Making Sense of Milling: the Role of Water on the Micro-Structural Relaxation-Like of Cryo-Milled Griseofulvin
Feng T1, Stanciu L2, and Carvajal MT1,3*
1Department of Industrial and Physical Pharmacy, Purdue University, West Lafayette, IN 47907, USA
2Material Sciences Engineering, Purdue University, West Lafayette, IN 47907, USA
3Agricultural and Biological Engineering, Purdue University, West Lafayette, IN 47907, USA
*Correspondence E-mail: tcarvaja@purdue.edu
Key Words: Griseofulvin, Relaxation, Temperature, Humidity, Annealing, Water, Crystal defects, Relative humidity
Received February 8th, 2012; Accepted March 25th, 2012; Published July 29th, 2012; Available online August 3rd, 2012
Summary
The purpose of this investigation was to study the effect of different annealing conditions (moisture and temperature) on the relaxation-like or rearrangement of cryogenic milled griseofulvin (cMG). Different analytical tools were used to characterize the structural changes and to monitor the stability of cMG. The cMG is thermodynamically unstable because it apparently contains some defective crystals. Due to the high Gibbs free energy acquired upon milling, the material was susceptible to relaxation or annealing upon exposure to favorable environmental conditions of temperature and humidity. Upon storage under certain conditions, the cMG restored some of its original arrangement of the molecules and/or crystal growth (sintering) which was dependent on the annealing temperature and duration. Possible relaxation-like regimes can be explored to explain the proposed findings. The isothermal annealing behavior of cMG may be important for milled powders where the material presents regions that are physically distinct from amorphous (totally disordered), hence the crystal defects (dislocated) are suggested.
Article Outline
Introduction
Results and Discussion
Conclusions
References
Discussion with Reviewers
Introduction
Micronization, also known as milling, is a common practice in the processing of medicines. Size reduction offers many potential advantages such as an increase in surface area and in dissolution rate giving as a result an enhancement on bioavailability of the final pharmaceutical products (Kraml et al 1962, Ober et al 1958, Parrott 1975, Macdonald and Himelick 1948, Kanig 1963, Fincher 1968). Additionally, micronization improves accessibility of inhalable products where the API is required to reach various parts of the lung (Patton and Platz 1992).
Milling has been generally associated with polymorphic transformations of various drugs such as indomethacin (Otsuka et al 1986a, Otsuka et al 1986b), chloramphenicol palmitate (Otsukaand and Kaneniwa 1986, Kaneniwaand and Otsuka 1985), and phenylbutazone (Matsumoto et al 1988). It has also been coupled with the dehydration of carbamazepine dihydrate (Otsuka 1999). Milling may affect the chemical reactivity of the solid-state Millard reaction between metaclopramide hydrochloride and lactose (Qiu et al 2005a, Qiu et al 2005b) and of piroxicam (Sheth et al 2005). The underlying mechanisms of the transformations induced by micronization are still unclear. It has been generally believed that the amorphous phase is the intermediate phase which plays a key role in subsequent transformations. However, Elamin (1994) reported that milling enhances the solubility of griseofulvin without the need to form an amorphous solid. This effect was suggested to be linked to the formation of defective crystals. Shalaev (2002) showed that milling of tetraglycine methyl ester induces a disordered phase, which was claimed as a crystalline mesophase with combined properties of the crystalline and amorphous phase. Bates et al. (2007) studied the crystalline defects introduced during the dehydration of raffinose pentahydrate using X-ray diffuse scattering and pair-wise distribution function (PDF).
Overall, little work has been found where researchers proposed that crystal defects are induced in organic crystalline materials during milling. Tao et al (2008) proposed that cryogenic milling of griseofulvin resulted in the formation of crystal defects rather than an amorphous phase. The authors also proposed an energy regime of the various solid states, crystalline raw material and amorphous (by mechanical processing or quench melt) vs defective crystal generated by milling. On this free energy landscape, there could be a continuum of various solid states between stable crystalline state and liquid state depending on the type and density of crystal defects. These states generally include all types of metastable states, such as different metastable polymorphs and amorphous solids relevant to food and pharmaceutical materials. Wunderlich (1998) reported the three types of crystalline mesophases: liquid crystal, plastic crystal, and conformationally disordered (condis) crystal. These mesophases may lose one or two of the three-molecular order: translation, orientation, and conformation.
Duncan-Hewitt and Weatherfield (1989) studied the mechanical properties of materials using microindentation techniques, one proposed phenomenon was the creation of dislocations in the crystal lattice. Dislocations occur when atoms are not spaced at their defined positions within a lattice and are a consequence of subjecting the material to mechanically strained process such as milling or as crystal growth causing further misalignments (Hiestand 2002, Juhasz 1998).
For a decade, a metastable state (defective state) has increased research interest, being noticed and discussed in the pharmaceutical literature (Otsuka et al 1986a, Elamin et al 1994, Shalaevet al 2002, Bates et al 2007, Feng et al 2008).
For purpose of clarification, defective crystals are referred to crystalline materials that contain certain amount of dislocations that practically impact its physical and chemical properties. Amorphous solids are non-crystalline, lack of long-range order characteristics of crystals where the disordered state increases molecular mobility. The use of crystal disorder and dislocations will be used in this work to refer to materials that are still crystals, even after milling but are not amorphous.
During product development, it is critical to identify potential risks of solid- state transformations throughout processing and storage. Under intensive mechanical processing, such as attrition, milling may lead to the generation and accumulation of crystal disorder or dislocations. This may result in a structurally unstable state that could revert to crystal growth (sintering – the process of fusing particles together by heating below melting point, use in ceramics and powder metallurgy) when exposed to favorable environmental conditions of temperature and/or humidity.
Thus, in order to avoid surprises during development or storage, it is crucial to understand the implications of milling, such as the structural relaxation under various conditions. The structural relaxation of amorphous solids has been extensively studied for pharmaceutical materials (Hodge 1994, Ediger 1996, Angell et al 2000, Vyazovkinand and Dranca 2005, Kawakamiand and Pikal 2005, Kakumanuand and Bansal 2002, Fukuoka et al 1991).
However, to our knowledge, research regarding the relaxation-like or sintering behavior of disordered crystals of organic materials is scarce. The focus and motivation of this research is to investigate the role of environmental conditions on the structural relaxation-like or sintering of cMG. This relaxation-like phenomenon can be proposed as a low-temperature sintering due to the fact that the dislocated/defected material is crystalline material that stabilizes by crystal growth. The conditions of temperature (T) and humidity (RH) are used as the basis to formulate a strategy to either prevent the defective crystal from stabilizing it or facilitate annealing; this will depend on the specific needs during product development.
In this work, relaxation-like or annealing will be used when referring to the molecular motion leading to the re-arrangement of the defective crystals as depicted in Figure 1 (modified and adapted from Hancock and Zografi 1997). The re-arrangement process alludes to the reorientation or relocation of local clusters of molecules that may lead to sintering or crystal growth process of the crystalline dislocated material. Again, this phenomenon is different from that of the relaxation of the amorphous phase where relaxation usually refers to the molecular motion which drives the glassy system to its equilibrium state.
