a brief summary of their ideas. Klotz in 1962 described the organization of water in contact with any surface. He proposed the ordering of water in a lattice around non-polar groups. Accordingly leading to extended regions of ice-like water on the surface of proteins. He suggested formation of pentagonal rings of water molecules that have now been reported over hydrophobic surface of protein crystals that extend into the solvent.
Ling’s theory is that protein surfaces with properly spaced negatively (i.e. carbonyl) and positively (i.e. imino) groups, as occurs on extended protein chains, serve as sites for polarized-oriented water molecules to form into polarized multilayers (PML). His theory is that a cardinal absorbent, like ATP, binds to a globular protein site and causes the unfolding of the protein to a more linear chain thus exposes a backbone of carbonyl and imino groups for PML formation. PML has size dependent solute exclusion properties and ion selective K+ over Na+ binding properties on the protein’s negative charge sites.
Chaplin’s idea is that carboxylate sites on two relatively immobile adjacent proteins (i.e. F-actin) bind K+ and form a clathate arranged water structure of 7 to 8 water layers (shell) around each surface. This interfacial water is said to have non-bulk freezing and osmotic properties (Garlid 2000). Chaplin’s theory is that more mobile proteins, (i.e. G-actin) loose K+ binding that may then help disrupt the clathate water structure. It is worth noting that K+ is a Hoffmeister series water structure breaker.
Drost-Hansen (2006) also has provided evidence for the presence of vicinal water layers over the surfaces of solids and of biopolymers of >2000 Daltons (MW). Vicinal hydration is said to be independent of the detailed chemistry of the macromolecular surface or solutes in the solution. Drost-Hansen agrees that surfaces with ionic sites electrostatically bind water molecules in their immediate vicinity causing a higher density than bulk water but he posits that there is structured (less dense 0.96-097 g/cm3) interfacial water layers that can extend out from 10 to more than 100 water layers, or from 5 to >50 nm.
Drost-Hansen described an interesting property of VW: the presence of well-documented thermal anomalies in several of its physical properties (viscosity, specific heat, solute distribution, diffusion coefficient, index of refraction, Bragg scattering) that occur at about 15, 30, 45 and 60˚C. Also VW is shear force sensitive and the high pressure generated in a spinning centrifuge tube can diminish or completely eliminate VW. The time to recover the vicinal water state can vary from minutes to hours or even days. Clearly centrifugal pressure and VW recovery time must be taken into account in experiment design.
Drost-Hansen concludes, “Since vicinal hydration can occur at all solid interfaces (including membranes) and with all large macromolecules in solution, it is little wonder that all cellular systems (i.e. all living systems) also show the effects of VW. Any biophysical or molecular biology theory that does not allow for – and specifically includes – VW must be judged to be incomplete.”
Conclusions
1) There is strong experimental evidence in literature for multiple subfactions of non-bulk water on tendon /collagen, globular proteins and in cells 2) The commonly held concept that water beyond a measured bound water fraction of 0.2 to 0.4 g/g on macromolecules has physical properties indistinguishable from bulk water is unwarranted. 3) A molecular stoichiometric hydration model (SHM), stemming from tendon/collagen measures appears to explain the four non-bulk water fractions that constitute the monolayer water coverage of collagen and of globular proteins (Table 2). The extent of monolayer water coverage for globular proteins averages 1.6 g/g but can increase due to globular protein unfolding or can decrease due to tighter protein folding, aggregation and crystallization. 4) Evidence that water beyond the first monolayer of water coverage over a proteins surface can have physical properties that distinguish it from that of bulk water is convincing. This type of water has been referred to as: multilayer, vicinal, unstirred and exclusion zone water and has been demonstrated to differ in a number of its physical properties from those of bulk water. 5) The physical properties of non-bulk water can be altered significantly by specimen preparative procedures such as centrifugation, shear force, agitation and heating and cooling (freezing). The delay time needed for recovery from such physical perturbation to return to the original unperturbed non-bulk water state is variable and ranges from minutes, to hours and even days. This fact must be taken into account in the design of experiments to measure the physical properties and the size of non-bulk water fractions.