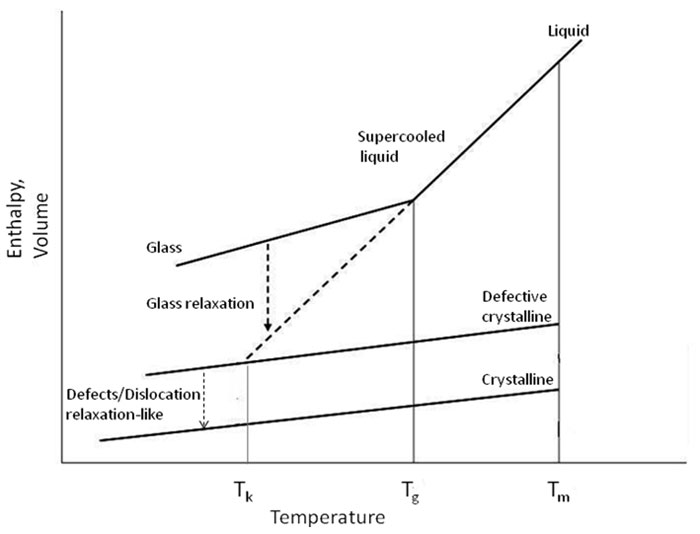
Figure 1: Schematic depiction of the variation of enthalpy (or volume) as a function of temperature for crystalline, amorphous (glassy) and defected (dislocated) crystalline material Tm is the equilibrium melting point, Tg is the glass transition temperature, Tk is Kauzmann (critical) temperature. Note the glass relaxation and the proposed defects relaxation-like are two different processes and marked on the diagram. (Modified and adapted from Hancock and Zografi 1997).
It is recognized that this type of system can be considered in the border line of amorphous and crystalline domains; this may cause conflict when referring to the milled material as defected or dislocated rather than either amorphous or crystalline. But the fact is that the mechanically stressed of some materials may not result in transformation to amorphous phase but rather present more similar solid state properties from those of the crystalline material than the amorphous counterparts.
Materials and Methods
Materials
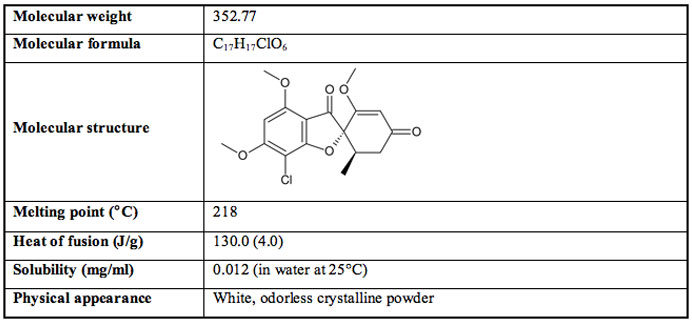
Crystalline griseofulvin was purchased from Sigma (St Louis, MO) and stored at 25°C in a desiccator over P2O5 before use. The relevant physicochemical properties of griseofulvin are summarized in Table 1.
Table 1: Physicochemical properties of Griseofulvin.
Cryogenic Milling of Griseofulvin
Cryogenic milling was reported previously (20), using a SPEX CertiPrep 6750 cryogenic impact mill (Metuchen, NJ). The samples were milled while completely submerged in a bath of liquid nitrogen. Milling time includes 10, 30 and 60 min. The samples were equilibrated for 30 min at room temperature in a desiccator over P2O5 then stored at 0ºC. The griseofulvin’s glass transition temperature is about 85ºC (29), and thus it is safe to keep milled griseofulvin sample below the glass transition temperature to inhibit possible aging effects, some have reported even 50ºC below the glass transition (Chryssikos et al 1991).
Stability Studies: Role of T and RH on the Relaxation of the Cryomilled Samples
a). Temperature – The 60-min cMG samples were stored in a P2O5 desiccator to allow annealing in the oven at two different temperatures (70ºC and 110ºC) for 10 and 30 min.
b) Humidity – The 60-min cMG samples were equilibrated overnight in the Qudrapack bottles at different RH using saturated salt solution at 25ºC. The RH levels investigated are: 11%, 22%, 33% and 75%. All samples were analyzed immediately after the equilibration period.
X-ray Powder Diffraction (PXRD)
Samples were analyzed using a Shimazu XRD-6000 X-ray powder diffractometer the conditions were the same as reported previously (20). Briefly, samples were scanned at 0.02º 2θ/s from 5 to 40º 2θ. Three scans of each sample were collected to check for data reproducibility. A silicon standard was analyzed to check the instrument alignment every time before sample measurements.
Differential Scanning Calorimetry (DSC)
A TA instrument Q10 DSC was used for thermal analysis of the samples. The DSC (TA Q10) was calibrated for enthalpy using high purity Indium. The temperature was calibrated by the three-point method. Nitrogen was used as the purging gas at a constant flow rate of 50 ml/min. Samples of 3 mg were weighed, placed and heated in aluminum hermetic pans at 10°C/minute to pass the melting point of 218°C.
Water Vapor Sorption Analysis
Water vapor sorption measurements were done with a symmetrical gravimetric analyzer Model 100 (SGA-100, VTI, FL). About 15~20 mg of the sample was placed in a glass sample holder using an empty sample holder as the reference. The sample was first dried at 0% RH under pure dry nitrogen at a flow rate of 180 ml/min for 2 hours and then exposed to the desired RH. At each RH, the maximum equilibration time was 3 hours. After the experiments, the time-course data was checked to make sure that at each RH, if a change in the sample weight occurred, equilibrium was achieved. At all times the unit was controlled at 25°C using a circulated water system.
Surface Area Measurement
The surface area was measured using Micrometrics Tristar 3000 Surface Area Analyzer (Norcross, GA) by adopting Brunauer-Emmett-Teller (BET) method. The cryo-milled griseofulvin samples were stored in the P2O5 desiccator at 0°C before starting the experiment. Three samples of around 150 mg were loaded into the glass tubes and jacketed with thermal insulators. The samples were initially cooled to the cryogenic temperature and then exposed to nitrogen at a series of precisely controlled pressures. In each measurement, eight points were collected linearly spaced between 0.05 and 0.4 of the partial pressure. With each incremental pressure increase, a volume of the nitrogen was adsorbed on the surface of the sample; this was recorded and used to calculate the surface area.
Inverse Gas Chromatography (IGC)
The IGC experiment was conducted using a commercial IGC system (iGC, Surface Measurements System Ltd., London, UK). The method is similar as previously reported (Otte and Carvajal 2010). The columns contain the samples under study, and then the columns were equilibrated with dry helium at 303K for prior to analysis. Helium was used as the carrier gas, probes of different polarity (from non-polar to polar) and methane as the inert gas (non-retention gas). The calculation of the dispersive and specific surface energy is from the data of triplicates.
Scanning Electron Microscopy
The samples were sputter-coated in a Hummer II sputter coater (Technics, Inc., Alexandria, VA) for 3 min at 100 mTorr and 10 mA with gold : palladium. SEM images were taken with nanoSEM200 field emission scanning electron microscope at 3 kV (FEI Company, Hillsboro, Oregon).