Acknowledgements
The assistance of Kerri Glaspie with manuscript preparation is gratefully acknowledged.
References
Albrecht-Buehler G and Bushnell A (1982). Reversible compression of the cytoplasm. Exp Cell Res 140: 173-189.
Ball P (2008). Water as an active constituent in cell biology. Chem Rev 108: 74-108.
Beranyi E, Szendrö Z, Rόzsahegyi P, Bogner P and Sulyok E (1996). Postnatal changes in water content and proton magnetic resonance times in newborn rabbit tissues. Pediatr Res 39: 1091-1098.
Born B and Havenith M (2009) Terahertz dance of proteins and sugar with water. J Infrared Milli Terahz Waves 30: 1245-1254.
Burgaard MC (2010). Effect of frozen storage temperature on quality-related changes in fish muscle. PhD Thesis, Technical University of Denmark www.food.dtu.dk.
Cameron IL, Fullerton GD and Lanctot AL (2011). The molecular stoichiometric hydration model SHM applied to tendon/collagen, globular proteins and cells. Cell Biol Int 35: 1205-1215.
Cameron IL, Fullerton GD (2008). Interfacial water compartments on tendon/collagen and in cells. Phase Transition in Cells: 43-50. Eds. Pollack GH, Chin WC. Springer, Berlin.
Cameron IL, Kanal KM, Keener CR and Fullerton GD (1997) A mechanistic view of non-ideal osmotic and motional behavior of intracellular water. Cell Biol Int 21:99-113.
Cameron IL (2010) Dye exclusion and other physical properties of hen egg white. WATER Journal 2: 83-96.
Cameron IL, Haskin CL and Fullerton GD (2013). Multiple unfrozen water fractions in biological tissues: freezing point and size. WATER Journal 5:45-56.
Cameron IL, Fullerton GD (2014). Lack of appreciation of the role of osmotically unresponsive water in cell volume regulation. Cell Biol Int 38: 610-614.
Creighton TE (2002). Proteins: Structure and Molecular Properties. 2nd ed. Freeman pp 141.
Ding T, Li R, Zeitler JA, Huber TL, Gladden LF, Middeelberg APJ and Falconer RJ (2010). Terahertz and far infrared spectroscopy of alanine-rich peptides having variable elliplicity. Optic Express 18: 27431.
Drost-Hansen W (2006). Vicinal hydration of biopolymers: cell biological consequences. Water and the Cell: 175-218. Eds. Pollack GH, Cameron IL and Wheatley DN. Springer, Berlin.
Ebbinghaus S, Kim JS, Heyden M, Yu K, Heugen U, Gruebele M, Leitner DM and Havenith M (2007). An extended dynamical hydration shell around proteins. Proc Natl Acad Sci 104: 20749-20752.
Fawcett DW (1966). The Cell. Saunders, Philadelphia.
Fels I, Orlov SN, and Grygorczyk R (2009). The hydrogel nature of mammalian cytoplasm contributes to osmosensing and extracellular pH sensing. Biophys J 96: 4276-4285.
Fullerton GD, Amurao M, and Cameron IL (2010). Micro-CT dilatometry measures of molecule hydration using bovine extensor tendon. Med Phys 37: 1-14.
Fullerton GD and Cameron IL (1986). An evaluation of the hydration of lysozyme by an NMR titration methold. Biochem et Biophys Acta 869: 230-246.
Fullerton GD and Cameron IL (2007). Water compartments in cells. Methods Enzymol 428: 1-28.
Fullerton GD, Nes E, Anurao M, Rohal A, Krasosselskaia L, Cameron I (2006a). An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol Int 30: 66-73.
Fullerton GD, Kanal KM, and Cameron IL (2006). On the osmotically unresponsive water compartment in cells. Cell Biol Int 30: 74-77.