Microstructural analysis by Transmission Electron Microscopy (TEM)
The cryo-milled griseofulvin crystals were imaged with an FEI CM-100 transmission electron microscope, operating at 80kV. Cryo-milling ensured the specimen has a thickness appropriate for TEM imaging (of less than 100 nm).
Results and Discussion
In a previous work, it was reported that the unit operation of milling may change the physico-chemical properties of the API due to the generation of disorder in crystalline materials during the process (Feng et al 2008). This work is a follow-up with particular interest in stability and further changes on the milled griseofulvin material. When the drug substance was milled to reduce particle size, it was also noticed that the crystal habit had changed as well (Figure 2). This seems to depend on the physicochemical properties of the material. These changes in morphology have been observed previously (Feeley et al 1998, Carvajal and Staniforth, 2006 and Chikhalia et al 2006). It has been also observed that griseofulvin crystal and properties are different from those of ibuprofen crystals, which convert to amorphous fairly quickly after milling (Crowleyand and Zografi 2002).

Figure 2: Scanning Electronic Micrograph of Griseofulvin. Un-milled plated and milled elongated and fused.
The surface energetics data are shown in Figure 3. The results seem to indicate that the surfaces of the three materials, un-milled, cryo-milled and amorphous, are different. Thus, it is reasonable to propose microcrystalline dislocations (defects) for the milled material since it presents characteristics that are physically distinct from amorphous (totally disordered). This is based on the energetic results (Figure 3), on previous DSC results (Feng et al 2009) where there is no Tg, on reported PXRD (Feng et al 2009) that shows crystallinity for the 30-min cryomilled griseofulvin and on similar observations using thermally stimulated current (TSC) for micronized substances where different milling energies were found (Forcino et al 2010).
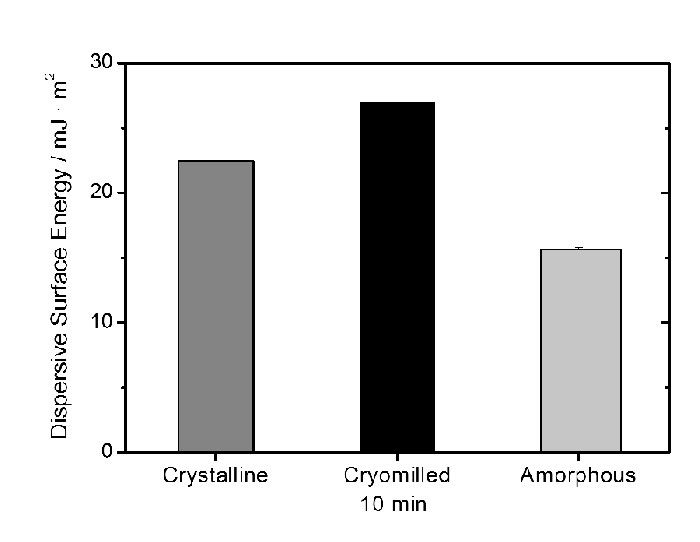
Figure 3: Dispersive surface energy for crystalline, cryomilled and amorphous
The defective crystal structure when exposed to conditions of temperature and/or humidity decreases its energy state to the crystalline form to some extent. The EFFECT under these conditions was investigated in terms of relaxation-like or annealing scenario of the milled material.
Effects of Temperature on Cryo-Milled Griseofulvin (cMG)
Reduction of crystallinity in the lattice as well as disordered molecules with high molecular mobility was the result after cryo-milling the material. The cMG is considered to be in a metastable state with higher Gibbs free energy than that of the ground state (crystalline phase). This results into molecular rearrangement and alignment to form a more ordered pattern under conditions of temperature and humidity. This process is referred to as annealing or relaxation that leads to re-crystallization. Annealing is typically used in metallurgy referring to a heat treatment that alters the microstructure of a material causing changes in properties such as strength and hardness (Askelandand and Phule, 2003). As shown in Figure 4, the cryomilled 60 min griseofulvin was annealed at 70°C and 110°C for 10 min and 30 min, respectively. The PXRD patterns evolved with different annealing conditions. The overall trend is that the crystallinity increases with higher annealing temperature and longer annealing time.
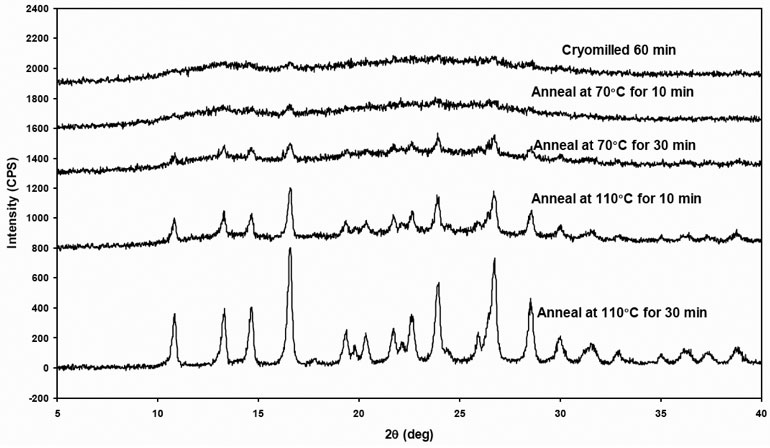
Figure 4: PXRD of freshly cryomilled griseofulvin followed by aged (annealed) at different temperatures and time lengths.
Figure 5 shows the DSC thermograms of cryomilled 60-min griseofulvin that was subjected to different annealing conditions. It is evident that with higher annealing temperature and longer annealing time, the recrystallization exotherm decreases. This trend agrees with what it was observed from the PXRD data. A decrease in the recrystallization exotherm reflected higher crystallinity which was gained during the annealing process. The experimental observation at the same heating rate was very reproducible in where the exotherms may show two relatively diffuse humps. These may change when changing the heating rate. However, focusing on this experimental set up, and observing the effect of temperature, it is hypothesized that these two parts could represent two different relaxation processes associated with dissimilar molecular mobilities. At low temperature (70°C), annealing can only rearrange the molecules which entail low mobility for relaxation. At high temperature (110°C), annealing can rearrange the molecules with higher mobility requirement. The PXRD data also suggest the same trend; the crystallinity is different at 70°C and at 110°C. Isothermal annealing at 70°C for 12 hours the DSC pattern was very similar to that of the shorter times, this could suggest that the event was thermodynamic activated rather than kinetics controlled.
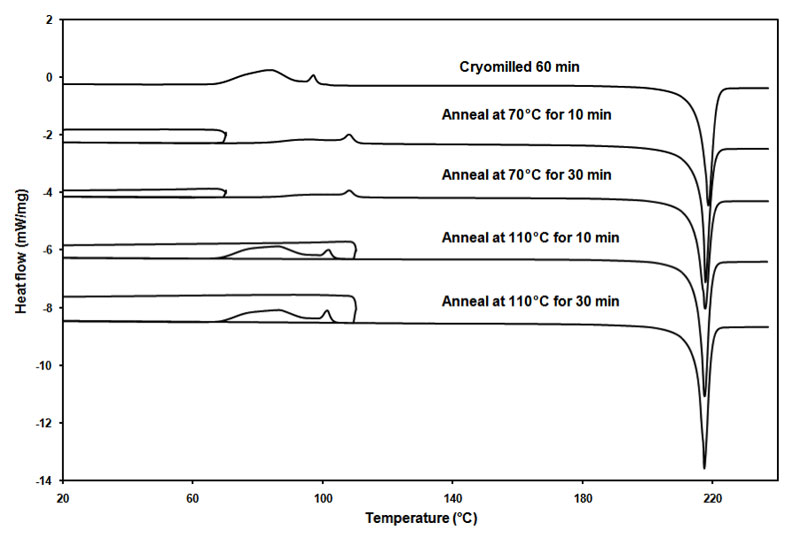
Figure 5: DSC thermograms of cryomilled 60-minute griseofulvin subjected to different annealing conditions.
Effect of Humidity on Cryomilled Griseofulvin (cMG)
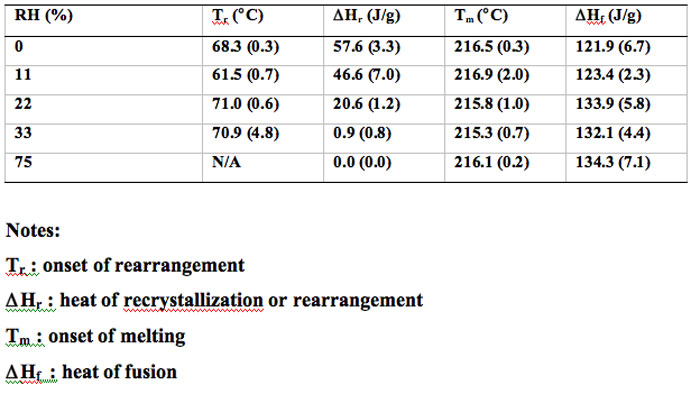
The disordered (defective/dislocated) crystal obtained after cryomilling griseofulvin is considered a highly energetic state therefore it has the tendency to revert to the ground state (native crystalline) due to the molecular mobility. Since water can act as plasticizer and diffuse into the interstices among molecules to increase the mobility of the host molecules, the cMG was subjected to various RH conditions to observe the relaxation and relaxation-like of this material. The PXRD data shows that by increasing RH, the crystallinity increases for both cryomilled 30 min and 60 min griseofulvin (Figure 6).
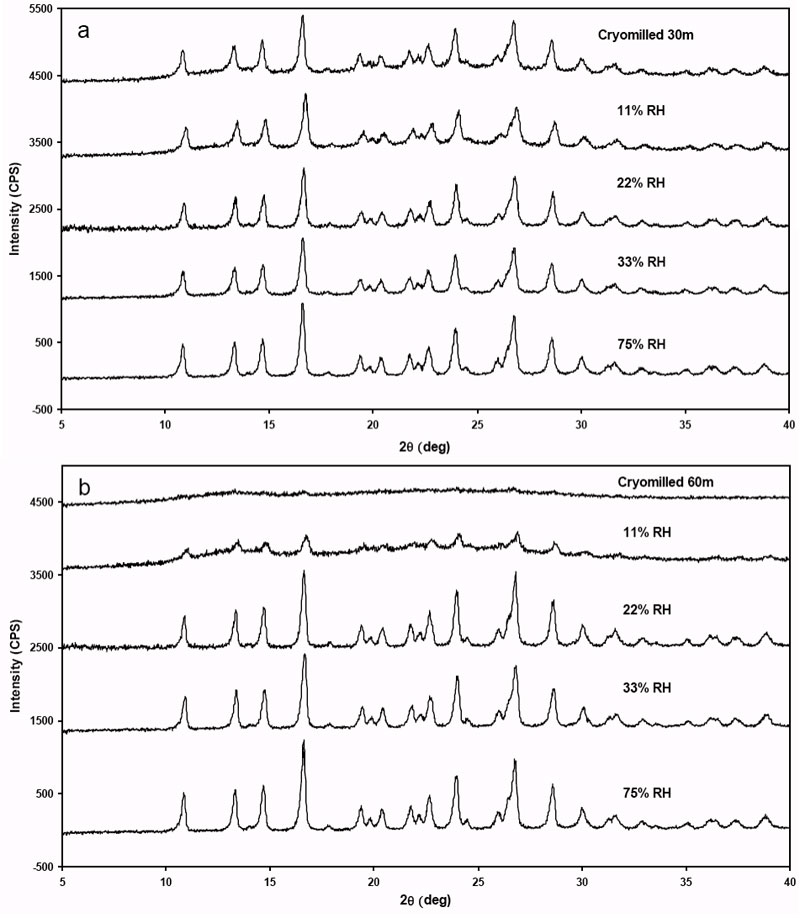
Figure 6: The PXRD for both cryomilled 30 minute and 60 minute griseofulvin as a function of % RH.
The DSC thermograms of cryomilled 30 min and 60 min griseofulvin are shown in Figure 7. The highly energetic cMG changed with increasing RH, the exotherm associated with the rearrangement decreases due to the annealing of griseofulvin at elevated RHs. The thermodynamic parameters, including the onset of rearrangement, recrystallization heat, the onset of melting and the heat of fusion, are included in Tables 2 and 3 for the cryomilled griseofulvin at 30 and 60-minute times, respectively.
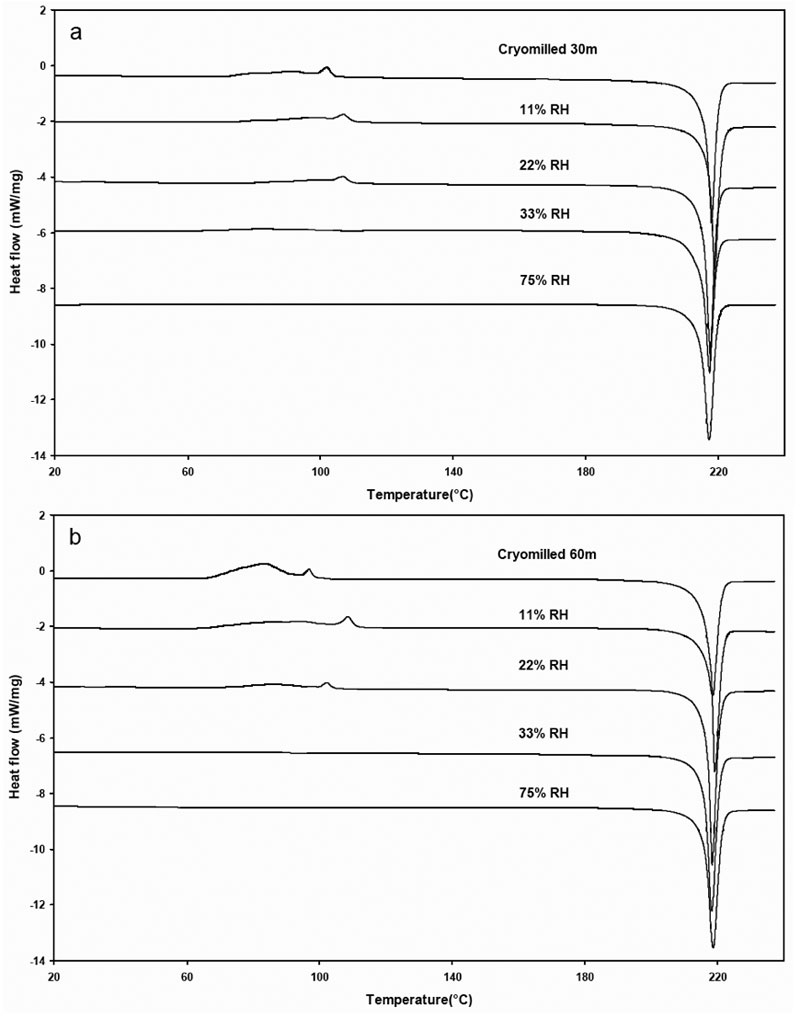
Figure 7: DSC thermograms of cryomilled 30 minute and 60 minute griseofulvin at various % RH.