Fullerton GD, Kanal KM, and Cameron IL (2006b). Osmotically unresponsive water fraction on proteins: non-ideal osmotic pressure of bovine serum albumin as a function of pH and salt concentration. Cell Biol Int 30: 86-92.
Fullerton GD and Rahal A (2007). Collagen structure: the molecular source of tendon magic angle effect. J Magn Reson Imaging 25: 346-361.
Fullerton GD, Zimmerman RJ, Cantu C, and Cameron IL (1992). New expression to describe solution nonideal osmotic pressure, freezing point depression, and vapor pressure. Biochem Cell Biol. 70: 1325-1331.
Gallyas F and Pál J (2008). Whole-cell phase transition in neurons and its possible role in apoptotic cell death. Phase Transition in Cell Biology: 63-71. Eds. Pollack GH and Chin WC. Springer, Berlin.
Garlid KD (2000). The state of water in biological systems. Int Rev Cytol 192: 281-302.
Havenith M (2011). Water and biological molecules protect by terahertz spectroscopy. www.hpsp.org/water-and-biological-molecules
Heugen U, Schwaab G, Brundermann E, Heyden M, Yu X, Leitner DM and Havenith M (2006). Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc Natl Acad Sci 103: 12301-12306.
Jasnin M, Moulin M, Haertlein M, Zaccai G, and Tehei M (2008). Down to atomic-scale intracellular water dynamics. EMBO reports 9: 543-547.
Kamitsubo E (1972). Motile protoplasmic fibrils in cells of the Characae. Protoplasma 74: 53-70.
Kim SJ, Born B, Haventih M and Gruebele M (2008). Real-time detection of protein-water dynamics upon protein folding by terahertz absorbance. Ange Chem Int Ed 47: 6486-6489.
Klotz IM (1962) Water. Horizons in Biochemistry: 523-550. Eds. Haska M and Pullman B. Acad. Press, NY.
Krise KM and Milosavljevic BH (2011). Mobility of molecules and ions solubilized in protein gels: diffusion in the thick fractions of hen egg white. Biomacromolecules 12: 2351-2356.
LeBard DN and Matyushov DV (2010). Ferroelectric hydration shells around proteins: electrostatics of the protein-water interface. J Phys Chem 114: 173-189.
Ling GN (2006). A convergence of experimental and theoretical breakthrough affirms the P M theory of dynamically structured call water on the theory’s 40th birthday. Water and the Cell: 1-52. Eds. Pollack GH, Cameron IL, Wheatley DN. Springer, Berlin.
Luukkonen P, Maloney T, Rantanen J, Paulapuro H, Yliruusi J (2001). Microcrystalline cellulose-water interactions – a novel approach using thermoporosimetry. Pharm Res 18: 15662-15669.
Maric K, Wiesner B, Lorenz D, Klussman E, Betz T and Rosenthal W (2001). Cell volume kinetics of adherent epithelial cells measured by laser scanning reflection microscopy: determination of water permeability changes of renal principle cells. Biophysical J 80: 1783-1790.
Mastro AM and Hurley DJ (1985). Diffusion of a small molecule in the aqueous compartment of mammalian cells. Organization of Cell Metabolism: 57-74. Eds. Welch R and Clegg JS. Plenum Press, NY.
Mayer F, Wheatley D, Hoper TM (2006). Some properties of interfacial water: determinants of cell architecture and function. Water and the Cell: 253-272. Eds. Pollack GH, Cameron IL, Wheatley DN. Springer, Berlin.
Mentré P and Hui BH (2001). The effects of high hydrostatic pressure on living cells: a consequence of the properties of macromolecules and macromolecular associated water. Int Rev Cytol 201: 1-84.
Mertré P (20102). Water in the orchestration of the cell machinery. Some misunderstandings: a short review. J Biol Phys 38: 13-26.