Table 2: Summary of thermodynamic parameters of cryomilled 30 minute griseofulvin subjected to different RH conditions.
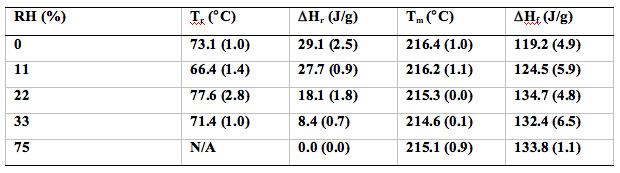
Table 2: Summary of thermodynamic parameters of cryomilled 60 minute griseofulvin subjected to different RH conditions.
When the cMG sample is exposed to higher RH, it is likely that water adsorbs onto the surface of crystal and diffuses into the crystal lattice. Water molecules interact with griseofulvin molecules increasing their mobility and facilitating their rearrangement. It is hypothesized that a portion of the distorted or disoriented molecules are rearranged and reverted to more ordered state.
When the conditioned sample was characterized under the DSC, the molecular rearrangement exotherm associated with the low temperature was lost because those molecules had already been reordered during the annealing process. However, when the RH is relatively low (11%), it may have the opposite effect. It seems that at low RH, only a small amount of water was adsorbed at the surface of the sample. These water molecules could only increase the mobility of griseofulvin to a very limited extent, which is possibly not high enough to initiate significant recrystallization or reordering of the griseofulvin molecules.
When this sample was analyzed by DSC, this phenomenon reflects the fact that subtle difference of moisture uptake could result in significantly change on relaxation behavior.
The crystallinity or rather the crystal growth achieved after subjecting the cMG to various RHs was estimated based on the DSC method described in previous work (Feng 2008) and plotted on Figure 8. The cryomilled 30 min and 60 min samples had similar trend and converged with increasing of RH. Both curves showed sigmoid type behavior, suggesting that the crystallinity restoration or crystal growth could be divided into three regions. At low RH (below 11% RH), there was only marginal crystal growth gained due to the small amount of water adsorbed mainly on the surface of the sample. At intermediate RH (between 11% and 33% RH), a significant amount of water was absorbed in the bulk of the sample and crystal growth increased quickly and almost achieved 100%. At high RH (above 33%), the recrystallization and rearrangement of the cryomilled samples completely recovered the crystallinity or that the crystals ended growing.
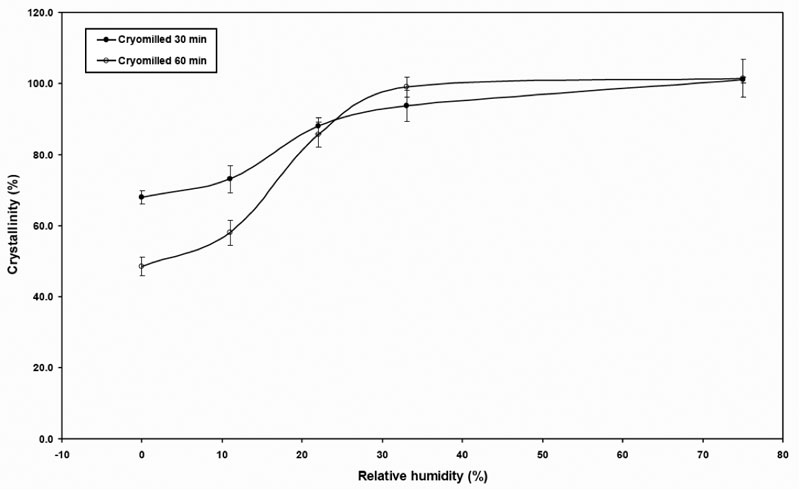
Figure 8: Crystallinity estimated based on the DSC data after subjecting the cMG to various RHs.
The vapor sorption isotherm profile of cMG is shown in Figure 9. It is apparent that the cryomilled samples showed much stronger water sorption compared to that of the unmilled sample. This suggests that water has much stronger interaction with the defective/disordered crystal than the native crystalline materials. With the increase in surface area and the accumulation of crystal defects, more free volume was created in the crystal lattice. Water molecules could be absorbed into the bulk of the defective crystals instead of being adsorbed at the surface. This led to significant increase of vapor sorption for cryomilled materials.
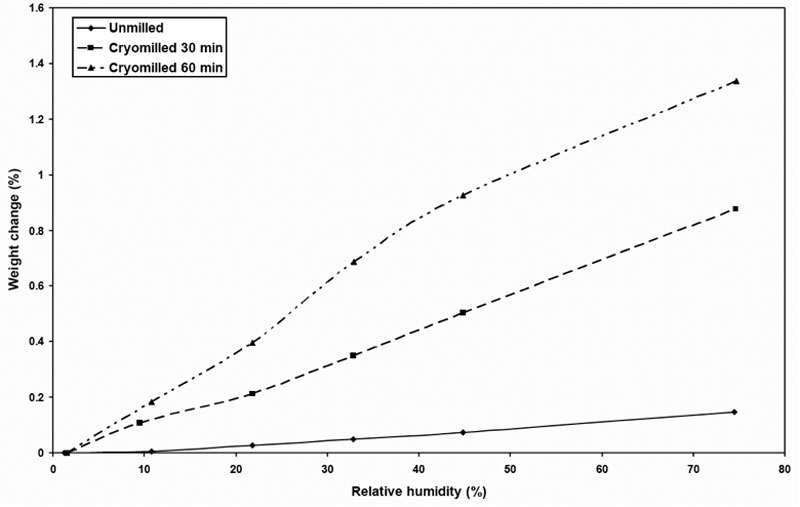
Figure 9: Vapor sorption isotherm profile of cMG.
Annealing Study
In an effort to better understand the relaxation-like effect of the defective crystals, the cryomilled 30-min griseofulvin sample was subjected to isothermal annealing at 75% RH at 10 min, 30 min, 60 min and 24 hr. Upon completion of annealing, the samples were immediately analyzed by DSC (Figure 10). The vapor sorption profiles of the samples at 75% RH against time were also collected to support interpretation of the relaxation process (Figure 11).
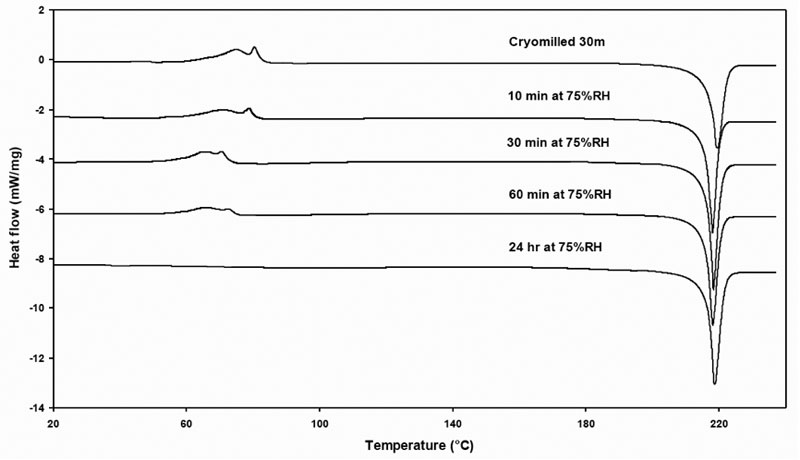
Figure 10: Thermogram profiles of annealed 30-minute cryomilled samples.
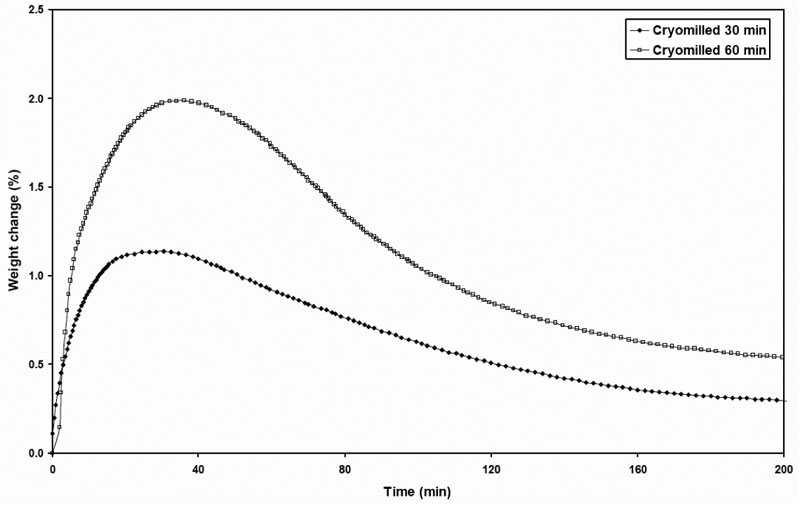
Figure 11: Vapor sorption profiles of the samples at 75% RH at various times for both 30- and 60 minute cryomilled material.
The vapor sorption profiles for both cryomilled 30-min and 60-min samples had an annealing process at 75% RH. The adsorption peak occurred at about 30 min for both samples, and then rearrangement started after that point. When comparing the equilibrium vapor sorption of cryomilled sample with that of the unmilled sample at 75% RH, it is observed that the cryomilled sample still adsorbed significantly more than that the unmilled crystalline griseofulvin even after the rearrangement. This is probably due to the surface area increase that material possessed from the milling process.
Comparing the vapor sorption behavior with the thermograms from the DSC, the results seemed to be on agreement. For the annealed 10 min sample, the rearrangement is almost unchanged, in terms of onset and amplitude. For the annealed 30 min sample, the onset for the crystal growth moves to lower temperature showing a diffuse hump. For the annealed 60 min sample, the onset for rearrangement also moves to lower temperature presenting a diffuse hump. For the annealed 24 hr sample, the whole crystal growth/rearrangement peak totally disappeared. We could speculate that the proposed dislocations move to the surface where they disappear by the rearrangement of the molecules.
Microstructural Analysis by TEM
The microstructural details on the crystal are apparent in Figure 12 despite the inherent low contrast of the images, specific to imaging organic molecules by TEM. The microstructure of the cryomilled griseofulvin indicates what appears to be a crystal defect due to the intensive mechanical stress during milling. The use of this technique was mainly as a support method for potential use in further observing the microstructural changes upon milling.
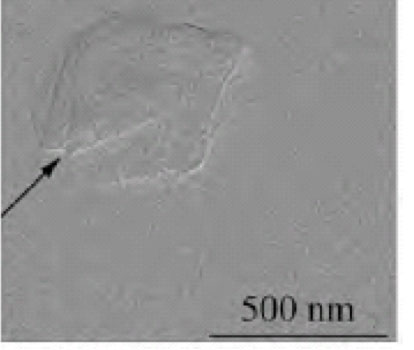
Figure 12: Imaging of griseofulvin by TEM the arrow indicates a crystal defect.
Conclusions
Cryogenic milling of crystalline griseofulvin resulted not only on the reduction of the particle size but also in decreasing crystallinity by the formation and accumulation of crystal defects (dislocation density) in the crystal lattice. After cryomilling griseofulvin, it gave the type XRPD halo pattern associated with amorphous samples. However, DSC analysis revealed remarkable difference from that of the amorphous phase. By exposing the defective crystals of griseofulvin to various conditions of temperature and humidity, the dislocated molecules gain certain mobility to rearrange and possibly to relax to a more ordered state. As the annealing temperature, time and RH increased, the cMG rearranged at various rates and extents. The relaxation-like processes are rather complicated and could be associated with molecular mobility. With subtle differences on the water vapor sorption, the relaxation-like or rearrangement of defective crystal could change drastically. The fundamental rearrangement and crystal growth mechanisms of defective crystals may lead to a better understanding and serve as the basis to formulate a strategy to either facilitate or inhibit the relaxation or rearrangement of milled materials, which would significantly affect the performance of the final product.
It is well-known that mechanical stress such as milling induces disorder, however this may not result in transformation to amorphous but rather to a crystalline material associated with crystal defects. As observed herein and before in the literature the physical attributes are different from the amorphous material; and the behavior of the defected dislocated materials is related to relaxation-like (for a lack of a better term) phenomenon to describe the rearrangement of molecules during the annealing process. Therefore, this phenomenon suggests manifesting the coalescence of crystal defects into clusters (crystal growth or sintering process) or migration of dislocations to the surface where these disappeared as a result of the rearrangement. However, evidence one way or another still needs additional investigation. In addition, the physical characteristics observed between the proposed defected crystal and amorphous form are different, this also deserves further examination. As a matter of fact, more recently, attention has been focused in the literature in interpreting an unusual DSC behavior for some organic crystals including griseofulvin. This atypical behavior is rather common but was not explored or paid attention in the past. It is believed that knowing and understanding the tendency behavior of milled material assists the formulator on the measures that must be taken to control environmental conditions during the mill unit operation process and during accelerated conditions of temperature and humidity for shelf-life stability assessment. This can potentially lead to a more rationale selection of the excipient partners (humidity interceptor, repellent or attractor), milling process and control of the environmental conditions based on the tendency for physical instability of the systems.