Merzel F and Smith JC (2005). High-density hydration layer of lysozyme: molecular dynamic decomposition of solution scattering data. J Chem Inf Model 45: 1593-1599.
Miller S, Lesk AM, Chothia C (1987b). Interior and surface of monomeric proteins. J Mol Biol 196: 641-56.
Miller S, Lesk AM, Janin J, Chothia C (1987a). The accessible surface area and stability of oligometric proteins. Nature 328: 834-836.
Mollenhauer HH and Morre DJ (1978). Structural compartmentation of the cytosol: zones of exclusion, zones of adhesion, cytoskeletal and intercisternal elements. Ed. Roodyn DB. Subcellular Biochemistry 5: 327-362. Plenum Press, NY.
Persson E and Halle B (2008). Cell water dynamics on multiple time scales. Proc Natl Acad Sci 105: 6266-6271.
Pollack GH and Clegg JS (2008). Unexpected linkage between unstirred layers, exclusion zones and water. Phase Transitions in Cell Biology: 143-152. Eds. Pollack GH and Chin WC. Springer, Berlin.
Qvist I, Persson E, Mattea C, and Halle B (2009). Time scales of water dynamics at biological interfaces: peptides, proteins and cells. Faraday Discuss 141: 131-144.
Record MT, Courtenay ES, Cayley DS, and Guttman HJ (1998). Responses of E. coli to osmotic stress: large changes in amount of cytoplasmic solutes and water. Trends Biochem Sci 23: 143-148.
Stebbings H and Hunt C (1982). The nature of the clear zone around microtubules. Cell and Tissue Res 227: 609-617.
Stebbings H and Willison JHM (1972). Structure of microtubules: a freeze-etched and negative stained microtubules from the ovaries of Notonecta. Z Zellforsh Mikrosk Anat 138: 387-396.
Sunde EP, Setlow P, Hederstedt L, Halle B (2009). The physical state of water in bacterial spores. Proc Natl Acad Sci 106: 19334-19339.
Trombitos K, Baatsen P, Schreuder J, Pollack GH (1993). Contraction-induced movement of the water in a single fiber of frog skeletal muscle. J Mus Res Cell Motil 14: 573-584.
Wolf J, Bryant C, Koster KL (2002). What is unfreezable water, how unfreezable is it and how much is there? Cryo Letters 23, 157-166.
Zhang J, Pollack GH (2006). Solute exclusion and potential distribution near hydrophilic surfaces. Water and the Cell: 155-174. Eds. Pollack GH, Cameron IL and Wheatley DN. Springer, Berlin.
Zheng JM, Chin WC, Khyniak E, Khyniak E Jr, Pollack GH (2006). Surfaces and interfacial water: evidence that hydrophilic surfaces have long-range impact. Adv Colloid Interface Sci 127: 19-29.
Zierold K (1986). The determination of wet weight concentration of elements in freeze-dried cryosections from biological cells. Scanning Electron Microsc 2: 713-724.
Discussion with Reviewers
Anonymous Reviewer: At higher osmotic pressure the extent of osmotically unresponsive water in cells is reduced. Is there an explanation?
Cameron I and Fullerton G: Motionally slowed or encapsulated water molecules that have been restricted in their ability to interact with the cells osmotic membrane become available for membrane interaction due to the higher pressure. Centrifugal force pressures also increase water motion as reported in Table 4.
Reviewer: There is a distinction between TEM zones lacking observable structural elements and zone of solute exclusion containing only structural water as reported by Pollack? Do you agree?
Cameron and Fullerton: We do agree. As you have pointed out to us, specialists of electron microscopy know that the opacity of a zone is a relative notion of contrast. Keith Porter explained that the clearest EM zones contain networks of macromolecules that he names hyaloplasm (J Cell Biol 99: 1-248, 1984). Thus hyaloplasm is better thought of as a sponge or gel saturated with interfacial water as the gel described by Gallyas and Pal 2008 and loses water upon cell damage (also see text of this report).