Acknowledgements
Funding was provided by the Purdue Research Foundation (PRF) and the National Science Foundation (NSF) Dane O. Kildsig Center for Pharmaceutical Processing and Research (CPPR) (EEC 0003064). We also thank the NSF Materials and Surface Engineering (CMMI- 0825994). We are grateful to Drs. Sai Chamarthy and Andrew Otte for their assistance in the surface energetics experiments.
References
Angell, C.A.; Ngai, K.L.; McKenna, G.B.; McMillan, P.F. and Martin, S.W. (2000). Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys., 88: 3113-3157.
Askelandand, D.R and Phule, P.P.(2003). The Three Stages of Annealing, The Science and Engineering of Materials, Brooks/Cole-Thomson Learning, Pacific Grove, CA, pp. 334-337.
Bates, S.; Kelly, R.C.; Ivanisevic, I.; Schields, P.; Zografi, G and Newman, A.W. (2007). Assessment of defects and amorphous structure produced in raffinose pentahydrate upon dehydration. J. Pharm. Sci., 96: 1418-1433.
Carvajal, M.T. and Staniforth, J.N. (2006). Interactions of water with the surfaces of crystal polymorphs. Int. J. Pharm. 307:216–224.
Chikhalia V, Forbes RT, Storey RA, Ticehurst M. (2006). The effect of crystal morphology and mill type on milling induced crystal disorder. Eur J Pharm Sci.27(1):19-26.
Chryssikos, G. D.; Kamitsos, E. I.; Bitsis, M. S.; Patsis, A. P. (1991). Journal of Non-Crystalline Solids, 131-133, 1068-1071.
Crowleyand, K.J. and Zografi, G. (2002). Cryogenic grinding of indomethacin polymorphs and solvates: Assessment of amorphous phase formation and amorphous phase physical stability. J. Pharm. Sci., 91: 492-507.
Ediger, M.D. (1996). Supercooled liquids and glasses. J. Phys. Chem. B, 100: 13200-13212.
Elamin, A.A.; Ahlneck, C.; Alderborn, G. and Nystrom, C.(1994). Increased metastable solubility of milled griseofulvin, depending on the formation of a disordered surface structure. Int. J. Pharm., 111: 159-170.
Feng, T.; Pinal, R and Carvajal, M.T. (2008). Process Induced Disorder in Crystalline Materials: Differentiating Defective Crystals from the Amorphous Form of Griseofulvin. J. Pharm. Sci., 97 (8): 3207-3221.
Feng, T., Bates, S. and Carvajal, M.T. (2009). Toward understanding the evolution of griseofulvin crystal structure to a mesophase after cryogenic milling. Int J Pharm.367(1-2):16-19.
Feeley, J.C., York, P., Sumby, B.S., Dicks, H. (1998). Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronization. Int. J. Pharm. 172, 89–96.
Fincher, J.H. (1968). Particle size of drugs and its relationship to absorption and activity. J. Pharm. Sci., 57: 1825.
Fukuoka, E.; Makita, M. and Nakamura, Y. (1991). Glassy state of pharmaceuticals.5. Relaxation during cooling and heating of glass by differential scanning calorimetry.Chem Pharm Bull 39: 2087-2090.
Hancock and Zografi, (1997). Characterization and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci., 86, 1-12.
Hiestand EN. 2002. Mechanics and physical principles for powders and compacts, 2nd edition. West Lafayette, IN: SSCI, Inc. p 110.
Hodge, I.M. (1994). Enthalpy relaxation and recovery in amorphous materials. J. Non-Cryst. Solids, 169: 211-266.
Juha`sz AZ. (1998). Aspects of mechanochemical activation in terms of comminution theory. Coll Surf A 141:449–462.
Duncan-Hewitt, W.C and Weatherly, G. C. (1989). Evaluating the hardness, Young’s modulus and fracture toughness of some pharmaceutical crystals using microindentation techniques. J. Mater. Sci. Lett. 8:1350–1352.
Kakumanuand, V.K. and Bansal, A.K. (2002). Enthalpy relaxation studies of celecoxib amorphous mixtures. Pharm. Res., 19: 1873-1878.
Kaneniwaand, N. and Otsuka, M. (1985). Effect of grinding on the transformations of polymorphs of chloramphenicol palmitate. Chem. Pharm. Bull., 33: 1660-1668.
Kanig, J.L. (1963). Pharmaceutical Aerosols. J. Pharm. Sci., 52: 513.
Kawakamiand, K. and Pikal, M.J. (2005). Calorimetric investigation of the structural relaxation of amorphous materials: Evaluating validity of the methodologies. J. Pharm. Sci., 94: 948-965.
Kraml, M.; Dubuc, J. and D. Beall (1962). Gastrointestinal absorption of griseofulvin.1. Effect of particle size, addition of surfactants, and corn oil on level of griseofulvin in serum of rats. Can. J. Biochem. Physiol., 40: 1449.
Macdonaldand, L.H.; Himelick, R.E. (1948). Effect of particle size, type of base, and a wetting agent on 3 antiseptic ointments. J. Am. Pharm. Assoc., 37: 368-369.
Matsumoto, T.; Ichikawa, J.; Kaneniwa, N. and Otsuka, M. (1988). Effect of environmental temperature on the polymorphic transformation of phenylbutazone during grinding. Chem. Pharm. Bull., 36: 1074-1085.
Ober, S.S.; Vincent, H.C; Simon, D.E. and Frederick, K.J. (1958). A rheological study of procaine penicillin-G depot preparations. J. Am. Pharm. Assoc. 47: 667-676.
Otsuka, M. Ofusa, T. and Matsuda, Y. (1999). Effect of environmental humidity on the transformation pathway of carbamazepine polymorphic modifications during grinding. Colloids Surf. B, 13: 263-273.
Otsuka, M.; Kaneniwa, N, and Matsumoto, T. (1986b). Relation between the transformation of indomethacin polymorphs during grinding and environmental temperature, and stability of noncrystalline solids obtained by grinding. J. Pharmcobio-Dyn, 9: S2-S2.
Otsuka, M.; Matsumoto, T. and Kaneniwa, K. (1986a). Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding. Chem. Pharm. Bull., 34: 1784-1793.
Otsukaand, M. and Kaneniwa, N. (1986). Effect of seed crystals on solid-state transformation of polymorphs of chloramphenicol palmitate during grinding. J. Pharm. Sci., 75: 506-511.
Parrott, E.L. (1975). Influence of particle-size on rectal absorption of aspirin. J. Pharm. Sci. 1975, 64: 878-880.
Otte, Andrew and Carvajal, M.T. (2010). Assessment of Milling-Induced Disorder of Two Pharmaceutical Compounds. J Phar Sci 100(5): 1793-1804
Pattonand, J.S.; Platz, R.M. (1992). Penetration enhancement for polypeptides through epithelia.D. routes of delivery – case studies.2.Pulmonary delivery of peptides and proteins for systemic action. Adv. Drug Deliv. Rev., 8: 179-196.
Qiu, Z.H.; Stowell, J.G.; Cao, W.J.; Morris, K.R.; Byrn, S.R. and Carvajal, M.T. (2005b). Effect of milling and compression on the solid-state Maillard reaction. J. Pharm. Sci., 94: 2568-2580.
Qiu, Z.H.; Stowell, J.G.; Morris, K.R.; Byrn, S.R. and Pinal, R. (2005a). Kinetic study of the Maillard reaction between metoclopramide hydrochloride and lactose. Int. J. Pharm., 303: 20-30.
Shalaev, E.; Shalaeva,M. and Zografi, G. (2002). The effect of disorder on the chemical reactivity of an organic solid, tetraglycine methyl ester: Change of the reaction mechanism. J. Pharm. Sci., 91: 584-593.
Sheth, A.R.;Lubach, J.W.; Munson, E.J.; Muller, F.X. and Grant, D.J.W. (2005). Mechanochromism of piroxicam accompanied by intermolecular proton transfer probed by spectroscopic methods and solid-phase changes. J. Am. Chem. Soc., 127: 6641-6651.
Vyazovkinand, S. and Dranca, I. (2005). Physical stability and relaxation of amorphous indomethacin. J. Phys. Chem. B, 109: 18637-18644 (2005).
Wunderlich, B. (1998). Condis crystals and their relation to liquid crystals, Conformational Motion and Disorder in Low and High Molecular Mass Crystals, Springer-Verlag, New York, NY, pp. 67
Discussion with Reviewers
Anonymous Reviewer: One could argue that the terminology used is not developed and quite undefined. For example, the use of defective crystals is not clear.
T. Feng, L. Stanciu and M. T. Carvajal: Defective crystals are referred to crystalline materials that contain certain amount of dislocations that practically impact its physical and chemical properties. Amorphous solids are defined as solids that lack of long-range order characteristics of crystals.
Reviewer: It seems that amorphous regions are formed in milling rather than defective crystals. Crystals give the x-ray diffraction, not the amorphous structures. Hence, there is some crystallinity and some noncrystalline regions.
Feng, Stanciu and Carvajal: The milling study generated some materials that do not have Bragg reflections. But lack of Bragg reflection does not necessarily suggest the amorphous nature of the materials. It has been proved that nanocrystalline material could also yield XRPD pattern without any Bragg reflections. In this case, we reported a phenomenon that defective crystals could also exhibit XRPD pattern that has no Bragg reflections. These observations are mainly discussed in a previous paper published by the same authors. (Process Induced Disorder in Crystalline Materials: Differentiating Defective Crystals from the Amorphous Form of Griseofulvin, J Pharm Sci:97(8):3207-3221 (2008).
Reviewer: The substance studied is poorly water soluble. How can the water plasticization of this substance be justified?
Feng, Stanciu and Carvajal: Based on the vapour sorption isotherm, the cryomilled 60 min griseofulvin can absorbs more than 1.2% moisture at 75% relative humidity. The absorbed moisture could diffuse into the voids in the materials and plasticises the API without necessary dissolving the API.
Reviewer: The glass transition measure-ments are missing or hidden.
Feng, Stanciu and Carvajal: The milled API obtained in this study is defective crystal instead of amorphous solid, Thus no glass transition temperature can be detected in the DSC experiment. (The details have been discussed in a previous paper published by the same authors. (Process Induced Disorder in Crystalline Materials: Differentiating Defective Crystals from the Amorphous Form of Griseofulvin, J Pharm Sci:97(8):3207-3221)
Reviewer: The title of the paper is misleading.
Feng, Stanciu and Carvajal: Indeed, we struggled to call it relaxation as it is a well-established term related to the phenomenon that occurs for amorphous materials. It seems, if permissible, to replace relaxation for relaxation-like for a lack of a better term for defective crystals or crystals with dislocations. Title has been re-worded.
Reviewer: No relaxations data are reported.
Feng, Stanciu and Carvajal: The relaxation-like is meant to refer to the molecular motion that leads to the re-arrangement of defective crystals or the re-distribution of the dislocation density. It is envisioned that if the dislocations are at or migrate (diffuse) to the surface, they tend to disappear when exposed to environmental conditions of temperature and humidity by transposing from high energy state to lower energy state. The relaxation-like or annealing was explored and characterized using a range of different analytical techniques such as XRPD, DSC, water sorption, SEM, and TEM.
Reviewer: The manuscript entitled “MAKING SENSE OF MILLING: THE ROLE OF WATER ON THE MICRO-STRUCTURAL RELAXATION OF GRISEOFULVIN” by T. Feng et al reports on a subject that seems suitable for the Water Journal and does appear to be an original contribution. The presentation, organization, and length are satisfactory and the references are adequate. While the data may have merit independent from the interpretations and conclusions, I have difficulty accepting the interpretations and conclusions as I do not believe alternative hypotheses have been adequately ruled out.
In a previous paper in the Journal of Pharmaceutical Sciences (Feng et al, 2008), the authors argued that defects produced by cryo-milling were not just intimate mixtures of amorphous and crystalline phases because physical mixtures of crystalline and amorphous griseofulvin exhibited different thermal behavior than cryogenic milled material. However, this may not have been an adequate test of the hypothesis. Cryo-milling may produce intimate mixutres of microscopic domains of amorphous material and seed crystals that cannot be reproduced by simply physically mixing bulk amorphous material with crystalline material. As domain sizes decrease, certain physical properties such as the temperature of recrystallization may be altered. The opinion of this reviewer is that the paper previous paper cited did not establish a firm foundation for the notion that defects produced by cryo-milling constitute a unique phase distinct from amorphous material. Therefore, the interpretations in the present manuscript lack foundation.
The depiction in Figure 1 of the amorphous glass and defective crystal as two different types of solids is also without a solid foundation. Some aspects of Figure 1 seem illogical or not well supported by experiments. For example, according to Figure 1, the crystalline solid and defective crystal have the same heat capacities – why should this be and what is the experimental support for this assertion? Is it possible for a glass to undergo relaxation to the extent that it would be equivalent to a defective crystal as suggested by the Figure? Figure 1 does not seem to be well supported by experimental data.
Generally, interpretation of the results of this paper would benefit from inclusion of results from parallel studies of amorphous systems stored under the same conditions.
Feng, Stanciu and Carvajal: The interpretation of our results and revision is based on the information provided in the present manuscript. Some references and results are added. The authors have proposed potential explanations to the atypical behavior of some milled powders, with the support of data and other publications in an attempt to address the observations. It is critical for the advancement and understanding to dare to speculate or hypothesize in a reasonable manner, therefore, opinions, questioning, disagreement and other explanations from the reviewers are welcomed, it is understandable as it seems that these type of systems are in the border line of amorphous and crystalline domains. It would be fantastic to have either our own data or from other researchers the final accounts such that these clarify and elucidate once and for all what it appears to lack in the present literature.
